OBJECTIVE: To study the effects of 6 months exercise training on ventricular remodelling and autonomic tone in patients with acute myocardial infarction and percutaneous coronary intervention.
DESIGN: Single-blinded randomized control trial.
PARTICIPANTS: Sixty patients with acute myocardial infarction who had undergone percutaneous coronary intervention.
METHODS: The exercise group followed a 6-month supervised exercise programme, while the control group received routine recommendations. All patients underwent an incremental cardiopulmonary exercise test and Doppler echocardiography at baseline and after 6 months.
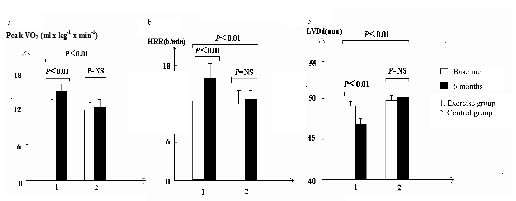
RESULTS: Three patients in the exercise group did not complete the programme. At 6 months follow-up, an improvement was seen in the exercise group compared with the control group regarding peak VO2 (p < 0.01), Powermax (p < 0.05), VO2 at anaerobic threshold (p < 0.01), time to reach anaerobic threshold (p < 0.05), heart rate recovery (p < 0.01), left ventricular end-diastolic diameter (p < 0.01) and left ventricular ejection fraction (p < 0.05).
CONCLUSION: Six months exercise training in patients with acute myocardial infarction and percutaneous coronary intervention with mild ventricular systolic dysfunction could prevent ventricular remodelling to a certain extent, and favourable modulating sympatho-vagal balance may be an important mechanism.
Key words: exercise training, acute myocardial infarction, ventricular remodelling, autonomic tone.
J Rehabil Med 2008; 40: 776–779
Correspondence address: Luo Ming, Department of Cardiology, Tongji Hospital of Tongji University, Shanghai, China, 200065. E-mail: lmfc84@yahoo.com.cn
Submitted December 22, 2007; accepted May 29, 2008
Introduction
It has been widely shown that exercise-based cardiac rehabilitation in patients with acute myocardial infarction (AMI) and percutaneous coronary intervention (PCI) has several beneficial effects on cardiovascular functional capacity, quality of life, risk factors modification, psychological profile and mortality (1). The degree of ventricular remodelling is regarded as an important prognostic factor associated with cardiac function after AMI, and an increasing number of studies have shown that, in patients with AMI with left ventricular dysfunction, exercise training does not worsen ventricular remodelling, and may even prevent this spontaneous deterioration (2). Supporters of this argument have put forward the hypothesis that improvement in impaired sympatho-vagal balance might be an important mechanism. Moreover, it has been reported that exercise training is associated with an improvement in heart rate recovery (HRR), an easily measured and powerful index of vagal tone in patients after AMI (3).
The aim of this study was to evaluate the effects of a 6-month supervised exercise programme for patients with AMI and PCI in terms of exercise capacity, left ventricular geometrical size and systolic function and HRR.
Methods
Study population
A total of 71 patients after AMI who were 3–7 days post-primary PCI treatment were referred to our centre. Of these, 60 were enrolled into the study, after taking into account the exclusion criteria listed below, and were assigned by alternate randomization in a 1:1 order into 2 groups, stratified by age and sex. Patients in the exercise group followed a 6-month exercise programme, while the other group received routine pharmacological therapy and lifestyle education. The 2 groups were similar with regard to age, extent of coronary artery disease brain natriuretic peptide, left ventricular ejection fraction (LVEF) and pharmacological therapy. The exclusion criteria were: post-infarction residual myocardial ischaemia, severe ventricular arrhythmias, atrioventricular block, severe reduction in LVEF (LVEF ≤ 45%), hypertrophic cardiomyopathy, valvular disease requiring surgery, pericarditis, acute systemic illness or fever, severe renal dysfunction (i.e. creatinine > 2.5 mg/dl), severe orthopaedic problems that would prohibit exercise, other metabolic problems, such as acute thyroiditis, hypokalaemia, hyperkalaemia, and hypovolaemia.
The study was approved by the local ethics committee. All patients gave their written informed consent.
Cardiopulmonary exercise test
All patients underwent an incremental cardiopulmonary exercise test (CPET) on a bicycle ergometer. To stabilize respiratory exchange, patients were asked to remain still on the ergometer for at least 3 min before starting exercise. After a 1-min warm-up period with no added work-load, a ramp protocol of 15 W/min was started and continued until exhaustion, including a 1-min cool-down period with no added work-load at the end. A 12-lead electrocardiogram was monitored continuously during the test, and cuff blood pressure was recorded manually every 2 min. Respiratory gas exchange measurements were recorded breath-by-breath by computerized metabolic monitoring (Inncor 5.0, Innovision Cor, Denmark). Peak VO2 was recorded as the mean value of VO2 during the last 20 sec of the test, and was expressed in ml × kg–1 × min–1. The ventilatory anaerobic threshold (AT) was assessed mathematically, the time of reaching AT(tAT) and VO2 (VO2AT) at AT were recorded. HRR was defined as the difference between peak heart rate (HR) and HR 1 min after CPET (4, 5).
Training protocol
The patients in the exercise group attended the exercise training as out-patients 3 times per week. Training sessions, performed under continuous electrocardiogram monitoring, were supervised by a cardiologist and a nurse. Each session was preceded by a 15-min warm-up and followed by a 15-min cooling-down period. Exercise was performed for 30 min on a bicycle ergometer at VO2AT (using a target rate determined by the results of the electrocardiogram) achieved at the initial symptom-limited CPET, and the work-load was adjusted according to the CPET results at the previous 3 months.
Doppler echocardiography
All patients underwent a Doppler echocardiographic study (Ultasomd Vivid 7, General Motor Cor., USA) at the beginning of the study and after 6 months. Standard views, including the parasternal long-axis, short-axis at the papillary muscle level, and apical 4- and 2-chamber views were recorded. Left ventricular end-diastolic (LVD) diameter, left ventricular end-systolic (LVS) diameter, and LVEF were measured, and left ventrical mass index (LVMI) was calculated according to the Devereux formula (6).
Statistics
All the data were normally distributed, and descriptive statistics are given in terms of mean value and standard deviation (SD). Comparison between groups for continuous variables was made using independent t-tests where data were interval or ratio level. A p-value of less than 0.05 was considered statistically significant. Differences between the 2 groups and changes over time within each group were assessed by 2-way repeated measures analysis of variance (ANOVA). All statistical analyses were performed using the software package SPSS, version 10.0 (SPSS Inc., Chicago, USA).
Results
Three patients in the exercise group did not complete the exercise programme because the timetable was inconvenient or they were unable to maintain the exercise regime. No statistically significant differences were found at baseline between the exercise group and the control group in clinical, angiographic characteristics and pharmacological therapy.
At the end of 6 months, no change in HRAT, HRrest, LVD diameter or LVMI was reported in either group. The exercise group demonstrated a greater increase in peak VO2 (p < 0.01), Powermax (p < 0.05), VO2AT (p < 0.01), tAT (p < 0.05), HRR (p < 0.01) and LVEF (p < 0.05) (Tables I and II, Figs 1a–b). Furthermore, LVD diameter decreased in the exercise group but increased in the control group (Table II, Fig. 1c).
| Table I. Cardiopulmonary exercise test parameters at baseline and after 6 months in the exercise training and control groups |
| | Exercise group n = 27 | Control group n = 30 | p-value |
| Baseline Mean (SD) | 6 months Mean (SD) | Baseline Mean (SD) | 6 months Mean (SD) |
| Peak VO2, ml × kg–1 × min–1 | 12.6 (1.5) | 15.7 (2.3) | 11.7 (1.9) | 12.0 (1.6) | 0.008 |
| Powermax, W | 64 (8) | 80 (6) | 65 (5) | 65 (4) | 0.021 |
| VO2AT, ml × kg–1 × min–1 | 9.2(2.9) | 12.7 (2.1) | 8.8 (2.6) | 9.0 (1.9) | 0.007 |
| HRAT, beats/min | 88 (4) | 86 (6) | 81 (3) | 81 (4) | NS |
| tAT, min | 6.8 (0.7) | 8.1 (1.0) | 7.1 (0.6) | 7.0 (0.9) | 0.011 |
| HRrest, beats/min | 74 (4) | 72 (3) | 70 (3) | 73 (2) | NS |
| HRR, beats/min | 12.1 (1.8) | 16.7(2.3) | 11.8 (2.0) | 12.4 (1.6) | 0.005 |
| Peak VO2: peak oxygen consumption; Powermax: maximal work-load; VO2AT: oxygen consumption at anaerobic threshold; HRAT: heart rate at anaerobic threshold; tAT: time from beginning to anaerobic threshold; HRrest: heart rate at rest; HRR: heart rate recovery, the difference between peak heart rate and heart rate one min after cardiopulmonary exercise test; NS: no significance; SD: standard deviation. |
| Table II. Doppler echocardiographic parameters at baseline and after 6 months exercise training |
| | Exercise group n = 27 | Control group n = 30 | p-value |
| Baseline Mean (SD) | 6 months Mean (SD) | Baseline Mean (SD) | 6 months Mean (SD) |
| LVDd, mm | 48.7(0.6) | 46.1 (0.8) | 49.2 (0.5) | 49.9 (0.9) | 0.018 |
| LVSd, mm | 34.2(1.1) | 33.9 (0.7) | 33.9 (1.2) | 34.1 (1.0) | NS |
| LVMI, g/m2 | 99.4 (1.8) | 99.0 (2.1) | 98.9 (2.0) | 99.4 (1.6) | NS |
| LVEF, % | 50.8 (4.0) | 53.0 (3.1) | 51.1 (2.9) | 50.9 (3.4) | 0.002 |
| LVDd: left ventricular end-diastolic diameter; LVSd: left ventricular end-systolic diameter; LVMI: left ventrical mass index; LVEF: left ventricular ejection fraction; NS: no significance; SD: standard deviation. |
Fig. 1. (a) Values of peak oxygen consumption (VO2 ml × kg–1 × min–1). (b) Values of heart rate recovery (HRR, beats/min). (c) Values of left ventricular diastolic diameter (LVD, mm) in the exercise group and at baseline and 6 months later. NS: no significance.
Discussion
To our knowledge, the present study is the first to evaluate the effects of exercise training on ventricular remodelling in patients with AMI adopting HRR, an easily measured and powerful indirect index. Some investigators have studied the mechanism underlying the HRR after low- or moderate-intensity exercise (below the anaerobic threshold). Imai et al. (7) showed that T30 (HR decay for the first 30 sec after exercise) was prolonged by a parasympathetic nerve blockade, but was almost completely independent of a sympathetic nerve blockade, and Perini et al. (8) reported that the decrease in plasma noradrenaline began 1 min after the cession of exercise. Both groups concluded that HRR immediately after exercise was mediated mainly by the reactivation of the vagal nerve.
Almost all the studies of exercise training in patients after AMI show an improved peak VO2, which is considered to be the best measure of cardiovascular fitness and exercise capacity. Giannuzzi et al. (9) investigated 126 patients after AMI, who were divided into 3 groups according to LVEF at the beginning of the study (≤ 35%, ≥ 45%, and between 35% and 45%), and there was a similar magnitude of improvement in exercise capacity in the 3 groups at the end of 3 months exercise training. In our study, we found that the exercise group patients’ peak VO2 and Powermax increased more than controls. Furthermore, we found that VO2AT and tAT increased, which suggested that aerobic metabolism was improved, because AT represents the point at which anaerobic metabolism starts, with production of lactic acid. Several previous studies have reached similar conclusions. Adamopoulos et al. (10) studied the effects of exercise on skeletal muscle metabolism in patients with heart failure classified as New York Heart Association (NYHA) class II–III. After 8 weeks of bicycle training, phosphocreatine depletion was reduced, adenosine diphosphate concentrations were increased, and phosphocreatine recovery time was shorter. These results suggest that moderate exercise training can improve the oxidative capacity of skeletal muscle.
Pooled data from clinical trials have shown significant improvement in LVEF after exercise training in patients after AMI. The Exercise in Left Ventricular Dysfunction (ELVD) study (11) reported that 6 months exercise training improved LVEF significantly in patients after AMI (34% (5%) vs 38% (8%) in controls), and this result was confirmed by Koizumi et al. (12) who found that after 3 months exercise in patients after AMI who had undergone successful PCI, LVEF during exercise was significantly improved compared with at rest in the trained group, whereas no difference was observed in the control group. In our study, 6 months exercise training increased LVEF more than controls.
Ventricular remodelling post-AMI is a process characterized by increased left ventricular wall thickness of non-infarcted left ventricular segments and enlargement of the left ventricular cavity, which is also a vital patho-progression from AMI to heart failure. Therefore, considerable attention has been directed to determine whether exercise training in the chronic phase of myocardial infarction could affect the process of ventricular remodelling. However, the influence of exercise training remains controversial. Jugdutt et al. (13) reported that patients with left ventricular asynergy of 18% or more showed topographic deterioration with low-level exercise training in the chronic phase of myocardial infarction. They hypothesized that the haemodynamic stress of exercise might precipitate further stretching of the scar, with chamber dilatation and aneurysmal bulging, in patients with an incompletely healed, large transmural infarction. Kubo et al. (14) investigated the effects of 3 months exercise training on ventricular remodelling after extensive anterior AMI with LVEF < 45% and found that control group patients' LVD volume index and LVS volume index improved, but there was no change in the rehabilitation group. Conversely, Otsuka et al. (15) reported that early exercise training did not deteriorate ventricular remodelling in mild, moderate and severe left ventricular dysfunction in patients. A similar study (16) reported that LVD diameter increased in the control group, but not in the exercise group, after 3 months' exercise training.
In our study, we found that 6 months' exercise reduced LVD diameter in the exercise group, which suggested that, to a certain extent, exercise could prevent ventricular remodelling in patients after AMI. As we know, 2 primary stimulating factors are mechanics overload (pressure or volume overload) and activation of neurohormones. The favourable contribution of exercise training on remodelling must be combined effects: such as autonomic tone adjustment (17), decreased left ventricular wall stress during exercise, and improved endothelial function (18).
A few recent controlled studies have been conducted to examine the influence of exercise on the autonomic regulation of cardiovascular function in patients with chronic heart failure. Pietilä et al. (19) reported beneficial changes in cardiovascular autonomic nervous control, evaluating the effects of exercise training on autonomic tone by baroreflex sensitivity, HR variability and cardiac pre-synaptic innervation by 11C-hydroxyephedrine positron emission tomography in 13 patients with heart failure. Given the hypothesis that moderate exercise could restore autonomic imbalance in patients with heart failure, we adopted HRR, an easily measured and powerful index, to clarify whether this beneficial effect could also be applied in patients after AMI. Our study demonstrated that HRR (beats/min) in the exercise group increased more than in the control group.
Recent data (20, 21) have demonstrated that HRR, an index of vagal activity, is an independent predictor of cardiovascular events and mortality, and patients after AMI usually present a delayed HRR. As we know, over-activation of the sympathetic system is harmful to patients’ ventricular remodelling, clinical symptoms and long-term survival, which is why beta-blockers are included as standard drug treatment for heart failure patients. In addition, several controlled trails (22–24) have confirmed that exercise training could produce a partial reversal of the autonomic imbalance in stable heart failure patients, with a reduction in sympathetic over-activation, and an enhancement of chronotropic responsiveness.
In conclusion, 6 months exercise training in patients with AMI and PCI with mild ventricular systolic dysfunction could prevent ventricular remodelling to a certain extent and favourable modulating sympatho-vagal balance evidenced by HRR may be an important mechanism.
References
1. Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med 2004; 116: 682–692.
2. van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail 2006; 8: 841–850.
3. Giallauria F, De Lorenzo A, Pilerci F, Manakos A, Lucci R, Psaroudaki M, et al. Long-term effects of cardiac rehabilitation on end-exercise heart recovery after myocardial infarction. Eur J Cardiovc Prev Rehabil 2006; 13: 544–550.
4. Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 2001; 285: 879–880.
5. Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation 2001; 104: 1911–1916.
6. Devereux KB, Alonso DR, Lutas EM. Ecocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450– 458.
7. Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994; 24: 1529–1535.
8. Perini R, Orizio C, Comandè A, Castellano M, Beschi M, Veicsteinas A. Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. Eur J Appl Physiol Occup Physiol 1989; 58: 879–883.
9. Giannuzzi P, Temporelli PL, Corrà U, Gattone M, Giordano A, Tavazzi L. Attenuation of unfavorable remodeling by exercise training in postinfarction patients with LV dysfunction. Circ J 1997; 96: 1790–1797.
10. Adamopoulos S, Coats AJ, Brunotte F, Arnolda L, Meyer T, Thompson CH, et al. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J Am Coll Cardiol 1993; 21: 1101–1106.
11. Giannuzzi P, Temporelli PL, Corrà U, Tavazzi L. ELVD-CHF Study Group. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure. Circulation 2003; 108: 554–559.
12. Koizumi T, Miyazaki A, Komiyama N, Sun K, Nakasato T, Masuda Y, et al. Improvement of left ventricular dysfunction during exercise by walking in patients with successful percutaneous coronary intervention for acute myocardial infarction. Circ J 2003; 67: 233–237.
13. Jugdutt BI, Michorowski BL, Kappagoda CT. Exercise training after anterior Q wave myocardial infarction: importance of regional LV function and topography. J Am Coll Cardiol 1998; 13: 362–372.
14. Kubo N, Ohmura N, Nakada I, Yasu T, Katsuki T, Fujii M, et al. Exercise at ventilatory threshold aggravates left ventricular remodeling in patients with extensive anterior acute myocardial infarction. Am Heart J 2004; 147: 113–120.
15. Otsuka Y, Takaki H, Okano Y, Satoh T, Aihara N, Matsumoto T, et al. Exercise training without ventricular remodelling in patients with moderate to severe LV dysfunction early after acute myocardial infarction. Int J Cardiol 2003; 87: 237–244.
16. Jiang AF, Zhang FC, Gao W, Li ZP, Zhao W, Li XW, et al. The impact of exercise rehabilitation on left ventricular remodeling and systolic function in acute myocardial infarction patients. Chin J Intern Med 2006; 41: 904–906.
17. Tiukinhoy S, Beohar N, Hsie M. Improvement in heart rate recovery after cardiac rehabilitation. J Cardiopulm Rehabil 2003; 23: 84–87.
18. Walther C, Gielen S, Hambrecht R. The effect of exercise training on endothelial function in cardiovascular disease in humans. Exerc Sport Sci Rev 2004; 32: 129–134.
19. Pietilä M, Malminiemi K, Vesalainen R, Jartti T, Teräs M, Någren K, et al. Exercise training in chronic heart failure: beneficial effects on cardiac (11) C-hydroxyephedrine PET, autonomic nervous control,and ventricular repolarization. J Nucl Med 2002; 43: 773–779.
20. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heat rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341: 1351–1357.
21. Wetanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality. Circulation 2001; 104: 1911–1916.
22. Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, et al. The effects of exercise training on sympathetic neural activation in advanced heart failure: A randomised controlled trail. J Am Coll Cardiol 2003; 42: 854–860.
23. Balardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure. Effects on functional capacity, quality of life and clinical outcome. Circulation 1999; 1173–1182.
24. Larsen AL, Gjesdal K, Hall C, Aukrust P, Aarsland T, Tickstein K. Effect of exercise training in patients with heart failure: a pilot study on autonomic balance assessed by heart rate variability. Eur J Cardilvasc Prev Rehab 2004; 11; 162–167.