Maping Huang, PhD1, Hui Chen, PhD1,2, Chonghe Jiang, PhD2, Keji Xie, PhD3, Ping Tang, PhD3, Rubiao Ou, PhD3, Jiangwen Zeng, PhD2, Qiuling Liu, MD1, Qingqing Li, MD1, Jiebing Huang, MD1, Tianhai Huang, MD1 and Weiwei Zeng, MD3
From the 1Department of Urology, Guangdong Provincial Work Injury Rehabilitation Hospital and Jinan University, Guangzhou, and 2Department of Urology, Qingyan City People’s Hospital, Jinan University, Guangdong and 3Department of Urology, Guangzhou First Municipal People’s Hospital, Guangzhou Medical University, China
OBJECTIVE: To evaluate the safety and effect of botulinum toxin A injection in the detrusor and external urethral sphincter in male patients with detrusor overactivity (DO) and detrusor external sphincter dyssynergia (DESD) secondary to spinal cord injury.
METHODS: A multicentre trial was conducted from June 2012 to August 2015. A total of 65 spinal cord injury patients with DO and DESD participated in the study. Of these, 59 received 200 U botulinum toxin intradetrusor and 100 U external urethral sphincter injections. The effective outcomes included maximum detrusor pressure at first DO and DESD, VDO-DESD, maximum urethral closure pressure, duration of first DO and DESD, Incontinence-Specific Quality-of-Life Instrument, voiding volume, urinary incontinence episodes and complete dryness. Adverse events were recorded.
RESULTS: All patients experienced a significant mean reduction in PdetmaxDO -DESD (46.60%), maximum urethral closure pressure (29.61%), duration of first DO and DESD (42.93%) and a significant mean increase in VDO-DESD (38.11%) 12-weeks post-injection. Significant (p < 0.001) improvement in mean Incontinence-Specific Quality-of-Life Instrument, voiding volume, urinary incontinence episodes and complete dryness were found in all patients at 2 weeks and were sustained at 8 weeks and 16 weeks.
CONCLUSION: Botulinum toxin A injection in the detrusor and external urethral sphincter is an effective treatment to protect the upper urinary tract and improve quality of life for patients with DO and DESD secondary to spinal cord injury.
Key words: detrusor overactivity; detrusor external sphincter dyssynergia; botulinum toxin A; spinal cord injury.
J Rehabil Med 2016; 48: 683–687
Correspondence address: Chen Hui, Department of Urology, Guangdong Provincial Work Injury Rehabilitation Hospital and Jinan University, 0086+510440 Guangzhou, China. E-mail: doc.chenhui @163.com
Accepted Jun 21, 2016; Epub ahead of print Aug 22, 2016
INTRODUCTION
It was reported in 2001 that the incidence of spinal cord injury (SCI) in China is 0.67–1.37 per 100,000 population (1). Since China has a population of 1.3 billion, this translates into more than 13,000 new SCI patients each year. However, in 2014, a new epidemiological investigation revealed that the incidence was approximately 50,000 new cases each year, mainly due to the increase in vehicle accidents with increasing numbers of cars and motor bicycles (2). Of these patients 60–95% will develop neurogenic lower urinary tract dysfunction (NLUTD), which can cause a range of long-term neuro-urological symptoms, such as autonomic dysreflexia (AD), hydronephrosis, recurrent urinary tract infection, vesicoureteric reflux (VUR) and damage to renal function, which is the most dangerous complication (3–5).
Intradetrusor injections of botulinum toxin A (BTX-A) (Botox, Allergan, Irvine, CA, USA) have resulted in long-lasting effects on detrusor overactivity (DO), and repeated injections appear to be possible without loss of efficacy (6–11). Some studies (12, 13) have reported that BTX-A injection in the external urethral sphincter could restore bladder emptying and reduce detrusor pressure. Of patients with combined suprasacral and sacral injuries, 30.95–67.7% developed secondary complications of both DO and detrusor external sphincter dyssynergia (DESD) (13, 14) . For these patients, high detrusor pressure results from both concurrent involuntary detrusor and urethra contractions. Therefore, encouraged by our satisfactory clinical effects, we performed this open, multicentre, treatment trial with pre- and post-injection evaluations to assess the effect of combined detrusor and external urethral sphincter BTX-A injections in patients with DO and DESD secondary to SCI.
MATERIAL AND METHODS
Study population
The trial subjects were inpatients in 3 different institutions which participated in the study from June 2012 to August 2015. All eligible inpatients over 18 years of age with chronic SCI (i.e. no progression in neurological symptoms in the previous 3 months) were screened for enrolment. Inclusion criteria were: (i) presence of DO and DESD; and (ii) inadequate response or intolerance to oral anti-muscarinic agent (oxybutynin, trospium, tolterodine, propiverine, darifenacin and solifenacin) or spasmolytic agents (hyoscine butylbromide), skeletal muscle relaxant (baclofen) and alpha blockers (doxazosin mesylate and terazosin). Exclusion criteria were: (i) allergy to BTX-A; (ii) coagulopathy disease and myasthenia gravis; (iii) acute urinary tract infection; (iv) other causes of bladder outlet obstruction (i.e. urethral stricture and benign prostatic hyperplasia); and (v) previous sphincterotomy. All patients were given a thorough explanation of the treatment and provided written informed consent before injection. The study was approved by the ethics committees of the 3 hospitals.
Study outcomes
All patients underwent a videourodynamic study at baseline evaluation and at week 12 after injection, using Siemens UROSKOP access X-ray system (Wittelsbacherplatz, Munich, Germany) and LaboriedelphisTM urodynamic system (Suite, Mississauga, Canada). The end-point outcomes included maximum detrusor pressure at first DO and DESD (PdetmaxDO-DESD), volume at first DO and DESD (VDO-DESD), maximum urethral closure pressure (MUCP), duration of first DO and DESD, Incontinence-Specific Quality-of-Life Instrument (I-QoL), voiding volume, urinary incontinence (UI) episodes and complete dryness. Detrusor overactivity is a urodynamic observation characterized by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked (15, 16). DESD is defined as detrusor contraction concurrent with an involuntary contraction of the external urethra (15, 16). MUCP is the maximum difference between the urethral pressure and the intravesical pressure (17). The I-QoL (18) contains 22 items evaluating problems related to incontinence. Items are scored on a 5-point scale, with values ranging from 1 (extreme) to 5 (not at all). Scores were then converted to a scale ranging from 0 (worst I-QoL) to 100 (best I-QoL). Voiding volume is defined as voided volume by clean intermittent catheterization (CIC) plus spontaneous voids. Complete dryness is defined as less than one incontinence episode per 24 h. All these outcomes were determined from 7 consecutive days of the patient’s bladder diary. Related adverse events were recorded throughout the study.
Injections
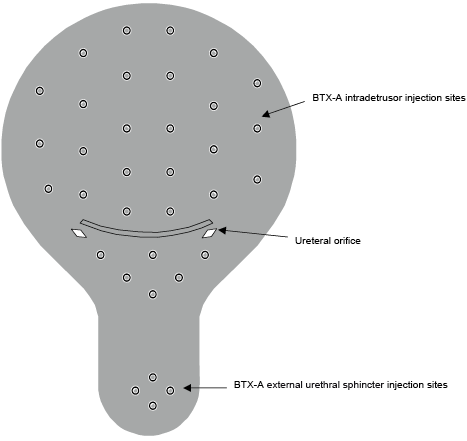
Injections were performed in the operating room with no anaesthesia or under epidural anaesthesia. The bladder was filled with 100–150 ml sterile saline to achieve adequate visualization so as to avoid blood vessels during injections. A 23-gauge needle (Cook Urological Incorporated, Bloomington, IN, USA) was used for injections through a 21F rigid cystoscope (Ackermann, Schaffhausen, Switzerland), to a depth of approximately 2 mm into the detrusor. Firstly, 200 U Botox® vials (100 U each) were reconstituted in 30 ml sterile saline (6.7 U/ml) and administered in 30 injections of 1 ml each, spaced 1 cm apart across the detrusor, and additional 4 1-ml (total 100 U) BTX-A injections into the external urethral sphincter to a depth of approximately 1 cm at 3, 6, 9 and 12 o’clock positions in approximately equal aliquots (Fig. 1) (19, 20). A 16 Foley catheter was inserted into the bladder and kept for 3–5 days. Oral prophylactic antibiotics (except aminoglycosides) were administered on the day of treatment.
Statistical analysis
Student’s paired samples t-test was used as appropriate to compare PdetmaxDO-DESD, VDO-DESD, duration of first DO and DESD, I-QoL, voiding volume, UI episodes of pre- and post-injection. The results are shown as mean values and standard deviation (SD). χ2 test was used to compare the rates of complete dryness. All statistical tests were 2-sided, and a p-value of 0.05 or less was considered statistically significant. Statistical analyses were performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Of the total number of 65 participants, 6 were lost to follow-up because they could not be contacted. Therefore, 59 cases completed this trial. Their mean age was 39.08 years, mean weight 60.42 kg and mean injury duration 11.74 months (Table I). The distribution of SCI levels was: 28 (47.46%) cervical, 25 (42.37%) thoracic, and 6 (10.17%) lumbar. The distribution of the American Spinal Injury Association Impairment Scale (AIS) scores was: 42 (71.19%) Grade A, 14 (23.73%) Grade B, and 3 (5.08%) Grade C.
|
Table I. Demographic characteristics of the participants |
|
|
Characteristics |
|
|
Number of patients |
59 |
|
Age, years, mean (SD) |
39.08 (16.29) |
|
Weight, kg, mean (SD) |
60.42 (18.31) |
|
Injury duration, months, mean (SD) |
11.74 (10.08) |
|
Neurological injury level, n (%) |
|
|
Cervical |
28 (47.46) |
|
Thoracic |
25 (42.37) |
|
Lumbar |
6 (10.17) |
|
AIS, n (%) |
|
|
Grade A |
42 (71.19) |
|
Grade B |
14 (23.73) |
|
Grade C |
3 (5.08) |
|
SCI: spinal cord injury; AIS: American Spinal Injury Association Impairment Scale. |
|
Fig. 2 shows that the significant difference resulted from pre-injection and post-injection PdetmaxDO-DESD, VDO-DESD, MUCP and duration of first DO and DESD (all p-value < 0.05). Comparing the urodynamic parameters at baseline, all patients experienced a significant mean reduction in PdetmaxDO-DESD (46.60%), MUCP (29.61%), duration of first DO and DESD (42.93%), and a significant mean increase in VDO-DESD (38.11%) at 12 weeks, respectively. Significant (p-value < 0.05) improvement in mean I-QoL, voiding volume, UI episodes and complete dryness were present in all patients at 2 weeks, and these clinical improvements were sustained at 8 and 16 weeks (Fig. 3). During the first week after injection, 8 patients had mild transient haematuria for 1–2 days. No patients required medication or surgical intervention.
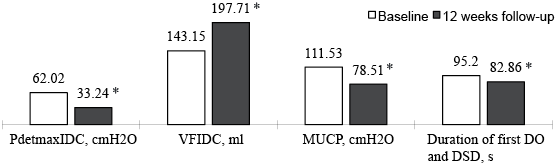
Fig. 2. Videourodynamic parameters of patients at baseline and at 12-weeks follow-up. A significant improvement (*p < 0.05) was noted at 12 weeks. DO: detrusor overactivity; DESD: detrusor external sphincter dyssynergia; MUCP: maximum urethral closure presure.
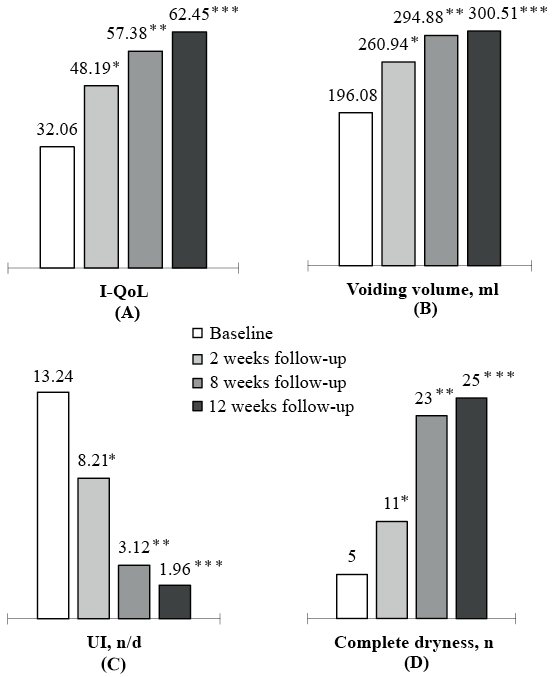
Fig. 3. I-QoL, voiding volume, urinary incontinence (UI) and complete dryness of patients at baseline, 2-, 8- and 12-weeks follow-up. A significant improvement (*p < 0.05) was presented at 2 weeks that was sustained at 8 weeks (**p < 0.05) and 12 weeks (***p < 0.05). I-QoL: Incontinence-Specific-Quality-of-life Instrument.
The causes of patients’ satisfaction and dissatisfaction with the treatments are shown in Table II. Significant satisfaction resulted from less autonomic dysreflexia (61.11%), decreased UI (100%), complete dryness (42.37%), less symptomatic urinary tract infection (42.86%) and less bladder pain syndrome (88.89%). However, the high cost of treatment, the requirement for repeated injection (45.76%), and CIC (30.51%) were noted as major problems for these patients.
|
Table II. Patients’ satisfaction and dissatisfaction with the botulinum toxin A injections |
|||
|
Satisfaction |
Patients n (%) |
Dissatisfaction |
Patients n (%) |
|
Less autonomic dysreflexia |
11(61.11) |
Needing CIC |
18 (30.51) |
|
Decreased UI |
59 (100) |
High cost of the injection |
15 (25.42) |
|
Complete dryness |
25 (42.37) |
Needing repeated injections |
27 (45.76) |
|
Less symptomatic UTI |
6 (42.86) |
|
|
|
At baseline, we observed 18 patients with symptoms of autonomic dysreflexia and 14 with prior symptomatic UTI (8 patients with fever and a temperature higher than 38°C, 5 with supra-pubic pain or tenderness). UI: urinary incontinence; UTI: urinary tract infection; CIC: clean intermittant catheterization. |
|||
DISCUSSION
According to the neuro-urology guidelines, the most important aim for treatment of neurological symptoms and their priorities is to protect the function of the upper urinary tract (4). Intradetrusor BTX-A injection was first introduced in 2000 by Schurch et al. (20) as a minimally-invasive treatment for patients with neurogenic detrusor overactivity (NDO) and urge incontinence resistant to anticholinergic drugs. According to their study, after treatment 19 patients had a significant decrease in mean maximum detrusor voiding pressure at 36 weeks follow-up. Furthermore, over the past 15 years there has been rapid progress in research into the clinical and urodynamic benefits of BTX-A for NDO secondary to SCI (21). Although the follow-up period was relatively short, our trial also reported an mean reduction of 28.78 cm H2O in maximum detrusor pressure (PdetmaxDO-DESD) and a mean increase of 54.56 ml in VDO-DESD. The explanations for the significant decrease in maximum detrusor pressure were as follows. Firstly, we chose to use BTX-A trigonal injections, which have been shown to be more effective than those excluding the trigone for patients with respect to NDO with incontinence. In 2008, Mascarenhas et al. (22) reported that, in 21 patients with NDO who underwent a detrusor injection of 300 units of BTX-A including the trigone, the maximum detrusor pressure (41.8%) had decreased significantly from baseline by 8 weeks after the injection. Similar results were observed by Manecksha et al. (23) and Abdel-Meguid (24). Secondarily, the MUCP at baseline (111.53 cm H2O) in the present trial was higher than in other published studies. This suggests that high bladder pressure in SCI patients with DO and DESD is probably connected not only to involuntary detrusor contractions, but also to spontaneous obstruction of the outlet. In 1988, Dykstra et al. (25) firstly adopted 100 U BTX-A urethral sphincter injection as the treatment for DESD in patients with SCI, and finally concluded that it reduced both the urethral and intravesical pressures. In 2005, Schulte-Baukloh et al. (26) also reported a significant reduction in maximal detrusor pressure for patients with overactive bladder (OAB) symptoms after injection of 200–300 U of BTX-A (Botox) into the detrusor muscle and external sphincter. In addition, 86% of patients in their study chose this procedure for their bladder condition again. A group of 10 children who had non-neurogenic or neurogenic bladder was treated with same procedure, as reported by Mokhless et al. (27). According to their trial, 100 IU BTX-A were administered into the external sphincter at the 3, 6 and 9 o’clock positions. At 2 weeks, 4 weeks, 3 months and 6 months, maximum detrusor pressure decreased from 66.3 mm H2O to 37.17 mm H2O, 41.67 mm H2O, 42.5 mm H2O and 43.5 H2O, respectively, with a 37% decrease at 6 months. Our findings, of a significant reduction in MUCP and duration of first DO and DSD (all p-value < 0.05), supported this result.
Combined detrusor and urethral external sphincter BTX-A injections also improved the quality of life of these patients, which is another important point in the treatment of neurogenic lower urinary tract dysfunction (4). The outcomes of I-QoL increased significantly between pre- and post-injection at the 3-month follow-up. The reasons we propose for this were that: (i) according to our trial, the patients showed greater improvement in I-QoL, voiding volume and UI episodes than those at baseline; (ii) 25 patients developed complete dryness, with very high I-QoL; and (iii) most importantly, 11 of 18 patients presented no symptoms of autonomic dysreflexia (AD), which is a life-threatening challenge for patients with SCI. Elkelini et al. (28) reported that intravesical BTX-A significantly blocked the dysreflexia response (high arterial pressure with bradycardia) induced by cystometrogram and significantly lowered nerve growth factor (NGF) concentrations in the bladder and the T4 spinal cord transaction in female rats. Kuo (12) also reported that, of the 7 patients with AD, 5 (71%) experienced significant decreases in their symptoms after BTX-A injection.
In our study, the relatively high cost of treatment, the requirement for repeated injection and CIC were challenging, but not insurmountable, obstacles. Limitations of this study included the lack of randomization and lack of a control group.
In conclusion, the current study demonstrates that BTX-A injection in the detrusor and external urethral sphincter is an effective treatment with respect to protection of the upper urinary tract and improvement in quality of life for patients with DO and DESD secondary to SCI.
ACKNOWLEDGEMENTS
This study was supported by Medical Scientific Research Foundation of Guangdong Province, China (grant number A2013477, A2015251, A2016433), National Science & Technology Pillar Program (2013BAI10B02), and Traditional Chinese Medicine of Guangdong Province, China (grant number A20152037). The authors thank Cliff S. Klein for providing assistance with comments on revising the manuscript.
The authors have no conflicts of interest to declare.
REFERENCES
