Séléna Lauzière, PhD1,2, Carole Miéville, MSc1,2, Martina Betschart, MSc1,2, Cyril Duclos, PhD1,2, Rachid Aissaoui, PhD1,2,3 and Sylvie Nadeau, PhD1,2
From the 1Centre de recherche interdisciplinaire en réadaptation (CRIR), Institut de réadaptation Gingras-Lindsay de Montréal (IRGLM), 2École de réadaptation, Université de Montréal and 3Centre de Recherche du Centre Hospitalier Universitaire de Montréal (CRCHUM), Quebec, Canada
OBJECTIVE: To determine if the level of effort in paretic plantar flexors during gait could be a factor in explaining locomotor asymmetry.
DESIGN: Cross-sectional study.
SUBJECTS: Twenty individuals with chronic stroke (mean age 49.4 years (standard deviation 13.2).
METHODS: Participants walked on a split-belt treadmill for 3 periods: baseline at self-selected speed; adaptation with the belt speed doubled on the non-paretic side; and post-adaptation at self-selected speed. Kinematic and kinetic data were recorded. The efforts were estimated with the muscular utilization ratio. Pearson correlation coefficients were used to assess the relationships between the paretic plantar flexor level of effort at baseline and changes in spatiotemporal gait parameters and joint moments after split-belt treadmill walking. In addition, in a subgroup of 12 asymmetrical individuals, paretic plantar flexor efforts were compared between periods (baseline (asymmetrical) and post-adaptation (symmetrical)) with paired Student’s t-tests.
RESULTS: Baseline level of effort in plantar flexors was negatively related to changes in paretic plantar flexion moments (r = –0.70; p = 0.001) and changes in non-paretic step length (r = –0.65; p = 0.003). A more symmetrical spatiotemporal gait increased the paretic plantar flexor effort from 73.7% to 86.6% (p = 0.007).
CONCLUSION: A more symmetrical gait increases paretic plantar flexor efforts. Individuals post-stroke presenting high plantar flexor efforts when walking have limited muscle capacity to increase non-paretic step after split-belt walking.
Key words: stroke; locomotor activity; kinetics.
J Rehabil Med 2016; 48: 576–582
Correspondence address: Sylvie Nadeau, School of Rehabilitation, Faculty of Medicine, Université de Montréal, PO Box 6128, Station Centre-ville, Montreal, QC, H3C 3J7 Canada. E-mail: sylvie.nadeau@umontreal.ca
Accepted Apr 22, 2016; Epub ahead of print Jun 20, 2016
INTRODUCTION
Spatiotemporal asymmetry is frequently reported in individuals following a stroke (1, 2). Whereas many present a longer paretic swing time compared with the non-paretic side, the direction of the double support time (DST) and step length (SL) asymmetry varies (2). Asymmetry is also observed in kinetic parameters where values are decreased on the paretic side in comparison with the non-paretic side. In particular, asymmetry in paretic plantar flexion joint moment and power that contributes to lower limb energy generation and forward propulsion of the body during gait (3–6) is expected to be related to spatiotemporal gait asymmetry. Indeed, recent evidence suggests plantar flexor (PF) muscles play a role in SL asymmetry (7–9). For example, Allen et al. (8) showed that the decrease in paretic plantar flexion moment impulse at late single stance in individuals with shorter non-paretic SL is greater than in individuals with longer non-paretic SL or with symmetrical SL (8). One explanation for this observation is that a reduction in paretic propulsion at late single stance could prevent the trunk from moving forward during the non-paretic swing, thus resulting in a shorter non-paretic step (10). The DST asymmetry is still not clearly explained in the literature.
With a split-belt treadmill protocol, it is possible to specifically modify the asymmetry of some gait parameters (11). For example, walking with the leg having a shorter SL (or shorter DST) on the fast belt during an adaptation period of 5–10 min leads to an after-effect characterized by an improvement in SL symmetry (or DST symmetry) when both belts are returned to the same speed (11). Furthermore, the results of a recent study with 20 individuals post-stroke showed that walking with the paretic leg on the slow belt (independently of the initial spatiotemporal gait asymmetry) increased the paretic plantar flexion moment in post-adaptation, and that this change was significantly related to the increase in the non-paretic SL (12). More precisely, the after-effects in SL or DST symmetry might be the consequence of different combinations of bilateral modifications (increased non-paretic step, decreased paretic step, or both). Furthermore, it was found that the amplitude of the after-effect observed in paretic plantar flexion net joint moment in the post-adaptation period also varied substantially among individuals post-stroke (12). One hypothesis that could explain this variation is that some individuals do not have the muscular capacity to increase their plantar flexion moment to increase the non-paretic SL or to reduce the paretic DST, since they already fully use their residual muscular capacity during comfortable gait. Consequently, these individuals could adopt a more spatiotemporal symmetrical gait pattern by reducing the paretic SL or increasing the non-paretic DST. In this case, the resulting improvement in spatiotemporal symmetry could ultimately lead to a reduced gait speed, which is probably not the preferred strategy of these individuals in everyday life. Since previous studies also suggested an important role of hip flexor (HF) muscles in SL asymmetry (8), this muscle group might compensate and help increase the non-paretic SL (by an increase in the non-paretic hip flexion moment) or reduce paretic DST (by an increase in the paretic hip flexion moment).
Findings by Lauzière et al. (12) concluded that, as a group, individuals with stroke having a shorter non-paretic step have the capacity to adopt a more symmetrical spatiotemporal gait pattern by increasing their paretic plantar flexion moment. Therefore, it is relevant to ask why these individuals do not walk more symmetrically spontaneously. One possible explanation is that a more symmetrical gait could induce too high a level of effort in the paretic PF to be sustained for an extended period of time. Insight into the level of effort during gait can be obtained through the use of the muscular utilization ratio (MUR) mechanical model, which provides an index of the percentage of maximal strength produced by a muscle group during gait (see Methods for details) (13).
In order to determine whether a more symmetrical gait pattern post-stroke is limited by an excessive PF level of effort (MUR) on the paretic side, we assessed whether PF MUR at baseline predicted the changes in spatiotemporal gait parameters and moments after split-belt treadmill walking. We expected a negative relationship between paretic PF MUR and changes in non-paretic SL, paretic DST and paretic plantar flexion moment and no relationship with changes in SL and DST asymmetry. We also hypothesized finding a positive relationship between PF MUR at baseline and the changes in bilateral hip flexion moment. Finally, we hypothesized that a subgroup of individuals post-stroke, presenting SL asymmetry (shorter non-paretic SL) or temporal asymmetry (longer paretic DST), would probably increase their paretic PF MUR and the bilateral HF MUR to walk more symmetrically after split-belt walking.
METHODS
Participants
Twenty individuals with stroke (14 with left hemiparesis, 7 women) participated in this study. The inclusion criteria were: (i) unilateral stroke for more than 6 months, (ii) capable of walking 10 m independently without gait aids (cane, walker or walking stick), and (iii) no severe cognitive or cardiovascular impairments that could affect their gait. Individuals with other neurological conditions or more than one stroke event were excluded. The ethics committee approved the experiment and participants provided written informed consent before the evaluation sessions.
Clinical assessment
Participants answered a general health questionnaire about medical conditions. The cognitive state of patients was assessed by the Folstein Mini-Mental State Examination (14). Sensation and proprioception at the ankle were, respectively, measured using Calibrated Semmes-Weinstein monofilaments for touch-pressure sensation (15), and an “up or down” segment position of the foot and hallux. The muscle tone and motor recovery of the leg and foot were assessed with the Composite Spasticity Index (16) and the Chedoke McMaster Stroke Assessment (CMSA) (17). Self-selected and maximal walking speeds were quantified by the 10-Meter Walk Test (18) and balance was assessed with the Berg Balance Scale (19).
Dynamometric assessment
In a first session, maximal voluntary contraction (MVC) in concentric mode was measured on both sides in plantar flexion (in sitting position) and hip flexion (in supine position) with a Biodex dynanometer system (Biodex Medical Systems, New York, USA) at different angular velocities (30°/s and 180°/s for PF and 30°/s, 90°/s and 120°/s for HF) to match those measured during gait (20, 21). All details of this assessment are described in a previous article (21). Two MVC with less than 10% difference were averaged in order to determine the maximal isokinetic strength at each degree in Newton-meters (Nm). This dynamometric assessment served as an estimate of the maximal potential moment required to calculate the MUR values.
Gait assessment
Instrumentation. Kinetic data were recorded with force plates embedded in a Bertec’s Fully Instrumented Split-Belt Treadmill (Bertec Corp., Colombus, OH, USA). These force plates record 3 orthogonal ground reaction forces (Fx, Fy, and Fz) and moments (Mx, My, and Mz) at a 600 Hertz (Hz) frequency. A fourth-order Butterworth zero-lag filter with a cut-off frequency of 10 Hz was used to filter the force data and these data were re-sampled at 60 Hz. An Optotrak Certus® Motion Capture System (Northon Digital (NDI), Waterloo, ON, Canada) was used to measure kinematic data by recording three-dimensional coordinates of 75 infrared markers placed bilaterally on the head, trunk, pelvis, upper limbs, and lower limbs (at 30 Hz). These data were filtered with a fourth-order Butterworth zero-lag filter with a cut-off frequency of 6 Hz and re-sampled at 60 Hz.
Locomotor tasks. The locomotor tasks were described in a previous study (12). Briefly, participants performed 3 walking periods: (i) baseline with both belts running at a self-selected speed for 3 min, (ii) adaptation with 1 belt speed set at twice the speed of the other (2:1 ratio) for 6 min, and (iii) post-adaptation with both belts running at a self-selected speed for 3 min. In the present study, the paretic leg was placed on the slow belt (self-selected gait speed) and the non-paretic leg on the fast belt during the adaptation period. During all walking periods, a harness was used for safety reasons without providing any weight support. Two handrails could be used to restore balance when required. However, participants were asked to look straight ahead and use the handrails only if they experienced a loss of balance. No participants held the handrail during the recording periods. Rest periods were allowed between gait periods and participants were instructed to rest in a sitting position until they were no longer fatigued.
Data analysis
Calculation of muscular utilization ratio. MUR was used to estimate the level of effort of a muscle group during concentric action in gait as described in previous studies (13, 22). An exhaustive description of the calculation of the MUR was presented in these aforementioned studies. The MUR is calculated during the phase of energy generation of each muscle group by the following equation: [walking moment/maximal potential moment × 100]. The walking moment (MUR numerator) is estimated during gait by an inverse dynamic analysis. The maximal potential moment (MUR denominator) is estimated in 2 steps. First, a regression equation (r2 ≥ 0.90) is derived from joint angle, angular velocity, and torque data arising from the MVC of each muscle group assessed in concentric mode at various velocities. Secondly, the maximal potential moment is obtained at a given time during the gait cycle by entering into the equation the velocity and the joint angle measured during gait. The peak MUR during the concentric action (push-off (A2) and pull-off (H3) phases for PF and HF, respectively) and the corresponding joint moments at peak MUR were retained for analysis. Data for one subject wearing a rigid ankle orthosis during walking was excluded for the analysis with the peak PF MUR, and for the peak HF MUR. One subject unable to produce a constant valid MVC in hip flexion was also excluded. Therefore, data analysis on peak MUR was performed with 19 values (instead of 20) for each muscle group.
Gait analysis
Biomechanical data (kinetic and kinematic) were collected for 30 s at baseline (from 90 s to 120 s) and post-adaptation periods (from 0 s to 30 s). Based on the vertical ground reaction forces, the Teager-Kaiser energy operator method was used to determine gait cycles, which were then normalized to 100% (23). Walking moments at the ankle and hip joints during gait were estimated by an inverse dynamic approach that used kinematic and kinetic data (see 12 for details). Customized software created by NI Labview synchronized the data between the Optotrak system and the split-belt treadmill. Step length was defined as the anterior-posterior distance between the heels of each foot at initial contact of the leading heel (24). Non-paretic SL corresponds to the SL measured at the non-paretic heel strike. Non-paretic DST corresponds to the DS phase ending at the non-paretic toe-off. Double support time was normalized to the duration of the gait cycle. For statistical analyses, SL and DST asymmetries were expressed as a ratio of the paretic side divided by the non-paretic side (paretic/non-paretic). A ratio of 1 corresponds to perfect symmetry. However, to classify individuals with stroke according to the direction of their spatiotemporal asymmetries in Table I, a ratio of the higher value to the lower value was computed. Participants were qualified as asymmetrical if their symmetry ratio was greater than 1.08 and 1.04, respectively, for SL and DST (see Table I) (2). To quantify the effect of a more symmetrical gait pattern on peak MUR, individuals with asymmetry ratios greater than these thresholds (paretic longer) formed a subgroup that was retained in the analysis. Finally, the amplitude of the after-effect was expressed as the percentage of change in gait parameters (paretic plantar flexion moment, paretic and non-paretic hip flexion moment, non-paretic SL and paretic DS time) for each leg between the baseline and the post-adaptation periods and was quantified with the following equation: (post-adaptation value – baseline value)/baseline value)*100.
|
Table I. Gait parameters at baseline and at post-adaptation periods (n = 20) |
|||||||
|
|
NP Mean (SD) |
P Mean (SD) |
Ratio (P/NP) Mean (SD) |
Ratio (High/Low) Mean (SD) |
NP High n |
SYM n |
P High n |
|
Baseline |
|
|
|
|
|
|
|
|
DS time, % |
16.4 (2.6) |
18.8 (2.6) |
1.17 (0.24) |
1.21 (0.21) |
3 |
5 |
12 |
|
Step length, m |
0.43 (0.10) |
0.45 (0.08) |
1.07 (0.18) |
1.13 (0.14) |
5 |
8 |
7 |
|
PF moments, Nm/kg |
1.18 (0.18) |
0.84 (0.26) |
0.70 (0.16) |
|
|
|
|
|
Post-adaptation |
|
|
|
|
|
|
|
|
DS time, % |
18.3 (2.50) |
17.1 (2.09) |
1.08 (0.19) |
|
|
|
|
|
Step length, m |
0.46 (0.10) |
0.40 (0.09) |
1.18 (0.20) |
|
|
|
|
|
PF moments, Nm/kg |
1.09 (0.22) |
0.97 (0.18) |
0.90 (0.18) |
|
|
|
|
|
NP: non-paretic; P: paretic; SD: standard deviation; m: metres; SYM: symmetrical; PF: plantar flexors. In the final 3 columns, individuals are classified as asymmetrical if ratio (high/low) was greater than 1.08 and 1.04, respectively, for step length and double support time (2). |
|||||||
Statistical analysis
Kolmogorov-Smirnov was used to assess the normality of the distribution of the variables and all variables were normally distributed. Relationships between PF MUR at baseline and changes in other gait parameters were assessed with Pearson correlations. Step length asymmetry ratios, DST asymmetry ratios, and bilateral peak MUR of PF and HF were compared between periods (baseline and post-adaptation) with paired Student t-tests. All statistical analyses were performed with the SPSS Version 20 software and the level of significance was set at 0.05.
RESULTS
Clinical evaluation
The clinical status of participants was described in a previous study (12). Briefly, the mean values and standard deviation (SD) of age, body mass, and height were 49.4 years (SD 13.2), 79.5 kg (SD 15.2), and 1.71 m (SD 0.07). The median and range values for Berg Balance Scale, CMSA of the leg, foot and ankle muscle tone were, respectively, 55.5 (44–56), 6 (3–7), 4.5 (1–7) and 6 (2–10). The mean self-selected and maximal treadmill gait speeds were, respectively, 0.63 ± 0.14 and 0.92 m/s (SD 0.25). Table I presents spatiotemporal gait parameters of individuals post-stroke during the baseline period.
After-effects in asymmetry ratios of gait parameters
Significant differences were found between baseline and post-adaptation periods for the asymmetry ratios of SL, DS time, and PF moment (p < 0.001). No differences between periods were found for asymmetry ratios of HF moment (p = 0.58). Table I presents SL, DST and PF moment symmetry ratios in baseline and post-adaptation periods.
Relationships between plantar flexor muscular utilization ratio at baseline and changes in spatiotemporal asymmetries
No significant association was found between PF MUR at baseline and changes in asymmetry ratio of SL ( = –0.30; p > 0.05; Fig. 1A). However, when one individual was removed from the analysis (data marked with circle in Fig. 1A), this relationship reached a level of significance (r = –0.51; p = 0.03). No significant association was found between PF MUR at baseline and changes in ratios of DST (r = 0.02; p = 0.94; Fig. 1B).
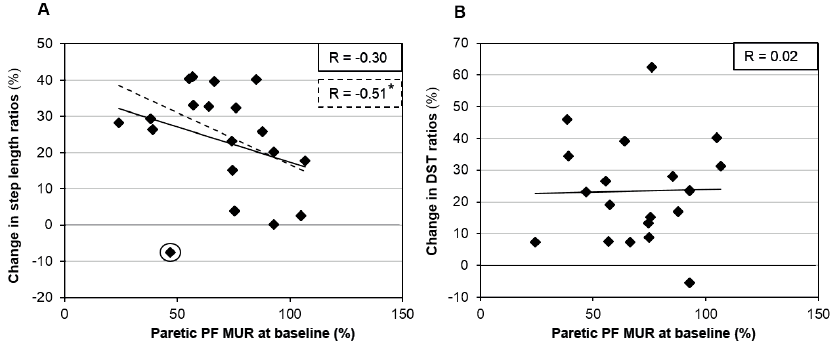
Relationships between plantar flexor muscular utilization ratio at baseline and changes in gait parameters
PF MUR at baseline was significantly related to changes in paretic PF moments (r = –0.70; p = 0.001; Fig. 2A) and changes in non-paretic SL (r = –0.65; p = 0.003; Fig. 2B). When one individual (the same previously removed in Fig. 1A) was removed from the analysis (data marked with circle in Fig. 2B), this relationship increased (r = –0.80; p < 0.001). No significant association was found between PF MUR at baseline and changes in paretic DST (r = 0.13; p = 0.61) and in paretic and non-paretic HF moments (r ≤ 0.33; p > 0.05).
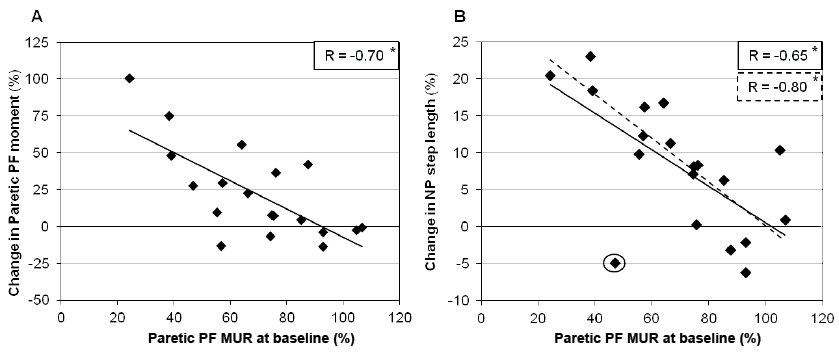
Effect of step length or double support time symmetry on peak muscular utilization ratio
A total of 12 individuals post-stroke presented spatial (SL asymmetry ratios >1.08) or temporal asymmetries (DST asymmetry ratios >1.04) that could be reduced by placing the paretic leg on the slow belt. Among them, 7 presented a shorter non-paretic SL and 11 presented a shorter non-paretic DST. The mean (and SD) step length symmetry ratios (P/NP) during baseline and post-adaptation periods were, respectively, 1.15 (SD 0.19) and 0.93 (SD 0.18) (p < 0.001; Fig. 3A). DST symmetry ratios (P/NP) during baseline and post-adaptation periods were, respectively, 1.30 (SD 0.22) and 1.04 (SD 0.12) (p < 0.001; Fig. 3B). Comparisons between baseline and post-adaptation periods for paretic PF MUR values showed that the paretic PF MUR value increased significantly from 73.7% (SD 25.3) to 86.6% (SD 19.12) (p = 0.007; Fig. 3C). No effect between periods was found for non-paretic PF MUR and bilateral HF MUR (p ≥ 0.25; Fig. 3C, D).
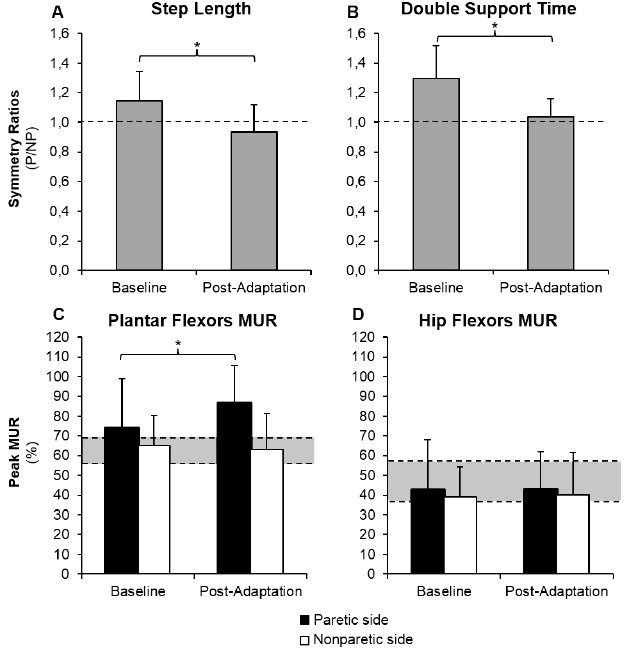
Fig. 3. Comparison between baseline and post-adaptation periods for symmetry ratios of (A) step length, (B) symmetry ratios of double support time, (C) peak muscular utilization ratio (MUR) of paretic and non-paretic plantar flexors, and (D) peak MUR of paretic and non-paretic hip flexors in a subgroup of 12 asymmetrical individuals. *Significant difference between conditions (p < 0.05). Dotted line: perfect symmetry in (A) and (B). Grey area: ( C) and (D) represents the 95% confidence interval of peak MUR values of 10 healthy individuals of the same age group during self-selected treadmill gait speed.

DISCUSSION
By quantifying levels of effort using the MUR method, the principal objective of this study was to determine whether PF weakness could be among the factors preventing individuals post-stroke from adopting a more symmetrical gait pattern. As hypothesized, we found that paretic PF MUR during comfortable gait speed was negatively related to the increase in the paretic PF moment and non-paretic SL following walking on a split-belt treadmill with the paretic side on the slow belt. Results also showed that, in individuals with asymmetrical gait pattern (shorter non-paretic SL or non-paretic DST), a more symmetrical gait significantly increased the paretic PF MUR. These combined results suggest that plantar flexor weakness (high MUR) could be among the factors preventing the adoption of a more symmetrical gait pattern in individuals with shorter non-paretic step.
After-effects in the symmetry ratios of step length, double support time, and plantar flexor moments
The after-effects in SL and DST asymmetry ratios observed in the present study are consistent with those previously found in other studies using this type of split-belt treadmill protocol (11, 25). Indeed, SL and DST of the leg that was on the fast belt during the adaptation period increased in the post-adaptation period. For joint moments, the results of the present study showed that asymmetry in PF moments at peak MUR were reduced in post-adaptation period, whereas no changes were observed for HF moments. In a previous study, we also showed that the PF moment of the leg on the slow belt increased and the PF moment of the leg on the fast belt decreased following walking on a split-belt treadmill in healthy individuals and in the participants post-stroke with non-paretic (dominant) leg on the slow belt (12).
Relationships between plantar flexor muscular utilization ratio and changes in gait parameters
The fact that paretic PF peak MUR was only slightly related to changes in asymmetry ratios of SL (and not at all to changes in DST ratios) is not surprising. Indeed, since it is possible to modify gait asymmetry by reducing the paretic step or increasing the paretic DST, the weakness of the paretic side is probably not a factor that will impair these strategies. These results showed that level of paretic PF MUR did not significantly affect the capacity to modify the symmetry of the gait pattern during split-belt treadmill walking. However, as previously noted in the introduction, the resulting changes in asymmetry are the consequence of different combinations of changes in each leg. Thus, the negative relationship between paretic PF MUR at baseline and after-effects in PF moments support our hypothesis that individuals walking with a high level of effort will show less increase in their paretic PF moment following walking on the split-belt treadmill with the paretic leg on the slow belt. Furthermore, a high level of association (negative relationship) was found between PF peak MUR and the increase in non-paretic SL. These combined results suggest that PF weakness (high peak MUR) could limit the capacity to increase paretic PF moment and subsequently limit the increase in the non-paretic SL. These results are important because to preserve gait speed, SL asymmetry must optimally be restored by increasing the shorter step rather than decreasing the longer step. For the same reason, the optimal way to restore DST asymmetry would be to decrease the longer DST. Our hypothesis stipulating that individuals with high MUR in the paretic PF would show incapacity to decrease the paretic DST was not supported since no significant association was found between PF MUR and the decrease in paretic DST. As previously indicated, it is possible that modification in DST following walking on the split-belt treadmill depends mostly on the timing (inter-limb coordination) rather than the level of muscular activity, and therefore high paretic MUR does not limit the decrease in paretic DST.
Muscular exigencies of a more symmetrical gait pattern
Among all participants, 12 individuals presented SL asymmetry (shorter non-paretic SL) or a DST asymmetry (shorter non-paretic DST), which was reduced by placing the paretic leg on the slow belt during the adaptation period. These individuals were analysed to verify whether a more symmetrical gait pattern would increase the paretic PF MUR. Following the adaptation period on the split-belt treadmill, both SL and DST were more symmetrical. This observation showed that these individuals are able to produce a more symmetrical gait pattern regarding spatiotemporal parameters. However, in the post-adaptation period, the paretic PF MUR was high (86.6%). For example, a previous study showed that the mean peak MUR during maximal gait speed in individuals post-stroke is approximately 85.9% (26). In comparison, the PF MUR during comfortable treadmill gait in healthy individuals is 62.6% (27) and during comfortable over-ground gait is 60.8% (13, 22). Thus, to conserve gait speed without requiring too high an effort in paretic PF, it can be suggested that these individuals preferred to reduce the utilization of the paretic PF, which then led to an asymmetrical gait pattern. Individuals post-stroke not included in the subgroup (because they presented a symmetrical gait pattern or shorter paretic SL or DST at baseline) also showed an increase in non-paretic step length, plantar flexion moment and PF MUR following walking on the split-belt treadmill with the non-paretic leg on the fast belt. However, data from these individuals were not presented in the actual study, since these individuals presented a more asymmetrical gait pattern in the post-adaptation period. Therefore, it was not the interest of the present study to describe the effort of a more asymmetrical gait pattern.
Role of hip flexors in step length changes
The regression analyses did not reveal significant association between change in hip flexion moment and change in SL. Furthermore, no association was found between paretic PF MUR at baseline and changes in hip flexion moment. Finally, in individuals post-stroke with asymmetrical gait pattern at baseline, the level of effort in bilateral hip flexors was not higher during the post-adaptation period. These results suggest that these individuals do not systematically use HF muscles to compensate for the weakness of the PF muscles in order to increase non-paretic SL. This result is also in accordance with a previous study showing that hip flexion moment was not modified following walking on the split-belt treadmill with the non-paretic leg on the fast belt (12).
Other factors that could explain spatiotemporal asymmetries
The results of the present study suggest that plantar flexor weakness (high paretic PF MUR) is one of the factors that could impair the ability to increase non-paretic SL in individuals post-stroke. However, some individuals in the present study walking with low MUR in paretic PF during gait also showed PF moment and spatiotemporal gait asymmetries. It is possible that PF moment asymmetry in some individuals was caused by other factors than weakness that prevented individuals from using their residual strength, such as impaired coordination, balance, proprioception, spasticity or range of motion at knee or ankle. Indeed, studies showed significant relationships between spatiotemporal asymmetry and many of these clinical characteristics (28–31). Furthermore, future studies are needed to explore other factors that could explain the heterogeneity of responses following walking on the split-belt treadmill as shown by the outlier in Fig. 1. Indeed, despite his low paretic PF MUR, this participant did not modify his asymmetry in the same direction as the other participants.
Study limitations
The principal limitation of the present study is the restricted number of individuals presenting SL asymmetry with shorter non-paretic SL. Since no significant relationship was found between initial SL asymmetry and change in SL (r ≤ 0.34; p ≥ 0.144), the heterogeneity of their asymmetry probably did not affect the relationship between PF MUR and the capacity to increase non-paretic SL. However, we cannot exclude the fact that a larger number of participants with a wider range of asymmetry would make it more likely to find a relationship between initial SL asymmetry and change in paretic and non-paretic SL. Future studies are needed with a larger cohort to assess the proportion of individuals post-stroke limited by their weaknesses. Furthermore, in the post-adaptation period, some individuals “over adapted” their initial SL asymmetry, which then resulted in an SL asymmetry in the other direction (longer non-paretic step). There is a need to identify patient characteristics and protocol that will better correct the level of asymmetry in post-adaptation period. Finally, other biomechanical parameters that might influence SL and DST, such as dorsiflexors, knee extensors and joint power, should be considered in future studies to explain modifications in spatiotemporal parameters.
ACKNOWLEDGEMENTS
Séléna Lauzière was supported by a Vanier Canada Graduate PhD scholarship from the Canadian Institutes of Health Research (CIHR). Carole Miéville is supported by PhD scholarships from the Fonds de recherche du Québec–Santé (FRQS) and the SensoriMotor Rehabilitation Research Team (SMRRT). Martina Betschart receives funding through a PhD scholarship from the SMRRT. This project was funded by the Ordre professionnel de la physiothérapie du Québec (OPPQ) and the Réseau provincial de recherche en adaptation-réadaptation (REPAR). The equipment and material required for the research conducted at the Pathokinesiology Laboratory was financed by the Canada Foundation for Innovation (CFI). We wish to thank Philippe Gourdou, Michel Goyette, Youssef El Khamlichi and Daniel Marineau for their technical support.
The authors declare no conflicts of interest.
REFERENCES
