Menno van der Holst, PT1,2, C. W. P. Gerco van der Wal, MSc1, Ron Wolterbeek, MD3, Willem Pondaag, MD, PhD4, Thea P. M. Vliet Vlieland, MD, PhD1,2 and Rob G. H. H. Nelissen, MD, PhD1
From the 1Department of Orthopaedics, Rehabilitation and Physical Therapy, Leiden University Medical Center, 2Rijnlands Rehabilitation Center, 3Department of Medical Statistics and 4Department of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands
OBJECTIVE: Irrespective of treatment history, shoulder dysfunction may occur in children with neonatal brachial plexus palsy. Following internal contracture release and/or muscle tendon transfer (ICR/MTT) shoulder function gain is possible. This study describes the outcomes of ICR/MTT for children with neonatal brachial plexus palsy, with or without prior nerve surgery (a group with prior nerve surgery and a group without prior nerve surgery).
Patients and methods: The study included children who underwent an ICR/MTT with a minimum follow-up of 6 months. Active/passive range of motion (aROM/pROM)/Mallet scores were recorded (pre-operatively, 6 months, and 1, 3, 5 and 10 years post-surgery). Changes over time within groups were analysed using a linear mixed model.
RESULTS: A total of 115 children (60 boys) were included, 82 with nerve surgery history, mean age 4.7 years (standard deviation (SD) 3.3 years), mean follow-up 6 years (SD 3.2 years). Pre-operatively active external rotation, abduction and forward-flexion were worse in the group with prior nerve surgery. aROM, pROM and Mallet scores, improved at all time-points in both groups. The course and magnitude of these improvements were largely similar in both groups. In the long-term, the effects of ICR/MTT decrease, but remain significant.
CONCLUSION: In children with neonatal brachial plexus palsy shoulder function improved after ICR/MTT, irrespective of treatment history. Pre-operative shoulder function was worse in the group with prior nerve surgery, resulting in less function in this group after ICR/MTT. Reporting on outcome after secondary shoulder surgery should be stratified into children with and without prior nerve surgery, in order to prevent over- or underestimation of results.
Level of evidence: This study concerned a retrospective treatment case series study. Level of evidence: IV.
Key words: brachial plexus neuropathy; joint capsule release; tendon transfer; treatment; outcome; paediatrics; nerve surgery; rehabilitation.
J Rehabil Med 2016; 48: 00–00
Correspondence address: Menno van der Holst, Department of Orthopaedics, Rehabilitation and Physical Therapy, Leiden University Medical Center, NL-2300 RC Leiden, The Netherlands. E-mail: m.van_der_holst@lumc.nl
Accepted Apr 5, 2016; Epub ahead of print May 27, 2016
INTRODUCTION
Neonatal brachial plexus palsy (NBPP) is the result of a birth stretch to the brachial plexus with an incidence of 0.38–5.10/1,000 (1–3). Most injuries are mild, and spontaneous recovery occurs in 70–80% of cases, leaving the remaining 20–30% with some functional deficit (4). When sufficient spontaneous recovery is lacking, nerve surgery at a young age (3–9 months) may be indicated (1, 2, 5–11). These nerve surgery treatments may not be sufficiently effective in some children, resulting in remaining functional deficits and muscular imbalance (2, 5, 7, 9). In particular, restoration of external rotation remains incomplete in a large proportion of nerve-surgically treated infants (12, 13). In conservatively treated children, functional deficits and muscular imbalance may develop due to incomplete spontaneous recovery.
As a result of muscular imbalance between the internal and external rotators of the shoulder, anatomical changes in the glenohumeral joint may develop, further limiting function (14, 15).
Irrespective of treatment history, limited functional recovery of the shoulder and/or anatomical changes to the glenohumeral joint can occur, and this can be an indication for secondary surgery in which an internal contracture-release and/or muscle tendon transfer (ICR/MTT) is performed (5, 6, 8, 9, 16–29). Observational studies on the outcome of such secondary surgical interventions show improvements in active and/or passive range of motion (aROM/pROM) and/or Mallet scores (16–22, 24–28, 30). A recent meta-analysis on the outcome of secondary shoulder surgery confirms the effectiveness of these interventions (31).
Two studies have employed subgroup analysis and reported outcomes separately for patients who have had prior nerve surgery and those who have not (22, 32). One study included 67 patients (mean age 6.4 years, mean follow-up 7.5 years, 37 had prior nerve surgery) who underwent secondary shoulder surgery (22). The group without prior nerve surgery had better outcomes regarding ROM. The second study reported 91 patients with a tendon transfer to the shoulder, divided into 4 subgroups (upper- and total plexus lesions were analysed separately, and divided with regards to: with/without prior nerve surgery (20 vs 71 patients, respectively)). The group without prior nerve surgery had better pre-operative ROM, but outcome of surgery over time was comparable for the groups (32). Two studies only included children who have had no prior nerve surgery (20, 33). In one study, only one child had prior nerve surgery and the outcomes for this child were described separately (24).
One study reported long-term results of abduction and external rotation (34). This specific study reported that abduction decreased starting 6 years after surgery, whereas external rotation did not decrease over time.
Thus far, no study has described the course of clinical outcome both in the long-term and in subgroups based on previous nerve surgery. Since children who have had nerve surgery are different from those who have not, in terms of early spontaneous recovery, these concern different subgroups of children within the NBPP population. Therefore, this long-term follow-up study aims to describe the course of ROM and function over time, as well as shoulder joint deformities pre-operatively, in 2 subgroups (with and without prior nerve surgery), in patients with NBPP undergoing an ICR and/or MTT.
PATIENTS AND METHODS
Study design
This study concerned a retrospective analysis of clinical data derived from paper or electronic medical records of children seen at the Leiden University Medical Center multidisciplinary brachial plexus clinic (1996–2014). All data were gathered during usual clinical care, according to a standardized (prospectively designed) protocol, and data extraction for the present study was performed between May 2013 and September 2014.
The medical ethics committee of Leiden University Medical Center waived informed consent for this prospective data collection, since it is part of good clinical practice for this tertiary referral clinic.
Patients
All children diagnosed with NBPP were eligible for the present study if they met the following inclusion criteria at the time of data extraction:
• treatment consisted of an internal contracture-release and/or muscle tendon transfer (ICR and/or MTT);
• an electronic or paper medical record was available;
• follow-up period of at least 6 months (first scheduled follow-up after surgery).
Surgical intervention and postoperative rehabilitation
Young children (under 4 years) received an ICR, whereas older children received an ICR and a MTT (mm. latissimus dorsi and teres major). The ICR was performed posteriorly as a subscapular muscle slide until 2002 (35). After 2002 an anterior ICR was performed.
ICR. The anterior ICR was performed through a 1–2-cm deltopectoral incision exposing the coracoid process. The coracohumeral ligament was released at the anterior capsule of the shoulder by an incision of approximately 3 mm (the width of a number 15 surgical knife blade).
MTT. Through a curved incision at the posterior axillary border, the mm. teres major and latissimus dorsi tendons were separately detached from the humerus. The humeral head was then exposed by a second incision cranial and posterior at the deltoid area, followed by a deltoid split. From the first incision, underneath the deltoid muscle the detached mm teres major and/or latissimus dorsi were transferred to the m. infraspinatus/supraspinatus footprint area at the humeral head. The tendon(s) were independently fixed at the greater tuberosity of the humerus with transosseous sutures.
Rehabilitation consisted of 6 weeks Baycast plaster in slight shoulder abduction and external rotation position, followed by physical therapy twice a week for at least 3 months. Physical therapy consisted of maintaining passive and improving active joint mobility and muscle strength and stimulating bimanual activities.
Assessments
Sociodemographic and disease characteristics. Age, gender, involved nerve roots, affected side and type of ICR/MMT: release or release and tendon transfer were recorded. History of nerve surgery prior to the ICR/MMT was extracted from the medical record and categorized.
Clinical follow-up. The following data were routinely recorded during the outpatient clinic visit according to a standardized protocol: pROM/aROM of the shoulder and Mallet score. Despite the follow-up protocol, exact timing of time-points differed among patients. Therefore, the following time-frames were defined for statistical analysis: pre-operatively (T0), 6 months (T1, range 0–9 months), 1 year (T2, range 10–18 months), 3 years (T3, range 19–42 months), 5 years (T4, range 43–66 months) and 10 years (T5, range 67–163 months). For analysis, follow-up time-points were defined as time windows about specified follow-up periods. The definition of time windows was based on completeness of data at all follow-up moments in a random selected number of 10 medical records and after consensus among the authors.
Glenohumeral joint deformity. Magnetic resonance imaging (MRI) was used to assess pre-operative glenohumeral joint deformity. From the MRI images the percentage of the humeral head anterior to the midscapular line (%PHHA) and glenoid version were measured (14).
Shoulder range of motion. aROM of the shoulder in the directions external rotation (in 0º and 90º abduction) abduction, scapulohumeral adduction and forward flexion were recorded with a 5º precision level. In addition, pROM in the directions external rotation (in 0º and 90º abduction), glenohumeral abduction and backward flexion were recorded. All measurements were made using a goniometer.
Mallet score. Shoulder movements of the affected arm were measured using the modified Mallet score. This score measures often used arm movements, including overhead movements, with scores ranging from 1 = no function to 5 = normal function. The aggregated Mallet score was computed as well, with scores ranging from 5 (minimum) to 25 (maximum) points (36–38).
Statistical analysis
Descriptive statistics were used for the clinical characteristics of the patients and the glenohumeral joint deformity at baseline (means with standard deviations (SD), frequencies with percentages, where appropriate). Difference over time for the clinical outcomes for the total group as well as for the 2 subgroups, were calculated by means of regression analyses using a linear mixed model, thereby taking into account the repeated measurements within-patients. Within the model follow-up time-points were the fixed effects and the patients the random effect. Outcomes were expressed as estimated means with standard errors and as mean change scores with 95% confidence intervals (95% CI). The level of statistical significance was set at p < 0.05 for all analyses. All analyses were carried out using SPSS 20.0 software (IBM SPSS Statistics for Windows, Version 20.0, IBM Corp., Armonk, NY, USA).
RESULTS
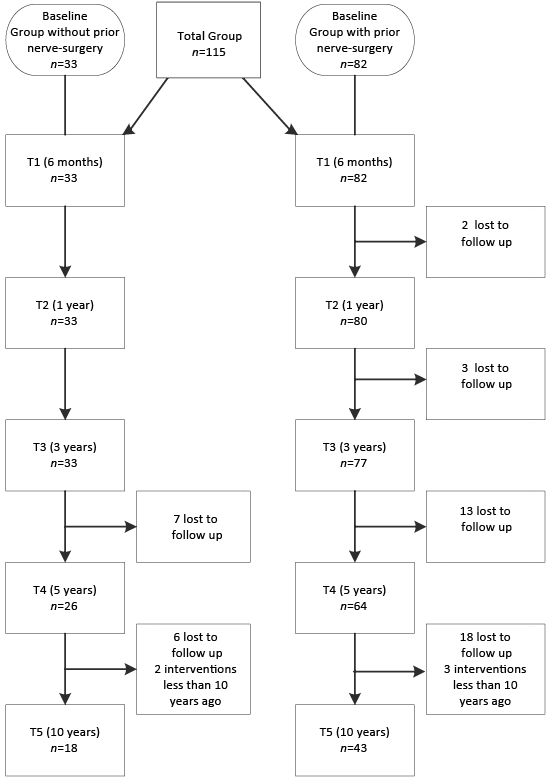
A convenience sample of 115 children met the inclusion criteria. The mean follow-up duration was 6 years (SD 3.2 years, range 6 months to 13 years). The mean follow-up within the time windows defined for T1–T5 was as follows: T1; 4.5 months (SD 2.5); T2; 13.1 months (SD 2.6); T3; 29.4 months (SD 7.1); T4; 53.6 months (SD 6.7); and T5; 96.9 months (SD 23.9). The numbers of patients at the follow-up moments are shown in Fig.1.
Fig. 1. Patients in the 2 subgroups at the different follow-up time-points.
The baseline patient characteristics are described in Table I.
|
Table I. Pre-operative characteristics of all included children with neonatal brachial plexus palsy undergoing an internal contracture release or a combined internal contracture release and muscle tendon transfer |
|||
|
|
Total group (n = 115) |
Group without prior nerve surgery (n = 33) |
Group with prior nerve surgery (n = 82) |
|
Gender, male, n (%) |
60 (52.2) |
18 (54.5) |
42 (51.2) |
|
Age at surgery, years, mean (SD) |
4.7 (3.3) |
6.8 (4.3) |
3.8 (2.3) |
|
Affected side, right, n (%) |
68 (59.1) |
16 (48.5) |
52 (62.7) |
|
Lesion extent, n (%) C5 C5–C6 C5–C7 C5–C8 C5–T1 |
2 (1.7) 66 (57.4) 40 (34.8) 4 (3.5) 3 (2.6) |
2 (6.3) 28 (84.8) 3 (9.4) 0 (0) 0 (0) |
0 (0) 38 (46.3) 37 (44.6) 4 (4.8) 3 (3.6) |
|
Surgical intervention, n (%) Release Release/tendon transfer |
32 (27.8) 83 (72.2) |
11 (34.4) 22 (66.7) |
21 (25.3) 61 (74.4) |
|
%PHHA, mean (SD) |
33.6 (13.3) |
36.0 (12.1) |
32.7 (13.7) |
|
Glenoid version, mean (SD) |
–18.1 (9.7) |
–19.2 (8.6) |
–17.6 (10.1) |
|
%PHHA: percentage of humeral head anterior to midscapular line; SD: standard deviation. |
|||
There were 60 boys and 55 girls with a mean age of 4.7 years (SD 3.3), with a total of 47 left sides and 68 right sides affected. Lesion extent was C5 (n = 2), C5/C6 (n = 66), C5–C7 (n = 40), C5–C8 (n = 4) and C5–T1 (n = 3). Eighty-two children (71.3%) had had prior nerve surgery (group with prior nerve surgery). Primary nerve surgery consisted of nerve reconstruction in 74 and neurolysis in 8 children. Depending on the severity of the nerve lesions and the availability of proximal stumps and/or graft material a reconstruction tailored to the individual was performed. The largest group consists of children in whom the superior trunk, or part of the efferents of the superior trunk were reconstructed (n = 64). Additional re-innervation was performed on the middle trunk (n = 9), the lower trunk (n = 1) or both (n = 1). The most frequent reconstruction was intraplexal grafting of the complete superior trunk (n = 46). The reconstruction of the suprascapular nerve and posterior division of the superior trunk were analysed, as these nerve elements innervate shoulder motion. The suprascapular nerve was reconstructed in 65 infants by means of grafting (n = 52) or transfer (n = 12). In 6 children reconstruction of the suprascapular nerve was not possible, in 4 children with partial lesions the trajectory to the suprascapular nerve was left intact, while other trajectories were reconstructed. The posterior division of the superior trunk was grafted in 64 children; no reconstruction of the posterior division had been performed in 5 children, and the trajectory to the posterior division was left untouched in 6. The remaining 33 children were conservatively treated (group without prior nerve surgery), usually consisting of contracture prevention and maintaining function by a physical therapist.
Pre-operative values for the group without prior nerve surgery and the group with prior nerve surgery differed in absolute values of ROM in terms of: active external rotation in 0° abduction, abduction, forward flexion and scapulohumeral adduction as well as in the aggregated Mallet score. These measures showed better results in the group without prior nerve surgery compared with the group with nerve surgery (more than 5° in ROM and more than one point in the aggregated Mallet score).
Overall, improvements in aROM, pROM and Mallet scores were seen in all groups. During follow-up these improvements were largely similar in both groups. The largest changes were found between T0 and T1. Almost all changes within the groups are significant at all time-points, with the exception of active scapulohumeral adduction. In addition, improvement in passive glenohumeral abduction was not significant in the group without prior nerve surgery. Backward flexion and Mallet “Hand to Back” decreased significantly over time, but only for the group with prior nerve surgery. Overall, there was a general tendency to a decrease in function from T1 onwards for both subgroups. Changes over time with 95% CI are shown in Tables II–IV.
Table II and Figs 2–4 show the course of aROM.
|
Table II. Active shoulder range of motion pre-operatively and at follow-up in children with neonatal brachial plexus palsy, with and without prior nerve surgery, undergoing an internal contracture release or a combined internal contracture release and muscle tendon transfer |
||||||
|
Active ROM (°) |
Pre-operative (T0) Estimated mean (SE) |
T0–T1 Change score (95% CI) |
T0–T2 Change score (95% CI) |
T0–T3 Change score (95% CI) |
T0–T4 Change score (95% CI) |
T0–T5 Change score (95% CI) |
|
Total group |
|
|
|
|
|
|
|
External rotation (0° abduction) |
–69.0 (3.3) |
73.0 (65.3–80.7) |
62.4 (54.5–70.2) |
65.0 (57.5–72.6) |
61.8 (53.7–69.8) |
59.0 (50.0–68.1) |
|
External rotation (90° abduction) |
4.3 (3.0) |
47.7 (41.2–54.3) |
41.8 (35.0–48.5) |
47.0 (40.5–53.5) |
42.2 (35.4–49.0) |
38.6 (31.2–46.0) |
|
Abduction |
74.4 (4.4) |
46.2 (38.1–54.3) |
41.1 (32.7–49.5) |
52.8 (44.9–60.7) |
50.5 (42.0–58.9) |
50.1 (40.8–59.4) |
|
Scapulohumeral adduction |
42.5 (1.7) |
–0.9 (–5.8–3.8) |
–0.8 (–5.2–3.6) |
–0.01 (–4.4–4.2) |
–6.2 (–11.1– –1.0) |
–6.4 (–11.7– –1.0) |
|
Forward flexion |
103.2 (4.2) |
28.0 (20.9–35.1) |
27.5 (20.3–34.7) |
33.8 (27.1–40.5) |
34.1 (27.0–41.2) |
29.0 (21.1–36.9) |
|
Group without prior nerve surgery |
|
|
|
|
|
|
|
External rotation (0° abduction) |
–56.5 (5.5) |
62.4 (49.8–74.9) |
47.8 (34.7–61.0) |
48.7 (36.4–60.9) |
41.9 (28.3–55.5) |
42.8 (27.7–57.8) |
|
External rotation (90° abduction) |
6.7 (5.5) |
41.1 (29.8–52.5) |
37.0 (25.3–48.8) |
41.0 (29.7–52.2) |
37.7 (25.6–49.8) |
25.4 (12.3–38.5) |
|
Abduction |
88.1 (8.5) |
45.6 (30.9–60.4) |
41.0 (25.1–57.0) |
46.6 (31.9–61.3) |
51.9 (35.7–68.0) |
57.7 (40.3–75.1) |
|
Scapulohumeral adduction |
37.2 (2.2) |
1.8 (–3.2–6.8) |
5.4 (0.45–10.4) |
1.6 (–3.1–6.4) |
1.1 (–4.2–6.5) |
–4.7 (–12.2–0.8) |
|
Forward flexion |
124.3 (7.7) |
20.7 (8.0–33.3) |
21.1* (7.3–34.9) |
20.6 (8.2–32.9) |
24.2 (10.7–37.7) |
21.0 (6.3–35.7) |
|
Group with prior nerve surgery |
|
|
|
|
|
|
|
External rotation (0° abduction) |
–74.5 (4.0) |
77.6 (68.2–87.1) |
68.6 (59.1–78.2) |
72.1 (62.9–81.4) |
69.8 (60.1–79.5) |
65.9 (54.9–77.0) |
|
External rotation (90° abduction) |
3.6 (3.5) |
50.3 (42.2–58.4) |
43.4 (35.2–51.7) |
49.2 (41.4–57.1) |
43.8 (35.6–51.9) |
43.7 (34.7–52.7) |
|
Abduction |
68.7 (4.9) |
46.4 (36.8–56.1) |
41.3 (31.4–51.2) |
55.1 (45.8–64.5) |
50.1 (40.1–60.1) |
46.9 (35.8–58.0) |
|
Scapulohumeral adduction |
44.8 (2.2) |
–1.4 (–8.1–5.4) |
–3.5 (–9.5–2.5) |
–0.9 (–6.7–5.0) |
–9.5 (–16.2– –2.9) |
–7.3 (–14.9–0.2) |
|
Forward flexion |
95.2 (4.8) |
30.3 (21.7–38.9) |
29.6 (21.0–38.1) |
38.7 (30.7–46.6) |
37.4 (29.0–45.8) |
31.6 (22.2–41.0) |
|
T1: 6-month follow-up, T2: 1-year follow-up, T3: 3-year follow-up, T4: 5-year follow-up, T5: 10-year follow-up; SE: standard error. |
||||||
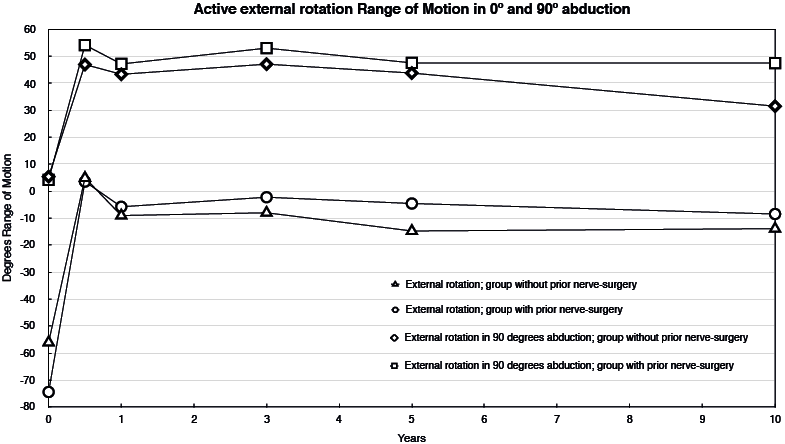
Fig. 2. Course of active external range of motion in 0º and 90º abduction over time in 2 subgroups, based on estimated means and mean changes from the linear mixed model; from pre-surgery (T0) to 6 months (i.e. T0+mean change T1), to 10 years post-surgery (i.e.T0 + mean change T5). Differences between T0 and all other time-points statistically significant for all groups and variables.
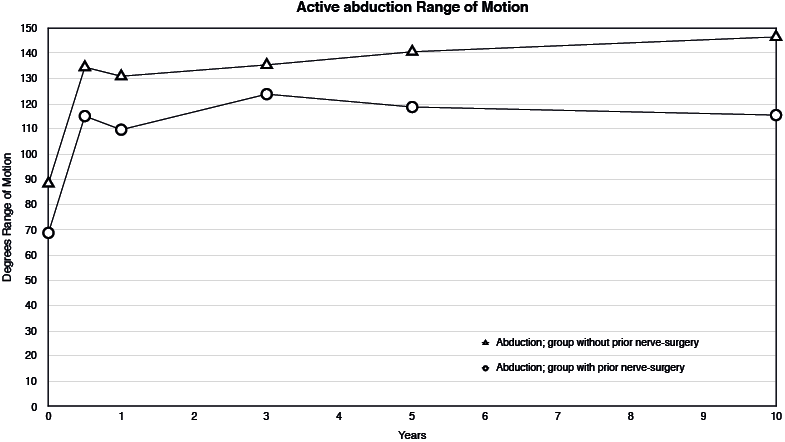
Fig. 3. Course of active abduction range of motion over time in 2 subgroups, based on estimated means and mean changes from the linear mixed model; from pre-surgery (T0) to 6 months (i.e. T0 + mean change T1), to 10 years post-surgery (i.e.T0+ mean change T5). Differences between T0 and all other time-points statistically significant for all groups.
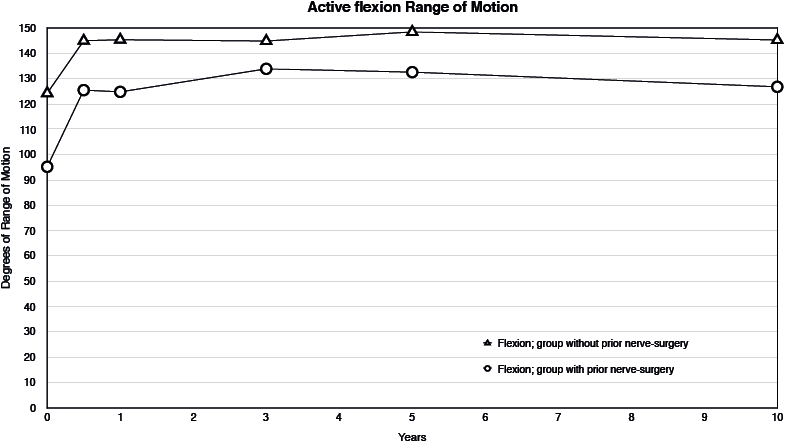
Fig. 4. Course of active forward flexion range of motion over time in 2 subgroups, based on estimated means and mean changes from the linear mixed model; from pre-surgery (T0) to 6 months (i.e. T0+mean change T1), to 10 years post-surgery (i.e. T0+ mean change T5). Differences between T0 and all other time-points statistically significant for all groups.
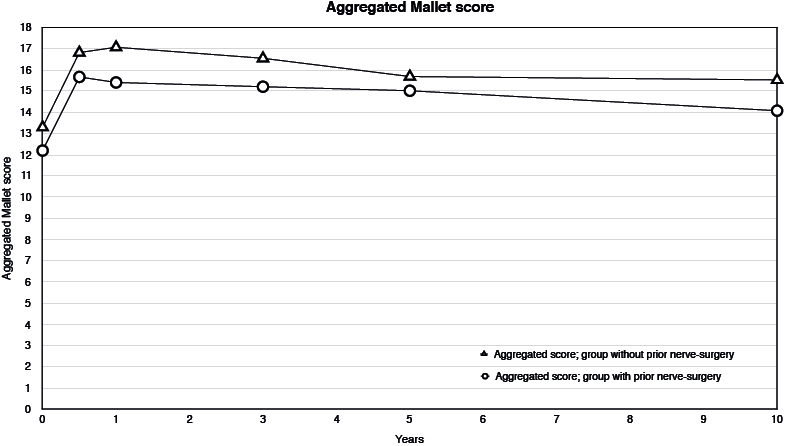
Fig. 5. Course of the aggregated Mallet score over time in 2 subgroups based on estimated means and mean changes from the linear mixed model; from pre-surgery (T0) to 6 months (i.e. T0 + mean change T1), to 10 years post-surgery (i.e.T0 + mean change T5). Differences between T0 and all other time-points statistically significant for all groups.
With the exception of active scapulohumeral adduction in all groups, aROM improved significantly at all time-points compared with baseline. The largest improvement was seen at T1, whereas at later time-points the differences with the pre-operative situation decreased. At all follow-up time-points, most absolute values of the aROM measures were more favourable in the group without prior nerve surgery than in the group with prior nerve surgery. Only absolute values for external rotation in 0° and 90° abduction were higher for the group with prior nerve surgery.
The course of pROM is shown in Table III.
|
Table III. Passive shoulder range of motion and muscle strength pre-operatively and at follow-up in children with neonatal brachial plexus palsy, with and without prior nerve surgery, undergoing an internal contracture release or a combined internal contracture release and muscle tendon transfer |
||||||
|
Passive ROM; ° |
Pre-operative (T0) Estimated mean (SE) |
T0–T1 Change score (95% CI) |
T0–T2 Change score (95% CI) |
T0–T3 Change score (95% CI) |
T0–T4 Change score (95% CI) |
T0–T5 Change score (95% CI) |
|
Total group |
|
|
|
|
|
|
|
External rotation (0° abduction) |
–7.7 (2.2) |
40.3 (35.0–45.5) |
38.0 (32.5–43.3) |
38.8 (33.5–44.0) |
30.6 (25.0–36.2) |
25.4 (19.1–31.8) |
|
External rotation (90° abduction) |
47.0 (1.7) |
28.1 (23.7–32.4) |
25.6 (21.1–30.1) |
28.8 (24.5–33.1) |
20.3 (15.7–24.9) |
17.4 (12.2–22.5) |
|
Glenohumeral abduction |
84.4 (1.3) |
3.4 (1.4–5.4) |
2.2 (0.2–4.2) |
4.5 (2.6–6.4) |
2.5 (0.5–4.5) |
0.2 (–2.0–2.5) |
|
Backward flexion |
50.5 (3.3) |
–14.5 (–20.7– –8.3) |
–13.9 (–20.7– –7.0) |
–15.8 (–22.8– –8.9) |
–16.3 (–24.8– –7.8) |
–13.5 (–24.9– –2.2) |
|
Group without prior nerve surgery |
||||||
|
External rotation (0° abduction) |
–4.4 (3.9) |
36.1 (26.6–45.6) |
34.6 (24.7–44.4) |
25.2 (15.9–34.5) |
15.6 (5.4–25.9) |
11.6 (0.4–22.9) |
|
External rotation (90° abduction) |
44.7 (3.1) |
31.1 (23.4–38.8) |
26.9 (18.2–33.7) |
26.0 (18.2–33.9) |
20.5 (12.0–29.0) |
18.0 (8.9–27.2) |
|
Glenohumeral abduction |
84.6 (1.6) |
2.8 (–0.3–5.9) |
–0.2 (–3.4–3.1) |
3.1 (0.1–6.0) |
0.6 (–2.7–4.0) |
2.4 (–1.3–6.2) |
|
Backward flexion |
52.0 (6.4) |
–14.0 (–26.6– –1.0) |
–9.1 (–23.7–5.4) |
–7.9 (–21.8–6.1) |
–0.2 (–17.7–17.2) |
–3.2 (–22.6–16.3) |
|
Group with prior nerve surgery |
||||||
|
External rotation (0° abduction) |
–9.1 (2.6) |
42.0 (35.8–48.2) |
39.5 (33.1–45.9) |
44.4 (38.2–50.5) |
36.3 (29.8–42.8) |
31.3 (23.8–38.8) |
|
External rotation (90° abduction) |
47.9 (2.1) |
26.7 (21.4–32.0) |
25.1 (19.6–30.6) |
29.9 (24.7–35.1) |
20.2 (14.6–25.7) |
17.1 (10.9–23.3) |
|
Glenohumeral abduction |
84.3 (1.6) |
3.7 (1.2–6.2) |
3.1 (0.6–5.6) |
5.1 (2.7–7.4) |
3.3 (0.8–5.7) |
–0.4 (–3.2–2.4) |
|
Backward flexion |
49.2 (3.7) |
–14.4 (–21.5– –7.4) |
–15.0 (–22.7– –7.4) |
–19.4 (–27.4– –11.4) |
–22.6 (–32.3– –13.0) |
–19.0 (–33.7– –4.2) |
|
T1: 6-month follow-up, T2: 1-year follow-up, T3: 3-year follow-up, T4: 5-year follow-up, T5: 10-year follow-up; SE: standard error. |
||||||
The pre-operative values of pROM were similar in the group without prior nerve surgery and the group with prior nerve surgery. Except for backward flexion and glenohumeral abduction in both groups all measures of pROM improved significantly at all time-points compared with baseline. Backward flexion decreased significantly at all time-points for the group with prior nerve surgery and for the group without prior nerve surgery only at T1.
Like the clinical course of aROM, after an initially large improvement directly following surgery, differences from baseline decreased gradually in both subgroups. This pattern was, however, not seen for backward flexion in the group without prior nerve surgery, which improved after an initial decline at T1.
The course of Mallet scores is shown in Table IV and Fig. 5.
Pre-operative Mallet scores were similar in the group without prior nerve surgery compared with the group with prior nerve surgery, except for the “Aggregated score”, which was 2 points greater in the group without prior nerve surgery.
|
Table IV. Shoulder function pre-operatively and at follow up in children with neonatal brachial plexus palsy, with and without prior nerve surgery, undergoing an internal contracture release or a combined internal contracture release and muscle tendon transfer |
||||||
|
Mallet score (1–5) |
Pre-operative (T0) Estimated mean (SE) |
T0–T1 Change score (95% CI) |
T0–T2 Change score (95% CI) |
T0–T3 Change score (95% CI) |
T0–T4 Change score (95% CI) |
T0–T5 Change score (95% CI) |
|
Total group |
|
|
|
|
|
|
|
Abduction |
3.2 (0.06) |
0.60 (0.46–0.75) |
0.47 (0.32–0.62) |
0.58 (0.44–0.72) |
0.44 (0.30–0.59) |
0.41 (0.24–0.57) |
|
External rotation |
1.03 (0.11) |
1.35 (1.10–1.61) |
1.34 (1.07–1.60) |
0.99 (0.75–1.24) |
0.91 (0.65–1.17) |
0.69 (0.41–0.98) |
|
Hand to Head |
2.51 (0.08) |
1.05 (0.87–1.22) |
1.10 (0.91–1.28) |
1.06 (0.89–1.23) |
0.99 (0.81–1.17) |
0.87 (0.67–1.06) |
|
Hand to Back |
3.20 (0.10) |
–0.50 (–0.70– –0.30) |
–0.32 (–0.52– –0.11) |
–0.36 (–0.54– –0.17) |
–0.44 (–0.64– –0.24) |
–0.43 (–0.65– –0.21) |
|
Hand to Mouth |
2.61 (0.07) |
0.92 (0.75–1.10) |
0.76 (0.58–0.94) |
0.79 (0.63–0.96) |
0.65 (0.48–0.83) |
0.43 (0.24–0.63) |
|
Mallet aggregated score |
12.49 (0.24) |
3.46 (2.91–4.02) |
3.32 (2.75–3.88) |
3.10 (2.57–3.61) |
2.73 (2.18–3.28) |
2.02 (1.41–2.63) |
|
Group without prior nerve surgery |
||||||
|
Abduction |
3.35 (0.11) |
0.55 (0.33–0.78) |
0.38 (0.13–0.64) |
0.44 (0.22–0.66) |
0.40 (0.16–0.64) |
0.56 (0.31–0.81) |
|
External rotation |
1.25 (0.20) |
1.19 (0.71–1.68) |
1.32 (0.80–1.84) |
0.89 (0.43–1.34) |
0.60 (0.10–1.11) |
0.24 (–0.30–0.77) |
|
Hand to Head |
2.70 (0.13) |
0.87 (0.54–1.19) |
1.02 (0.66–1.37) |
0.94 (0.63–1.25) |
0.86 (0.52–1.20) |
0.98 (0.62–1.33) |
|
Hand to Back |
3.56 (0.14) |
–0.38 (–0.74– –0.03) |
–0.14 (–0.52–0.25) |
–0.07 (–0.41–0.27) |
–0.34 (–0.71–0.03) |
–0.21 (–0.60–0.18) |
|
Hand to Mouth |
2.57 (0.12) |
1.15 (0.85–1.45) |
0.89 (0.56–1.21) |
1.00 (0.72–1.29) |
0.80 (0.48–1.12) |
0.60 (0.27–0.93) |
|
Mallet aggregated score |
13.29 (0.42) |
3.53 (2.62–4.43) |
3.78 (2.76–4.80) |
3.26 (2.39–4.12) |
2.40 (1.42–3.37) |
2.23 (1.23–3.23) |
|
Group with prior nerve surgery |
||||||
|
Abduction |
3.10 (0.08) |
0.63 (0.45–0.82) |
0.51 (0.32–0.69) |
0.64 (0.47–0.81) |
0.47 (0.29–0.65) |
0.34 (0.14–0.54) |
|
External rotation |
0.94 (0.14) |
1.43 (1.12–1.74) |
1.37 (1.06–1.69) |
1.05 (0.76–1.33) |
1.04 (0.73–1.35) |
0.90 (0.56–1.25) |
|
Hand to Head |
2.43 (0.09) |
1.13 (0.91–1.34) |
1.14 (0.92–1.35) |
1.12 (0.92–1.32) |
1.05 (0.84–1.26) |
0.82 (0.58–1.05) |
|
Hand to Back |
3.04 (0.11) |
–0.55 (–0.80– –0.30) |
–0.38 (–0.62– –0.14) |
–0.45 (–0.67– –0.22) |
–0.46 (–0.70– –0.23) |
–0.50 (–0.77– –0.24) |
|
Hand to Mouth |
2.63 (0.08) |
0.83 (0.61–1.04) |
0.71 (0.49–0.92) |
0.70 (0.50–0.90) |
0.58 (0.37–0.79) |
0.35 (0.11–0.59) |
|
Mallet aggregated score |
12.19 (0.28) |
3.47 (2.80–4.16) |
3.21 (2.53–3.89) |
3.00 (2.36–3.64) |
2.83 (2.16–3.35) |
1.88 (1.12–2.63) |
|
T1: 6-month follow-up, T2: 1-year follow-up, T3: 3-year follow-up, T4: 5-year follow-up, T5: 10-year follow-up; SE: standard error. |
||||||
Except for the “Hand to Back” item in all groups, there was a significant improvement compared with baseline for all Mallet items, including the aggregated score. The “Hand to Back” item decreased significantly at all time-points for the group with prior nerve surgery and for the group without prior nerve surgery only at T1. This is in line with the previous pROM findings.
The largest improvements in Mallet scores were seen at T1 and T2, whereas at later time-points the differences in the preoperative situation overall decreased. At all follow-up time-points, all absolute Mallet scores, except for the “External Rotation” item at T4 and T5, were more favourable in the group without prior nerve surgery than in the group with prior nerve surgery, leaving the group without prior nerve surgery with better function according to the Mallet score.
DISCUSSION
This long-term follow-up study (over a mean of 6 years) reported the outcomes of secondary shoulder surgery in 115 children with NBPP. In children both with and without prior nerve surgery, shoulder passive and active external rotation, (glenohumeral) abduction and forward flexion ROM, as well as almost all Mallet score items, improved significantly.
Children without prior nerve surgery had overall better pre-operative shoulder function. The positive effects of surgery decreased over time, to some extent, but differences from baseline remained statistically significant. Only backward flexion and the Mallet “Hand to Back” item decreased significantly. The children who were conservatively treated before secondary shoulder surgery had an overall better shoulder function at all follow-up time-points than the children who had undergone nerve surgery prior to shoulder surgery. Only active and passive external rotation, both in 0° and 90° abduction, are slightly better at all follow-up time-points after secondary shoulder surgery for children who had undergone prior nerve surgery.
The favourable effect on ROM and Mallet scores in children with NBPP in the current study is in line with the results of several other studies (16–22, 24–30, 39, 40) and a recent meta-analysis (31). The same holds for the negative effect on backward flexion and the possibility of bringing the arm to the back (20, 22).
In contrast to the current study, most other studies regarding the outcome of secondary shoulder surgery in children with NBPP did not take previous nerve surgery into account (21, 41) or reported the outcomes for both groups as a single series (16–20, 24–30, 39, 40). Only 2 studies on the outcomes of secondary shoulder surgery described the outcomes for the 2 groups separately (22, 32). One study found, similar to the current study, that those children who have had previous nerve surgery had worse ROM at baseline (32). The other study only stated that improvement in ROM was greater for the group without prior nerve surgery (22), which is opposed to the findings in the current study, where improvements were similar. However, the absolute values of all endpoint measures, except external rotation, in the current study were more favourable in the group without prior nerve surgery. The number of included patients in the present study who had nerve surgery was relatively high compared with other studies. This is related to the fact that, in the Netherlands, this surgery is performed in 3 centres, of which Leiden is the largest and is also a “last resort” facility for babies with NBPP (29, 30).
Secondary surgery is performed in children with NBPP with limited shoulder function and possible joint deformities, irrespective of previous nerve surgery. The differences in pre-operative characteristics of the group without prior nerve surgery and the group with prior nerve surgery, and the clinical course over time after surgery made it clear that these 2 groups concern different subgroups of patients. Moreover, children in the group with prior nerve surgery were, on average, 3 years younger at the time of surgery. This indicates that these children show shoulder problems earlier in life, possibly because of worse function and/or neurological recovery, and this again shows that both groups differ from each other. Primary nerve surgery is performed only in those children who show no, or insufficient, recovery of function around 3–6 months after birth (11), thus constituting a selected group of children. This phenomenon is usually designated as “confounding by indication”, and this makes the outcomes of these subgroups not directly comparable (42).
Regarding the long-term outcomes of secondary shoulder surgery, most other studies do not show the course of clinical outcome over time at different time-points, but only give pre-operative and post-operative values for the outcomes at a single point in time, which may vary largely among individual patients (16–22, 24–28, 39, 40). The present study included multiple time-points, which made it clear that the beneficial effect decreases with time, except for backward flexion, which after an initial decline, improved only in the group without prior nerve surgery. The largest decrease was seen for shoulder external rotation ROM, especially in 0° abduction, and for the Mallet “external rotation” item after 6 months follow-up.
Decrease in shoulder function after secondary surgery has been described previously by one study, in particular for abduction 6 years after surgery (34). In the current study, a gradual decrease was also seen for other outcomes. The decrease in effect might be related to the fact that patients may stop doing exercises at some time after surgery. The question is whether the decrease is clinically relevant, as patients may not always need the full extent of their gained ROM to perform daily activities. Moreover, despite the decrease, more than 5 years after surgery, shoulder function was overall still significantly better than pre-operatively.
This study has a number of limitations. First, there was a variation in follow-up moments between individuals, due to the fact that data were gathered in routine clinical care (e.g. sometimes appointments were rescheduled). Therefore, for analysis, follow-up windows (combining follow-up moments) were defined. The chosen time windows were wide, thus aggregating all available data. Nevertheless, missing data of some patients were present at certain time-points. Between 5 and 10 years after surgery, a number of patients were lost to follow-up; perhaps this group of patients had good clinical function and did not see the necessity of follow-up, or had other reasons not to participate in follow-up. Thus, the group remaining at long term follow-up is prone to selection bias. To a certain extent statistical analyses of the data by means of a linear mixed model deals with missing data. Measurements were made prospectively with a goniometer during regular patient care by 3 dedicated clinicians over time. Thus intra- and inter-observer variability might be present. A long-term prospective outcome study with fixed time-points, to which patients and parents adhere, could solve this limitation. Even so, children may become ill, resulting in rescheduling and thus possible missing data.
Secondly, some of the pre-operative patient characteristics, other than the clinical outcomes, varied in terms of type and extent of the lesion within and between both subgroups. The group without prior nerve surgery include only C5/C6±C7 lesions and the group with prior nerve surgery also had 7 children with involvement of C8 and/or T1.
Thirdly, 2 types of secondary surgical interventions were used within both groups and a change in operating technique for the ICR was made in 2002. Because all procedures (ICR and ICR/MTT) are designed to improve aROM, pROM and function, no subgroup analyses was done based upon the chosen intervention and/or technique.
Fourthly, the size of the 2 subgroups were different, with more patients in the group with prior nerve surgery (82 vs 33). However, these patients differ in lesion severity by definition and clinical outcomes of the secondary surgical intervention may not be directly compared between these groups.
Fifthly, no patient reported outcome measure or functional assessment was included, besides the Mallet score. The Mallet score, however, only measures function and not activities. Future studies should include analyses of activities and participation according to ICF standards (43) to further comprehend the outcome of secondary surgery around the shoulder.
In conclusion, the present study shows that, in children with NBPP, shoulder function improves after an ICR/MTT, irrespective of whether they have had prior nerve surgery. Over the course of time the effects of secondary surgery decreased, but differences from baseline remained significant, indicating permanently improved shoulder function. However, this study also showed that pre-operative and postoperative shoulder function with respect to active external rotation in 0° abduction, abduction, forward flexion and scapulohumeral adduction ROM and the aggregated Mallet score, were better at all time-points in children without prior nerve surgery compared with children who had nerve surgery, indicating that both groups are different entities, and should be reported separately. Reporting the outcomes for the 2 groups separately on multiple time-points, will prevent an over- or under-estimation of the results of the orthopaedic intervention and is a good option to provide more accurate, detailed information.
More detailed information on the expected treatment outcome over time, taking into account previous nerve surgery, is important for parents and children and can contribute to the quality of the decision-making process for parents of patients and treating physicians.
The authors declare no conflicts of interest.
REFERENCES
