Elisabeth Ekstrand, RPT, MSc1,2, Jan Lexell, MD, PhD1,2 and Christina Brogårdh, RPT, PhD1,2
From the 1Department of Health Sciences, Lund University and 2Department of Neurology and Rehabilitation Medicine, Skåne University Hospital, Lund/Malmö, Sweden
OBJECTIVE: To evaluate the test-retest reliability of isometric and isokinetic muscle strength measurements in the upper extremity after stroke.
DESIGN: A test-retest design.
SUBJECTS: Forty-five persons with mild to moderate paresis in the upper extremity > 6 months post-stroke.
METHODS: Isometric arm strength (shoulder abduction, elbow flexion), isokinetic arm strength (elbow extension/flexion) and isometric grip strength were measured with electronic dynamometers. Reliability was evaluated with intra-class correlation coefficients (ICC), changes in the mean, standard error of measurements (SEM) and smallest real differences (SRD).
RESULTS: Reliability was high (ICCs: 0.92–0.97). The absolute and relative (%) SEM ranged from 2.7 Nm (5.6%) to 3.0 Nm (9.4%) for isometric arm strength, 2.6 Nm (7.4%) to 2.9 Nm (12.6%) for isokinetic arm strength, and 22.3 N (7.6%) to 26.4 N (9.2%) for grip strength. The absolute and relative (%) SRD ranged from 7.5 Nm (15.5%) to 8.4 Nm (26.1%) for isometric arm strength, 7.1 Nm (20.6%) to 8.0 Nm (34.8%) for isokinetic arm strength, and 61.8 N (21.0%) to 73.3 N (25.6%) for grip strength.
CONCLUSION: Muscle strength in the upper extremity can be reliably measured in persons with chronic stroke. Isometric measurements yield smaller measurement errors than isokinetic measurements and might be preferred, but the choice depends on the research question.
Key words: outcome assessment; muscle, skeletal; reproducibility of results; rehabilitation.
J Rehabil Med 2015; 00: 00–00
Correspondence address: Elisabeth Ekstrand, Department of Health Sciences, Lund University, Box 157, SE-221 00 Lund, Sweden. E-mail: elisabeth.ekstrand@med.lu.se
Accepted Apr 24, 2015; Epub ahead of print Jul 16, 2015
INTRODUCTION
Stroke is one of the main causes of disability worldwide (1). It often leads to a variety of sensorimotor impairments; up to 70% of stroke survivors have reduced arm and hand motor function in the acute phase after stroke (2, 3). Decreased muscle strength is the most common impairment in the upper extremity after stroke (4, 5), which can impact the ability to perform many daily activities (6).
To evaluate recovery of muscle strength and the effects of interventions, reliable outcome measures are needed. Today, isokinetic dynamometers are considered the gold standard for accurate strength measurements in healthy persons as well as in persons with neurological diseases (7, 8). These dynamometers enable measurements of both isometric and isokinetic muscle strength. Isometric measurements are easier to perform, as they are made in a stable position, whereas isokinetic measurements assess the dynamic torque development and therefore better reflect activities in real life (9). While, traditionally, hydraulic hand-held dynamometers are used to measure isometric grip strength (10), electronic dynamometers can provide more precise and detailed information about grip strength.
It has been shown that isokinetic muscle strength in the upper extremity, as measured with electronic dynamometry, can be reliably assessed in healthy persons (11–13), but to the best of our knowledge, no study has evaluated whether isokinetic muscle strength in the upper extremity can be reliably measured after stroke. A few studies have evaluated the reliability of isometric muscle strength in the upper extremity after stroke. However, these studies have limitations, such as small sample sizes (10–18 persons) (14–16), inclusion of participants in the acute phase, when spontaneous recovery can still be expected (15, 17), large variation between test occasions (6–84 days) (12) or very short intervals between measurements (1 h) (15). Moreover, these studies have used different statistics to evaluate the test-retest reliability and the measurement error, such as the intra-class correlation coefficient (ICC) (16, 17), standard error of measurement (16), coefficient of variation (14, 15) and smallest real difference (15, 17). This makes it difficult to compare the results between the studies. In addition, none of the previous studies have fully evaluated the reliability, i.e. assessed test-retest reliability and systematic and random measurement errors, for a group of individuals and for a single individual.
The ICC is commonly used to evaluate test-retest reliability. However, it is generally agreed that the ICC is insufficient as a single measure of reliability. The ICC evaluates the agreement between repeated test occasions, and thereby only evaluates the variance between individuals. A high ICC does not always mean that the measurement is reliable and relevant for clinical use. Measurement errors (systematic and random) should also be small and measurements should be sufficiently sensitive to detect clinically real changes.
Therefore, the aim of this study was to evaluate the test-retest reliability of strength measurements in the upper extremity (isometric shoulder abduction, isometric elbow flexion and isokinetic elbow extension/flexion and isometric hand grip) in persons with chronic stroke and to assess the measurement errors in order to define limits for the smallest change that indicates a real change, both for a group of individuals and a single individual.
METHODS
Participants
Forty-five participants were recruited from a university hospital in the south of Sweden during the period April to December 2013. They had all been diagnosed with a cerebral infarction or cerebral haemorrhage and had been treated as inpatients or outpatients at the Department of Neurology and Rehabilitation Medicine. The participants were at least 6 months post-stroke and were considered to have mild to moderate paresis in their more affected upper extremity. This included a self-reported decrease in muscle strength, reduced dexterity and/or difficulties in performing daily hand activities, but an ability to bring the hand to the forehead and to grasp and release 1 block of the Box and Block test (18).
Exclusion criteria were: persons with self-reported full recovery of arm and hand function after stroke onset; other diseases that could have affected their arm and hand muscle strength; and an inability to understand and follow test instructions due to cognitive impairments or communication difficulties.
Ethics
Prior to inclusion, information about the purpose of the study was provided and each individual gave his or her written consent to participate. The principles of the Declaration of Helsinki were followed and the study was approved by the Regional Ethical Review Board, Lund, Sweden (Dnr 2012/591).
Procedures
Muscle strength in the upper extremity was measured on 2 occasions, one week apart. All assessments were performed at the same time of the day in a quiet separate room of the hospital by an experienced physiotherapist (first author). The less affected upper extremity was measured before the more affected. First, isometric shoulder abductor strength was measured, followed by isokinetic elbow extensor and flexor strength, isometric elbow flexor strength and, finally, grip strength. On each test occasion the arm strength measurements took approximately 45 min and the grip strength measurements 10 min to perform. All participants performed the measurements in the same order during both test occasions in order to secure standardization of the measurements and to avoid fatigue. During the measurements the participants were guided by standardized verbal instructions and encouragements, but were not allowed to see the computer display. A summary of the test protocols for the different measurements is presented in Table I.
|
Table I. Set-up and testing positions for isometric and isokinetic maximal muscle strength measurements of the upper extremity in persons with chronic stroke |
||||
|
Muscle group |
Mode |
Subject and upper extremity positions |
Dynamometer and chair positions |
Measurements |
|
Shoulder abductors |
Isometric |
Sitting upright, shoulder 15° abducted in the scapular plane, elbow extended, forearm in neutral position |
Dynamometer (Biodex System 3 PRO) rotated to 0° and tilted 10°, chair rotated to 75°, movement axis aligned with the axis of the acromio-clavicular joint |
Two maximal muscle contractions, lasting 3 to 5 s, 60 s rest interval Verbal encouragement: ”push, push, push” |
|
Elbow extensors and flexors |
Isokinetic |
Sitting upright, shoulder in 30° flexion and slight abduction, elbow supported, forearm supinated |
Dynamometer (Biodex System 3 PRO) rotated 30° away from the measured arm, chair rotated to 0°, movement axis aligned with the centre of the trochlea and the capitulum of the humerusa (cf Fig. 1) |
Three reciprocal extension/flexion maximal contractions 60°/s, no rest interval Verbal encouragement: ”extend and flex, extend and flex, extend and flex” |
|
Elbow flexors |
Isometric |
Sitting upright, shoulder in 30° flexion and slight abduction, elbow supported and 90° flexed, forearm supinated |
See isokinetic elbow extensors and flexors |
Two maximal muscle contractions lasting 3 to 5 s, 60 s rest interval Verbal encouragement: ”pull, pull, pull” |
|
Hand grip |
Isometric |
Sitting upright, forearm resting on a foam cushion, forearm in neutral position, shoulder in 30°, elbow in 90°, wrist in 0° to 15° dorsiflexion |
Dynamometer (Grippit) consisting of a vertical cylinder on a foot, placed on the table (cf Fig. 2) |
Three maximal muscle contractions lasting 3 s, 60 s rest interval Verbal encouragement: ”press, press, press” |
|
aGravity correction applied to the Biodex software for the isokinetic strength measurements. |
||||
To characterize the participants’ upper extremity function, assessments of the sensorimotor impairments in both upper extremities were performed during the first test occasion. Muscle tone was measured by the response to resistance of passive movement according to the Modified Ashworth Scale (MAS) (19) and classified as present if the elbow, wrist or fingers had a score on the MAS larger or equal to 1. Light touch and proprioception in the arms and hands were assessed according to the Fugl-Meyer Assessment of Sensorimotor Recovery After Stroke (FM-UE) (20). Dexterity was assessed by the modified Sollerman Hand Function Test (mSHFT) (21) as a sum score between 0 and 12 points (where 12 indicates normal dexterity). The MAS and FM-UE tests were performed before the arm strength measurements and took approximately 10 min to complete and the mSHFT was performed between arm strength and hand strength measurements and took approximately 10 min to complete.
Arm strength measurements
Measurements of shoulder and elbow muscle strength were performed with a Biodex System 3 PRO dynamometer (Biodex Medical Systems Inc., NY, USA; http://www.biodex.com) (Fig. 1).
Fig. 1. Set-up and testing position for isokinetic elbow extension and flexion strength using the Biodex System 3 PRO. A written permission is given from the patient to publish this figure.
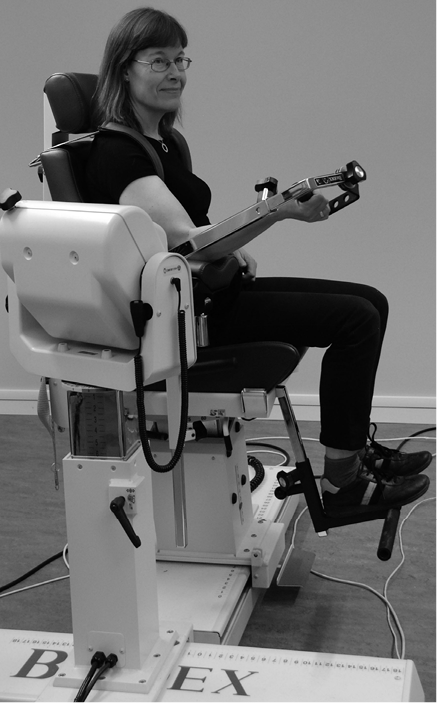
The protocol to measure arm strength was developed from the Biodex manual (22) with regard to the chair and dynamometer positions. The angle positions were chosen to allow safe and pain-free movements of the joints and to enable measurements of specific muscle groups. The muscle groups were selected based on the study by Harbo et al. (23). Before each test session, the system was calibrated to be within allowable limits recommended by the manufacturer. The isometric strength measurements were performed twice and the isokinetic strength measurements included 3 trials (Table I). The number of trials was based on previous protocols used in our research group (24, 25). The highest maximal voluntary isometric and isokinetic contractions from the Biodex measurements were recorded as the highest peak torques in Newton metres (Nm).
During the measurements, the participants were seated in the Biodex adjustable chair with a hip flexion of 85°, back and foot support and stabilized with straps across the shoulders and waist. The Biodex chair and dynamometer were adjusted (in height, rotation and tilt) and positioned with regard to each other on 2 travellers so that the joint lines were aligned with the movement axis of the dynamometer. For each participant the details of the individual adjustments were recorded and used during the second test session. Prior to each measurement the participants practiced the movement approximately 5 times and then performed 1 or 2 submaximal contractions to warm-up and to become familiar with the procedures.
Grip strength measurements
Isometric grip strength measurements were performed with the computerized wireless grip strength dynamometer Grippit (Catell, Hägersten, Sweden, http:/www.catell.se) (Fig. 2). The handgrip measurements were standardized according to the manufacturer’s recommendation with regard to the test position and test procedure. Before testing, the dynamometer was calibrated and the signal strength was checked. The grip strength measurements were repeated 3 times (14–17). The highest maximal voluntary contraction was recorded as the maximal isometric grip strength in Newton (N); the highest grip strength value is often used in the clinical setting to represent maximal hand strength (15).

Fig. 2. Set-up and testing position for isometric grip strength using the Grippit dynamometer.
Statistical methods
Data were analysed with the IBM SPSS Statistics version 22 (IBM Corporation, Armonk, New York, USA). p-values less than 0.05 were considered statistically significant.
Demographic data and clinical characteristics are presented as frequencies, means and standard deviations (SD) or medians, minimum and maximum. All muscle strength measurements were judged to be symmetrically distributed and therefore presented as mean and SD, and as ratios between the more affected and the less affected upper extremity.
The test-retest reliability was evaluated with the intra-class correlation coefficient, ICC2.1. The strength of the ICC values was interpreted according to Fleiss et al. (26) (< 0.40 poor, 0.40–0.75 fair to good, > 0.75 excellent agreement). Changes in the mean were defined from the 2 test occasions. To determine if there was a true systematic difference between the values from the 2 test occasions (e.g. due to a learning effect), the paired mean difference (đ) with 95% confidence interval (95% CI) for đ was calculated between the 2 measurements (test 2 minus test 1). If zero was included in the CI, corresponding to p ≥ 0.05 in a paired t-test, it was inferred that there was no systematic change in the mean (27).
Prior to the evaluation of the measurement error, an analysis of heteroscedasticity (i.e. if participants with higher strength measurements had more dispersed measurement errors than those with lower values) was performed to determine the correct statistics of measurement error. The heteroscedasticity analysis was performed in 3 steps. In the first step, the mean of the 2 test occasions was correlated to the absolute difference between the 2 test occasions for all strength measurements. If Kendall’s tau (т) was positive and т > 0.1 (28), data were then analysed in a second step. Here, the differences between the 2 test occasions were plotted against the mean of the 2 test occasions for each participant, to assess if there was a visible heteroscedastic pattern (i.e. if higher values gave a higher dispersion and a fan-shaped pattern). In the third step, the means of the differences (test occasion 2 minus test occasion 1) were divided into quartiles and the SD were analysed to determine whether there were trends of increased standard deviations from quartile 1 to 4. The analyses showed that 4 variables had a correlation т > 0.1. However, further analysis of the plots and the trends of the standard deviations showed no clear heteroscedastic pattern, and the data were therefore considered to be homoscedastic. Thus, the measurement errors were calculated as standard error of measurement (SEM) and smallest real difference (SRD).
The SEM indicates the extent of the measurement error caused by a random variation for a group and was calculated from the standard deviation around đ, i.e. as the square root of the total within subject variance, SEM=√ total WMS (29). The SRD, which can be estimated from the SEM, determines whether a single individual achieves a real improvement beyond measurement error at a 95% confidence level. SRD is defined as 1.96 * SEM * √2 (30).
Since SEM and SRD in relative terms are easier to interpret and to compare with other studies, SEM and SRD were also expressed as a percentage of the mean of each strength measurement for the entire group (SEM% and SRD%). Benchmarks for acceptable relative measurement errors are, however, lacking in the literature, but in stroke studies SEM% values less than 10% and SRD% values less than 30% have been suggested as acceptable (17, 31).
To visually present the systematic change and random variation of the test-retest data, Bland–Altman graphs were formed for the 10 measurements. The Bland–Altman graphs show the difference from the 2 test occasions plotted against the mean of the 2 test occasions for each participant, including the paired mean difference đ together with 95% CI and the 95% limits of agreement (LOA).
RESULTS
In Table II, the demographic and clinical characteristics of the 45 participants (8 women and 37 men) are presented. Their mean age was 65 years (SD 7) and the mean time from stroke onset to the first test occasion was 44 months (SD 28). All participants except 3 were right-handed and the dominant hand was affected in 58% of the participants. One-third of the participants had spasticity and 38% had sensory impairments in their more affected upper extremity. None of the participants had spasticity or sensory impairments in their less affected upper extremity.
|
Table II. Characteristics of the 45 participants with chronic stroke |
|
|
Characteristics |
|
|
Sex, n (%) |
|
|
Male |
37 (82) |
|
Female |
8 (18) |
|
Age, mean years (SD; min – max) |
65 (7; 44–76) |
|
Type of stroke, n (%) |
|
|
Cerebral infarction |
32 (71) |
|
Cerebral haemorrhage |
13 (29) |
|
Months from stroke onset to first test occasion, mean (SD; min – max) |
44 (28; 10–116) |
|
Paretic side, n (%) |
|
|
Right |
25 (56) |
|
Left |
20 (44) |
|
Handedness, n (%) |
|
|
Right-handedness |
42 (93) |
|
Left-handedness |
3 (7) |
|
Spasticity in the more affected UE ≥1, n (%)a |
15 (33) |
|
Light touch absent or diminished in the more affected UE, n (%)b |
17 (38) |
|
Proprioception absent or diminished in the more affected UE, n (%)b |
9 (20) |
|
Dexterity (score 0–12) in the more affected UE, median (min – max)c |
7 (0–11) |
|
aModified Ashworth Scale; bFugl-Meyer Assessment of Sensorimotor Recovery After Stroke; cModified Sollerman Hand Function Test. SD: standard deviation; UE: upper extremity. |
|
All participants except 2 were able to perform all strength measurements. One participant was unable to perform the isometric shoulder abduction in the more affected arm due to muscle weakness and another participant was unable to perform the isokinetic elbow extension/flexion due to spasticity in the more affected arm. Thus, the statistical analyses were based on all 45 participants except for the isometric shoulder abduction in the more affected arm (n = 44) and the isokinetic elbow extension and flexion in the more affected arm (n = 44).
In Table III, the mean values (SD) for all muscle strength measurements in the upper extremity at the 2 test occasions are presented, as well as the ratios between the more affected and the less affected upper extremity. The ratios between the more affected and the less affected upper extremity ranged from 0.68 to 0.78.
|
Table III. Isometric and isokinetic maximal muscle strength measurements of the upper extremity in 45 participants with chronic stroke |
||
|
Test occasion 1 Mean (SD) |
Test occasion 2 Mean (SD) |
|
|
Isometric shoulder abduction (Nm) |
||
|
Less affected arm |
47.4 (15.8) |
46.5 (15.7) |
|
More affected arma |
32.0 (16.5) |
32.0 (17.5) |
|
Ratio (more affected/less affected) |
0.68 (0.28) |
0.70 (0.32) |
|
Isokinetic elbow extension at 60°/s (Nm) |
||
|
Less affected arm |
30.4 (9.7) |
31.9 (10.7) |
|
More affected arma |
21.1 (9.9) |
22.9 (10.7) |
|
Ratio (more affected/less affected) |
0.69 (0.23) |
0.72 (0.25) |
|
Isokinetic elbow flexion at 60°/s (Nm) |
||
|
Less affected arm |
37.2 (12.7) |
37.3 (12.9) |
|
More affected arma |
27.1 (11.3) |
28.5 (12.1) |
|
Ratio (more affected/less affected) |
0.73 (0.20) |
0.76 (0.22) |
|
Isometric elbow flexion (Nm) |
||
|
Less affected arm |
52.3 (17.0) |
51.9 (17.3) |
|
More affected arm |
39.1 (17.0) |
40.1 (17.2) |
|
Ratio (more affected/less affected) |
0.75 (0.23) |
0.78 (0.24) |
|
Grip strength (N) |
||
|
Less affected hand |
347.7 (120.9) |
351.5 (122.0) |
|
More affected hand |
238.1 (112.6) |
244.3 (113.9) |
|
Ratio (more affected/less affected) |
0.71 (0.28) |
0.71 (0.28) |
|
aNumber of participants = 44. SD: standard deviation; Nm: Newton metre; N: Newton. |
||
In Table IV, data for the reliability analyses are presented. The ICCs for the isometric and isokinetic strength measurements ranged from 0.92 to 0.97 (95% CI 0.83–0.98). The calculation of the change in the mean was significant (i.e. the 95% CI for đ did not include zero) for 3 isokinetic measurements: the isokinetic elbow extension in both arms and the isokinetic elbow flexion in the more affected arm.
|
Table IV. Reliability of isometric and isokinetic maximal muscle strength measurements of the upper extremity in 45 participants with chronic stroke |
|||||||||
|
Grand mean |
ICC2.1 |
95% CI for ICC |
đ (T2–T1) |
95% CI for đ |
SEM |
SEM% |
SRD |
SRD% |
|
|
Isometric shoulder abduction, (Nm) |
|||||||||
|
Less affected arm |
46.93 |
0.97 |
0.95–0.98 |
–0.92 |
–2.04–0.21 |
2.7 |
5.8 |
7.5 |
16.0 |
|
More affected arma |
32.02 |
0.97 |
0.94–0.98 |
0.05 |
–1.25–1.36 |
3.0 |
9.4 |
8.3 |
26.1 |
|
Isokinetic elbow extension at 60°/s, (Nm) |
|||||||||
|
Less affected arm |
30.90 |
0.92 |
0.85–0.96 |
1.56 |
0.34–2.66 |
2.9 |
9.3 |
8.0 |
25.9 |
|
More affected arma |
22.21 |
0.92 |
0.83–0.96 |
1.78 |
0.64–2.91 |
2.9 |
12.6 |
8.0 |
34.8 |
|
Isokinetic elbow flexion at 60°/s, (Nm) |
|||||||||
|
Less affected arm |
37.03 |
0.95 |
0.92–0.97 |
0.40 |
–0.79–1.58 |
2.8 |
7.4 |
7.7 |
20.6 |
|
More affected arma |
28.20 |
0.95 |
0.91–0.98 |
1.34 |
0.32–2.38 |
2.6 |
9.2 |
7.1 |
25.5 |
|
Isometric elbow flexion, (Nm) |
|||||||||
|
Less affected arm |
52.13 |
0.97 |
0.95–0.98 |
–0.42 |
–1.66–0.82 |
2.9 |
5.6 |
8.1 |
15.5 |
|
More affected arm |
39.62 |
0.97 |
0.94–0.98 |
1.05 |
–0.21–2.31 |
3.0 |
7.6 |
8.4 |
21.2 |
|
Grip strength, (N) |
|||||||||
|
Less affected hand |
349.60 |
0.95 |
0.92–0.97 |
3.88 |
–7.42–15.18 |
26.4 |
7.6 |
73.3 |
21.0 |
|
More affected hand |
241.17 |
0.96 |
0.93–0.98 |
6.20 |
–3.19–15.59 |
22.3 |
9.2 |
61.8 |
25.6 |
|
aNumber of participants = 44. ICC: intraclass correlation coefficient; CI: confidence interval; đ: difference between test occasion 2 minus test occasion 1; SEM: standard error of measurement; SEM%: SEM in relative terms of the mean of the cohort; SRD: smallest real difference; SRD%: SRD in relative terms of the mean of the cohort; Nm: Newton metre; N: Newton. |
|||||||||
In Table IV, the measurement errors, SEM/SEM% and SRD/SRD%, are presented. The absolute and relative strength measurement errors for a group of individuals, SEM (SEM%), ranged from 2.7 to 3.0 Nm (5.6–9.4%) for isometric arm strength, 2.6–2.9 Nm (7.4–12.6%) for isokinetic arm strength, and 22.3–26.4 N (7.6–9.2%) for isometric grip strength. The absolute and relative measurement errors for a single individual, SRD (SRD%), ranged from 7.5 to 8.4 Nm (15.5–26.1%) for isometric arm strength, 7.1–8.0 Nm (20.6–34.8%) for isokinetic arm strength, and 61.8–73.3 N (21.0–25.6%) for isometric grip strength. For all measurements, the SEM% and SRD% values were higher for the more affected arm compared with the less affected.
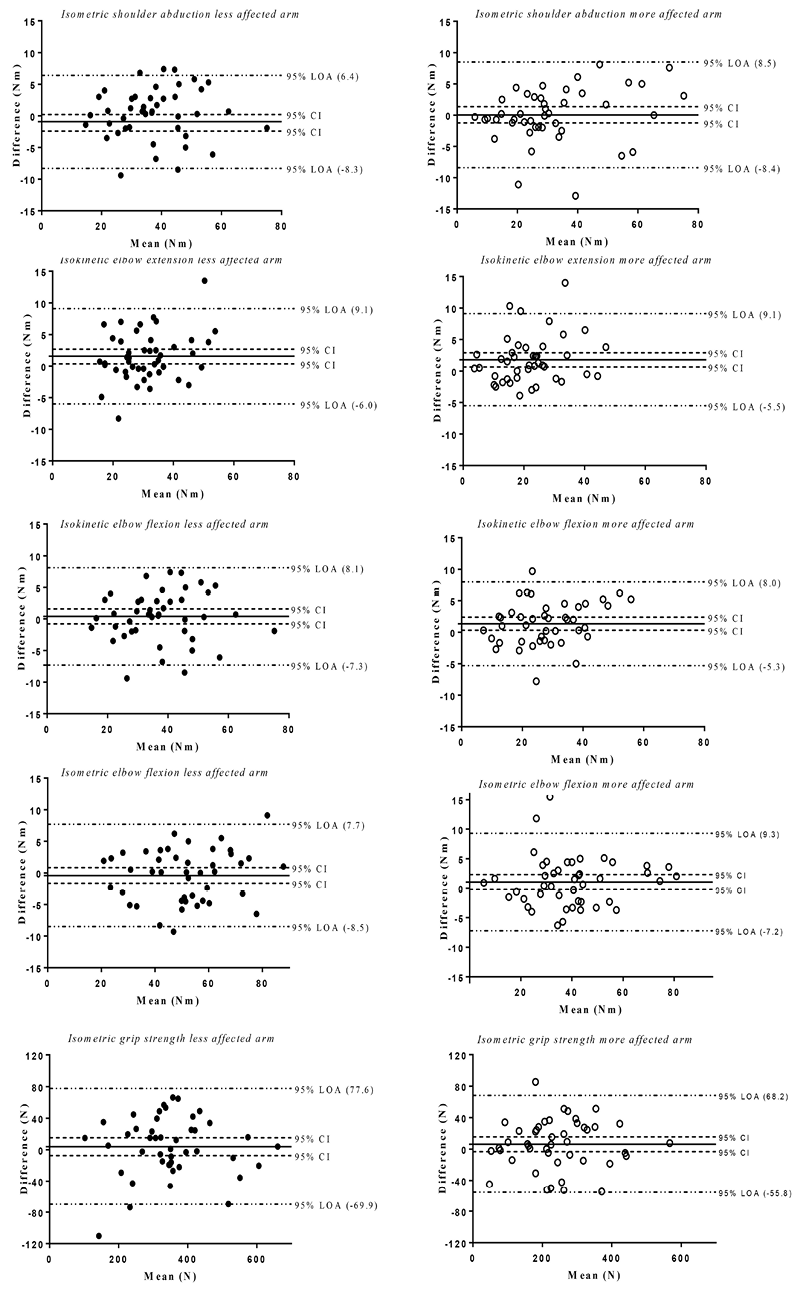
The Bland –Altman graphs (Fig. 3) show that the mean differences (đ), including the 95% CI (i.e. the systematic bias), were generally small for all 10 strength measurements. The 95% LOA were –8.5 to 9.3 Nm for the arm strength and –69.9 to 77.6 N for the grip strength.
Fig. 3. Bland–Altman graphs for the 10 strength measurements, including reference lines for the mean difference (test occasion 2 minus 1) and 95% confidence interval (95% CI), and the 95% limits of agreement (LOA).
DISCUSSION
This study evaluated the test-retest reliability of isometric and isokinetic muscle strength measurements in a group of persons with chronic stroke and mild to moderate paresis in their more affected arm. The main findings were that test-retest reliability was high and measurement errors were acceptable to evaluate changes post-stroke in muscle strength in the upper extremity, both for a group of individuals and for a single individual.
The ICCs ranged from 0.92 to 0.97, which can be considered excellent according to Fleiss et al. (26). Our ICCs are in agreement with previous reliability studies of muscle strength measurements in the upper extremity after stroke (16, 17) and in healthy subjects (11–13, 32, 33), even though different types of dynamometers were used. The ICC values in the present study are also in line with the ICCs from muscle strength measurements in the lower extremities after stroke (31, 34, 35).
The systematic bias of the measurements was generally small (Table IV and Fig. 3). However, a significant systematic change in the mean was revealed for 3 isokinetic measurements: the isokinetic elbow extensions for both arms and the isokinetic elbow flexion for the more affected arm. The participants performed slightly better on the second test than on the first, which could be a learning effect since they had practiced once during the first test occasion. The isokinetic tests were perceived as more difficult to perform, probably because of the reciprocal movements and the need to shift between agonist and antagonist during measurements. In clinical practice it may therefore be necessary to include more than 1 practice session to reduce such a potential learning effect.
Fig. 3 presents the mean difference with 95% CI (i.e. the systematic bias) together with 95% LOAs. The LOA was originally proposed by Bland–Altman and used in studies of differences between methods for individual patients (36). However, use of the LOA as a measure of reliability has been criticized and the measurement errors (SEM and SRD) have been proposed as better measures of variability (37, 38). This is mainly because the LOA is dependent on the sample size, whereas the measurement error has an expected value independent of the sample size. However, in a test-retest situation the 95% SRD and the 95% LOA yield approximately the same values (38). In practice, the 2 approaches complement each other. SEM and SRD represent the smallest change that indicates a real change for a group of individuals and for a single individual, respectively, whereas the Bland–Altman analysis is an excellent visual tool and an easy approach for disentangling bias from imprecision.
In the present study, the absolute measurement errors (SEM) for arm strength measurements did not differ much between the isometric and isokinetic measurements (Table IV). The relative measurement errors (SEM% and SRD%) for the isometric arm and grip strength (Table IV) were all acceptable (SEM% < 10% and SRD% < 30%) (17, 31). Our SEM% values are in agreement with the SEM% values in previous stroke studies by Bertrand et al. (14) (isometric elbow strength 4–9% and isometric grip strength 4–13%) and Hammer et al. (15) (isometric grip strength 6–10%), and our SRD% values for the isometric grip strength are also in line with the SRD% values in the study by Chen et al. (17) (isometric grip strength 19–24%). Furthermore, the relative measurement errors for the isokinetic arm strength (Table IV) were also within the suggested acceptable limits (SEM% < 10% and SRD% < 30%) (17, 31), except for the isokinetic elbow extension of the more affected arm. One explanation for the larger measurement error could be that after stroke the elbow extensor muscles are often more difficult to activate in isolation and the stronger flexor muscles are difficult to inhibit during reciprocal movements.
Taken together, the measurement errors (systematic and random) for the isokinetic strength measurements were somewhat higher than the isometric strength measurements. Isometric strength measurements might therefore be preferred when evaluating the recovery of muscle strength and effects of interventions. Nevertheless, the isokinetic strength measurements are valuable as they reflect dynamic force development and reciprocal movements in real life. Future research should investigate how isometric and isokinetic strength measurements are related to real life activities in the upper extremity.
In the present study, the shoulder abductors were only measured isometrically since many persons after stroke have a risk of impingement when raising the arm above the horizontal plane. The contractions were performed in a slightly abducted position (15°) in the scapular plane to secure a pain-free position. The elbow, which could be considered as a more stable joint than the shoulder, was measured both isokinetically and isometrically. For measuring arm strength the participants were stabilized with trunk and pelvic straps, but the measured arm was not fixated (in agreement with the Biodex protocol). This could have impacted the measurement errors between the 2 test occasions due to perturbations in the alignment of the axis of the joint and the dynamometer. For measuring grip strength the participants were seated in a standardized position with their arm resting on a foam cushion, but the trunk and the arm were not fixated. To further standardize the position it would have been desirable to fix the position of the forearm.
A limitation of the present study was that only individuals with mild to moderate paresis in the upper extremity after stroke were included. In addition, we did not include persons with any major cognitive impairments or difficulties in communicating, and more men than women volunteered to participate. Therefore, the results cannot be generalized to the entire stroke population. A strength of the study is that it included 45 participants, which can be considered a sufficiently large number when the reliability of measurements is evaluated (39). Furthermore, care was taken to standardize the test situation; the test protocols were described in detail, and the tests were performed at the same time of day, at the same location and with the same time interval between tests.
In conclusion, isometric and isokinetic muscle strength in the upper extremity can be measured reliably, both for a group of individuals with chronic stroke and for single individuals. This study indicates that isometric strength measurements yield smaller relative measurement errors and might be preferred when evaluating muscle strength after stroke, but the choice of measurement mode depends on the research question.
ACKNOWLEDGEMENTS
The authors would like to thank all the volunteer participants, Dr Ulla-Britt Flansbjer, Lund University, Lund, Sweden for guidance with the Biodex measurements, and Professor Jonas Björk, Lund University, Lund, Sweden for valuable support with the statistical analyses. The study was supported by grants from Skåne county council’s research and development foundation, Vårdakademin at Skåne University Hospital, the Norrbacka Eugenia Foundation and the Swedish Stroke Association.
REFERENCES
