Anupam Datta Gupta, MD, FAFRM1 and Renuka Visvanathan, FRACP, PhD2
From the 1Department of Rehabilitation Medicine and 2Aged and Extended Care Services, Queen Elizabeth Hospital, Woodville South, South Australia
BACKGROUND: Six patients with Parkinson’s disease with deep brain stimulation who were experiencing disabling foot dystonia were referred to the spasticity clinic for a trial of botulinum toxin. The foot and ankle muscles were injected with onabotulinum toxin (Botox) to determine the effects on foot dystonia, pain and lower limb functional outcomes.
DESIGN: Case series.
SUBJECTS/PATIENTS: Six patients with Parkinson’s disease having deep brain stimulation experiencing disabling foot dystonia.
METHODS: Dystonic foot and ankle muscles were identified and injected with 250–400 units botulinum toxin and re-coded pre- and 3 weeks post-injection with the Burke Fahn Marsden Dystonia score, visual analogue score of pain, Unified Parkinson’s Disease Rating Scale (UPDRS) – lower limb score, Timed up and Go test (TUG), 6-Minute Walk Test (6MWT), gait velocity, cadence in an instrumented walkway, and Goal Attainment Scale (GAS).
RESULTS: Three weeks after botulinum toxin injection, significant improvements were noted in dystonia, pain, UPDRS, 6MWT, gait velocity, and cadence. Five out of 6 patients improved on the TUG test. Patients also reported improvements in their GAS goals.
CONCLUSION: Botox injection significantly improved foot dystonia, pain and lower limb functional outcomes in patients with Parkinson’s disease with deep brain stimulation.
Key words: foot dystonia; Parkinson’s disease; deep brain stimulation.
J Rehabil Med 2016; 48: 00–00
Correspondence address: Anupam Datta Gupta, Department of Rehabilitation Medicine, Queen Elizabeth Hospital, 28 Woodville Road, Woodville South, 5011, South Australia. E-mail: adattagupta86@gmail.com
Accepted Mar 8, 2016; Epub ahead of print Apr 28, 2016
INTRODUCTION
Foot dystonia (FD) is described as involuntary, repetitive and twisting muscle contraction leading to abnormal posturing of the foot. FD can be part of generalized dystonia or, in isolation, a focal dystonia involving only the foot and ankle.
FD can occur in idiopathic Parkinson’s disease (PD) (1). Deep brain stimulation (DBS) is now an important adjunctive treatment for control of debilitating tremor, rigidity and bradykinesia in PD (2). Dystonia has been reported to be a problem even in some surgically treated PD patients, but the true incidence of dystonia in DBS-treated patients is unknown (3). FD, when present, may not only be painful, but also causes difficulty in wearing shoes, standing and walking. Six patients with PD having DBS were referred to the spasticity clinic with disabling foot dystonia for consideration of treatment with botulinum toxin (Botox, Allergan, Australia).
Botox injection is effective and safe to treat FD, such as striatal toe and remains the treatment of choice for limb dystonia (4, 5). There is, however, no published evidence to date on the effectiveness of Botox in the treatment of FD in PD patients treated with DBS.
The aim of this pilot study of 6 patients with PD having DBS experiencing FD is 2-fold:
• to describe the involved muscles;
• to investigate the effectiveness of Botox in reducing dystonia, pain and improving lower limb function.
METHODS
The ethics committee of Basil Hetzel Research Institute of the Queen Elizabeth Hospital, South Australia, approved the study as a quality assurance project. All subjects gave their informed consent.
Setting
The study was conducted at the Spasticity Clinic in the Department of Rehabilitation Medicine, at the Queen Elizabeth Hospital, South Australia. Patients were referred by neurologists from the major teaching hospitals in South Australia where DBS is performed.
Patients
Six patients with PD who developed significant FD, causing pain and difficulty walking despite DBS, were enrolled in this study between January and December 2014. A score of 3 or more on the Burke Fahn Marsden Dystonia (BFMD) rating scale (range 0–4) was defined as clinically significant dystonia.
Inclusion criteria. Age 30–75 years; bilateral or unilateral FD in patients with PD having DBS; no other associated organic or psychiatric disorder; no history of treatment with Botox.
Exclusion criteria. Atypical PD patients; PD patients without DBS; contraindication to Botox; FD due to other causes; significant memory impairment; and non-ambulatory patients.
Intervention
Dystonic muscles were identified from the history and repeated clinical observations. Dantec Clavis (Natus Neurology, Middleton, WI, USA) was used for EMG/stimulation guided injection in to the dystonic muscles. The involved muscles were injected with 250–400 units onabotulinum toxin, i.e. Botox.
Outcome measurements
Primary outcome. Dystonia measured by BFMD rating scale, a validated scale for quantification of severity of dystonia (6).
Secondary outcomes. Pain measured by visual analogue scale (VAS) 0–10 scale, Unified Parkinson’s Disease Rating Scale (UPDRS) – lower limb motor score, Timed Up and Go (TUG) test, 6-Minute Walk Test (6MWT), gait velocity and cadence and Goal Attainment Scale (GAS).
Assessments
Patients were assessed by a rehabilitation physician (ADG, an accredited botulinum toxin injector), a physical therapist and a nurse. Dystonic muscles were identified from the history of posturing of feet and from repeated direct observation during functional activities, such as standing and walking.
The following initial assessments were carried out and repeated, 3 weeks after the Botox injection was performed:
• FD measured by BFMD rating scale: a 4-point ordinal scale, from 0 = no dystonia present, 1 = slight dystonia, but not causing impairment; clinically insignificant, 2 = mild dystonia – walks briskly and unaided, 3 = moderate dystonia – severely impairs walking or requires assistance, 4 = severe – unable to stand or walk on involved leg.
• VAS- 0–10 scale, 0 being no pain and 10 intolerable pain; a score of 3 and above was considered clinically significant pain.
• UPDRS lower limb motor score – 5 items: Leg Agility, Arising from chair, Posture, Gait, Postural stability. Each can be scored from 0 to 4, thus the total score is 20. A higher score indicates more severe impairments.
• TUG test – the time taken for standing up from a seating position, walking a distance of 3 m then turning around, walking and sitting to the chair. A higher TUG score indicates increased risks of fall.
• The 6MWT was performed by asking the patients to walk for 6 min and the distance was recorded in metres (m).
• Gait velocity (in m/s) and cadence (steps per min) measured in an instrumented walkway (GAIT rite, Electronic Walkway, version 4, CIR Systems Inc, NY, USA).
• The GAS goals scoring –2: Failure to achieve expected goal; –1: partially achieved the expected goal; 0: achieved the expected goal; +1: achieved the expected goal plus a bit more; +2: way surpassed the expected goal.
Safety consideration
Adverse events during the study, such as weakness, bleeding and infection, were recorded.
RESULTS
Patients
The 6 patients had an age range of 46–68 years and the mean appearance of foot dystonia following DBS was 3 years. There were 3 male and 3 female patients (1:1 ratio).
Two patients had bilateral FD, and the others had unilateral FD. The muscles involved in causing FD were the extensor hallucis longus, extensor digitorum longus, flexor hallucis longus, flexor digitorum longus, tibialis posterior, gastrocnemius and soleus, flexor digitorum brevis and first dorsal interossei (Table I).
|
Table I. Patients’ demography, date of deep brain stimulation (DBS), appearance of foot dystonia and muscle characterization |
||||||
|
Patient |
Age, years |
Sex |
DBS |
Dystonia |
Muscles involved |
Botulinum toxin injected, units |
|
1 |
63 |
M |
2010 |
2011 |
Bilateral TP |
150, in each muscle |
|
2 |
66 |
M |
2007 |
2011 |
Right FDB |
100 |
|
|
|
|
|
|
Right TP |
150 |
|
|
|
|
|
|
Right 1st DI |
50 |
|
3 |
62 |
F |
2009 |
2010 |
Right FHL |
100 |
|
|
|
|
|
|
Right FDL |
100 |
|
|
|
|
|
|
Left EHL |
100 |
|
|
|
|
|
|
Left EDL |
100 |
|
4 |
46 |
M |
2008 |
2014 |
Left EHL |
100 |
|
|
|
|
|
|
Left FDL |
150 |
|
5 |
60 |
F |
2007 |
2010 |
Right FHL |
100 |
|
|
|
|
|
|
Right FDL |
150 |
|
6 |
68 |
F |
2010 |
2013 |
Left GS |
200 |
|
|
|
|
|
|
Left TP |
150 |
|
TP: tibialis posterior; FDB: flexor digitorum brevis; DI: dorsal interossei; FHL: flexor hallucis longus; FDL: flexor digitorum longus; EHL: extensor hallucis longus; EDL: extensor digitorum longus; GS: gastrocnemius and soleus; M: male; F: female. |
||||||
Primary outcome
Foot dystonia. Pre- and post-injection FD, measured with the BFMD scale, improved from a baseline score of 4 to 2, 3 to 1, 4 to 2, 4 to 2, 4 to 0, and 3 to 1 in the 6 patients, respectively.
Secondary outcomes
Pain. Pre- and post-injection reduction in pain score (VAS) in 6 patients were as follows: 4 to 0, 5 to 0, 5 to 1, 2 to 0, 7 to 2, and 2 to 0.
Lower limb functional measures
Improvements in lower limb UPDRS motor score was noted in all 6 patients, with the following scores: –11 to 7, 9 to 7, 11 to 4, 15 to 11, 14 to 10, and 12 to 4. The pre- and post-injection 6MWT were 461 to 486, 101 to 118, 465 to 472, 294 to 307, 364 to 382, and 275 to 331 m. Gait velocity increased, respectively, from 127.3 to 128.9, 73 to 104.5, 79.2 to 119.6, 78.1 to 94.7, 48.1 to 54, and 72.6 to 76.9 cm/s. The pre- and post-injection cadence (steps/min) were as follows: 122.3 to 126.5, 82.2 to 90.6, 94.8 to 120.6, 97.5 to 107, 85.5 to 96.4, and 110.3 to 114.1 steps/min. TUG values were 9.7 to 7.8. 17 to 12.5, 8.7 to 7.8, 14 to 12, 11 to 10 and 13 to 14 s, respectively (Table II). All patients also noted improvement in their GAS goals (Table III). Patients did not report any adverse events.
|
Table II. Pre- and post-injection outcomes: Fahn-Marsden-Dystonia rating scale (FMD), pain by visual analogue scale (VAS), Unified Parkinson’s Disease Rating Scale (UPDRS)-lower limb scores, Timed Up and Go (TUG) test, 6-Minute Walk Test (6MWT), gait velocity (GV) and cadence |
||||||||||||||||||||
|
Patients |
FMD |
|
VAS |
|
UPDRS |
|
TUG |
|
6MWT |
|
GV |
|
Cadence |
|||||||
|
Pre- 0–4 |
Post- 0–4 |
|
Pre- 0–10 |
Post- 0–10 |
|
Pre- Lower limb -20 |
Post- Lower limb -20 |
|
Pre- s |
Post- s |
|
Pre- m |
Post- m |
|
Pre- cm/s |
Post- cm/s |
|
Pre- Steps/min |
Post- Steps/min |
|
|
1 |
4 |
2 |
|
4 |
0 |
|
11 |
7 |
|
9.7 |
7.8 |
|
461 |
486 |
|
127.3 |
128.9 |
|
122.3 |
126.5 |
|
2 |
3 |
1 |
|
5 |
0 |
|
9 |
7 |
|
17 |
12.5 |
|
101 |
118 |
|
73 |
104.5 |
|
82.2 |
90.6 |
|
3 |
4 |
2 |
|
5 |
1 |
|
11 |
4 |
|
8.7 |
7.8 |
|
465 |
472 |
|
79.2 |
119.6 |
|
94.8 |
120.6 |
|
4 |
4 |
2 |
|
2 |
0 |
|
15 |
11 |
|
14 |
12 |
|
294 |
307 |
|
78.1 |
94.7 |
|
97.5 |
107 |
|
5 |
4 |
0 |
|
7 |
2 |
|
14 |
10 |
|
11 |
10 |
|
364 |
382 |
|
48.1 |
54 |
|
85.5 |
96.4 |
|
6 |
3 |
1 |
|
2 |
0 |
|
12 |
4 |
|
13 |
14 |
|
275 |
331 |
|
72.6 |
76.9 |
|
110.3 |
114.1 |
|
Table III. Goal Attainment Scale (GAS) score: scoring interpretation: –2 = Failure to achieve expected goal, –1 = Partially achieved the expected goal, 0 = Achieved the expected goal, +1 = Achieved the expected goal plus a bit more, +2 = Way surpassed the expected goal |
|||
|
Patients |
GAS |
Pre- |
Post- |
|
1 |
Reduce pain |
–2 |
1 |
|
1 |
Improve quality |
–2 |
0 |
|
1 |
Improve distance/endurance |
–2 |
0 |
|
2 |
Reduce pain |
–1 |
0 |
|
2 |
Improve walk distance |
–1 |
0 |
|
2 |
Efforts in putting on shoes |
–1 |
0 |
|
3 |
Foot dystonia |
–1 |
1 |
|
3 |
Morning feet pain |
–1 |
0 |
|
4 |
Walking longer distance |
–1 |
–1 |
|
4 |
Walking quicker |
–1 |
2 |
|
5 |
Foot pain |
–1 |
0 |
|
5 |
Toe curling after walking |
–1 |
0 |
|
6 |
Reduce dystonia |
–1 |
0 |
|
6 |
Improve gait |
–1 |
–1 |
|
6 |
Assistance with mobility |
–1 |
–1 |
DISCUSSION
This study may be the first report on new onset intractable FD following DBS treatment in patients with PD. It also demonstrates effective treatment of FD with Botox injection into the dystonic foot and ankle muscles in a number of patients with PD who were otherwise successfully treated by DBS. In addition to a significant reduction in FD following Botox injection, we also noted improvement in pain and number of lower limb functional outcomes (7), such as UPRDS lower limb motor score, 6MWT, gait velocity, and cadence. The GAS goals reported by the patients also showed improvements. Five out of 6 patients improved with the TUG test and 1 patient did not show any improvement. This justifies doing the study with larger subjects. The patients did not receive any formal physiotherapy. This pilot study primarily examined the effects of botulinum toxin on foot dystonia. The duration of the effectiveness of Botox was approximately 5 months.
None of these patients had FD during the time of their diagnosis of PD. These patients continued experiencing FD even after optimization of their medication and DBS settings. FD caused significant pain, discomfort and lower limb functional impairments in these patients.
A number of abnormal foot posturing, caused by the dystonic muscles, were identified (Fig. 1). Extensor hallucis longus caused striatal big toe (hyperextension). Flexor hallucis longus and flexor digitorum longus caused clawing of the toes. The extensor digitorum longus caused extension of the 2nd, 3rd, 4th and 5th toes. Dystonic gastrocnemius and soleus muscle caused equinus and an equinovarus deformity (inversion and plantar flexion) when combined with dystonic tibialis posterior muscle during walking. Flexor digitorum brevis caused first metatarsophalangeal flexion and the first dorsal interossei causing big toe abduction. The most common muscles involved were tibialis posterior, extensor hallucis longus and flexor digitorum longus, respectively, causing equinovarus deformity, hyperextension of the big toe (striatal deformity) and clawing of the toes.
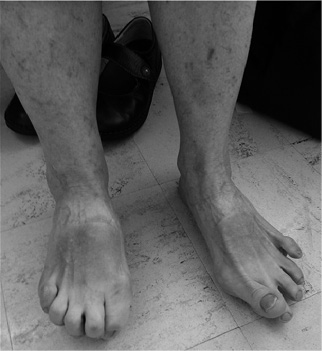
FD is reported in patients with untreated and advanced PD and in the chronic phase of levodopa therapy. Studies have also reported that patients with PD treated with DBS may experience a gradual decline in the control of axial symptoms, contributing to the development of postural instability and other complications, such as falls and fracture (8). Postoperative gait deterioration following DBS has been reported (9). Recent long-term studies of DBS show a gradual decline in the effectiveness of STN-DBS on gait and axial symptoms, such as posture over 3, 5, 8 or 10 years, in contrast to tremor, rigidity and bradykinesia with no specific mention of FD (10). FD following DBS in patients with PD is poorly recognized and therefore under-reported in the literature (3). The mechanism of FD following DBS is beyond the scope of this study and, from this small study, it is difficult to say whether foot dystonia is caused by DBS. This report shows that FD can occur and has significant functional impact on patients, if untreated. A prevalence study of this problem amongst these patients should be carried out.
The limitations of this study include the small sample size and the non-inclusion of a control arm. Investigators and patients were all aware of the treatment, and this might have introduced a bias.
In conclusion, this study highlights that, despite optimal DBS treatment, patients with PD can still experience debilitating FD. Treatment with Botox appears to be effective, but further confirmation through a randomized control trial is warranted.
ACKNOWLEDGEMENTS
Part of this study was published as a conference abstract in the Journal of Rehabilitation Medicine 2015; Suppl 54. (Abstracts – The 9th World Congress of International Society of Physical and Rehabilitation Medicine, June 19–23, 2015, Berlin, Germany).
The clinic is partly funded (towards the payment of the physiotherapist and the nursing staff) by Allergan Australia.
The authors would like to acknowledge the help of Associate Professor Thomas Kimber, consultant neurologist at Royal Adelaide Hospital for referring the patients to the Spasticity Clinic at Queen Elizabeth Hospital and the Spasticity Clinic team members – physiotherapist, occupational therapist and nursing staff.
The authors declare no conflicts of interest.
REFERENCES
