Andrea Greisberger, MSc1, Hanna Aviv, MSc2, Sven F. Garbade, PhD3 and
Gudrun Diermayr, PhD4,5
From the 1Department of Physical Therapy, University of Applied Sciences FH Campus Wien, Vienna, Austria,
2Clinic for Neurology and Neurological Rehabilitation Braunfels, BDH-Klinik, Braunfels, 3School of Psychology,
4School for Therapeutic Sciences, SRH Hochschule, Heidelberg, Germany and 5Department of Neurology,
Medical University Vienna, Vienna, Austria
OBJECTIVE: To evaluate the evidence for, and clinical relevance of, immediate and long-term effects of trunk restraint during reach-to-grasp training poststroke on movement patterns and functional abilities within the framework of the International Classification of Functioning, Disability and Health.
DATA SOURCES: PubMed, Web of Science, CINAHL, Embase, PEDro, Cochrane Library (publication dates January 1985 to March 2015).
STUDY SELECTION: Randomized controlled trials comparing training using trunk restraint with any other exercise training.
DATA EXTRACTION: Data were extracted by one researcher and checked by two other researchers. The Cochrane Collaboration’s tool for assessing risk of bias and the Physiotherapy Evidence Database scale were used by two researchers to assess study quality and risk of bias.
DATA SYNTHESIS: Eight studies met the inclusion criteria. Five studies found better recovery of movement patterns (trunk displacement, elbow extension, and/or shoulder flexion – body function/structure) at post-test in the experimental compared with the control groups. Functional abilities (activity/participation) improved more in the experimental groups in 3 studies at post-test. Long-term effects were found in one study after 4 weeks.
CONCLUSION: Trunk restraint has immediate and some long-term effects in adults with chronic stroke. However, these effects are not consistently clinically relevant when referring to minimal detectable change or minimal clinically important difference values.
Key words: stroke; rehabilitation; exercise therapy; upper extremity; outcome assessment.
J Rehabil Med 2016; 48: 00–00
Correspondence address: Gudrun Diermayr, School of Therapeutic Sciences, Maria-Probst Strasse 3, DE-69123 Heidelberg, Germany. E-mail: gudrun.diermayr@hochschule-heidelberg.de
Accepted Jan 27, 2016; Epub ahead of print Mar 22, 2016
Introduction
An essential aspect of arm function is the ability to reach and grasp for objects. Moving the hand to a target within arm’s length primarily involves elbow extension and shoulder flexion (1, 2). Cirstea & Levin (3) observed a significant correlation between decreased elbow extension and shoulder flexion and increased movement of the trunk poststroke. Therefore, Cirstea & Levin (3) hypothesized that restricting degrees of freedom in the trunk might enhance recovery of movement in the affected arm. Specifically, restraining the trunk during reaching exercises would force individuals to use the arm’s unexploited capacity. In addition, studies support the hypothesis that training with trunk restraint (TR) can help to improve functional abilities (4–6).
A recent meta-analysis by Wee et al. (7) (including 6 studies) showed that reach-to-grasp training with TR has a moderate significant effect on reduction of upper extremity impairment measured by the Fugl-Meyer Assessment/Upper Limb Section (FMA/ULS) and on improvements in shoulder flexion. Furthermore, a large, but not significant, effect for reduction in excessive trunk movement was shown. However, it has been suggested that the interpretation of observed change using statistical significance and effect sizes (ES) should be accompanied by an interpretation concentrating on detectable and important change in order to meet patients’ and clinicians’ needs (8, 9). This has not been done so far. Traditionally, detectable change is expressed with minimal detectable change values (MDC90 with 90% confidence interval (CI) or MDC95 with 95% CI). Important change is reported with minimal clinically important difference values (MCID) (9). These measures were suggested to quantify clinically relevant change (10).
Detectable change values are calculated based on test-retest methodology and indicate reliable change. Distribution- and anchor-based methods are used to calculate important change. Distribution-based methods are statistically-derived estimates and anchor-based methods refer to an external standard in order to indicate important change. This external standard can be patients’ or therapists’ ratings of subjectively perceived change. Triangulation of all methods is recommended in order to capture clinically relevant change (9, 10).
The immediate effects of a training with TR compared with the same training without TR have also been analysed in a systematic review by Pain et al. (11). In their analysis of 5 studies, they classified outcome measures according to the International Classification of Functioning, Disability and Health (ICF), a framework introduced by the World Health Organization (WHO): they concluded that TR has an immediate effect within the domain of body function/structure, whereas its immediate effects on activity/participation remain unclear. Neither of the published reviews (7, 11) evaluates the long-term effects of TR, although this would indicate motor learning and they do not analyse the results with MDC/MCID values, which is crucial for patients and clinicians alike. Furthermore, the comparison with usual care or neurodevelopmental treatment (NDT) has not been included in their analysis.
Therefore, this systematic review evaluates the scientific evidence for immediate and long-term effects of TR in reach-to-grasp training. Our main question, formulated according to the PICO (population, intervention, comparison, outcome) principle, is: “Does reach-to-grasp training while restraining compensatory trunk movements result in greater recovery of arm movement patterns in adults with chronic hemiparesis poststroke compared with any other exercise training of the arm?” We also evaluated the effects of TR on functional ability (secondary research question). Importantly, we interpret the immediate and long-term effects of TR in the context of MDC and MCID.
Methods
Data sources
This review has been registered at “PROSPERO – International prospective register of systematic reviews” (www.crd.york.ac.uk/PROSPERO/) (registration number CRD42012003464) and is reported in accordance with the PRISMA Checklist (12).
An extensive literature search was performed by 2 authors (HA & AG) in the following databases: PubMed, CINAHL, Embase, Web of Science, PEDro, and the Cochrane Library (including the Register of Controlled Trials). The search strategy for PubMed is shown in Appendix I and was modified for the other databases. Inclusion criteria were: experimental studies, comparing reach-to-grasp training with TR vs any other training in adults with chronic hemiparesis (> 6 months) due to stroke. TR was defined as a mechanical restraint that restricts movement of the trunk during training. Only randomized controlled trials (RCTs) or quasi RCTs (i.e. trials using quasi random methods of allocating participants to different interventions, e.g. alternation or assignment based on date of birth) published between January 1985 and March 2015 were considered. The studies had to be in English and published in peer-reviewed journals. Studies were excluded if they included participants younger than 19 years.
Study selection
The literature was separately screened by 2 authors (HA & AG). Their results were compared and discussed. In case of disagreement the last author (GD) was consulted. After removing duplicates, the titles and abstracts of the remaining citations were screened. The identified articles were assessed for eligibility by reading the full text. Subsequently, the reference lists of the included articles were screened. In addition, a publication bias analysis was performed to estimate the possibility of a systemic bias in selected studies due to over-reporting of positive results. Publication bias analysis was computed with the metafor package for R (13).
Data extraction
The studies were listed according to authors, number of participants, brain lesion side, time poststroke, type and duration of intervention, and outcome measures by HA (Table I). AG and GD verified the extracted data, and disagreements were resolved by discussion. Authors were contacted when further information was needed (4–6, 14–16). We obtained the raw data of the Reaching Performance Scale (RPS) and Wolf Motor Function Test (WMFT) used by Thielman in his original study (15) and in the follow-up study (17). As his research question for the analysis of the follow-up data (17) did not focus on between-group differences we performed further statistical tests: a 2 (group) by 3 (pre-test, post-test, retention) repeated measures analysis of variance (ANOVAs) were calculated using IBM SPSS Statistic 20.
HA and AG independently evaluated the potential risk of bias in the studies using the Cochrane Collaboration risk of bias tool (18) and the study’s methodological quality using the Physiotherapy Evidence Database (PEDro) scale (19). Discrepancies were resolved by discussion with the last author (GD). A classification suggested by Foley et al. (20) was used to interpret the PEDro scores. Studies scoring between 9 and 10 are considered “excellent”, a score of 6–8 “good”, 4–5 “fair”, and < 4 “poor” quality.
Table I. Characteristics of the included studies |
|||
Authors/Reference |
Sample size Brain lesion side Time poststroke (mean and SD) |
Intervention Duration and intensity |
Outcome measures Testing schedule |
Michaelsen & Levin (24) |
28 EG: 11 left, 3 right CG: 8 left, 6 right EG: 36 (28) m CG: 22 (15) m |
Reach & grasp a cylinder EG: TR using a harness, fixed with an electromagnet to the back of chair CG: no TR 60 trials, single session |
Reaching kinematics: velocity peaks, movement time, peak wrist velocity, time-to-peak velocity, temporal coordination index, trunk displacement & rotation, elbow extension, shoulder horizontal adduction, shoulder flexion Pre-test Post-test: immediately after intervention Retention test: next day |
Michaelsen et al. (4) |
30 EG: 6 left, 9 right CG: 9 left, 6 right TR: 16.7 (9.1) m CG: 18.2 (10.7) m |
Supervised home-training of task-specific, repetitive unimanual & bimanual reach-to-grasp tasks EG: TR using body and shoulder belt attached to the back of the chair CG: no TR 15 sessions, 1 h each, 3 × weekly, 5 weeks |
Reaching kinematics: peak arm velocity, hand trajectory straightness, smoothness, trunk displacement, elbow extension, shoulder flexion Clinical measures: FMA/ULS, TEMPA, dynamometry, BBT Pre-test Post-test: immediately after intervention Retention test: 1 month after intervention |
Woodbury et al. (16) |
11 EG: 3 left, 3 right CG: 5 left EG: 36.3 (35.3) m CG: 32.4 (33.7) m |
mCIMT EG: TR using a padded shield in front of the trunk CG: no TR 10 sessions, 6 h each, 5 × weekly, 2 weeks |
Reaching kinematics: segmentation - number of peaks, trunk displacement, shoulder flexion, elbow extension, hand path trajectory Clinical measures: FMA/ULS, WMFT, MAL/AOU, MAL/QOM Pre-test Post-test: within 1 week after intervention |
Thielman (15) Retention-data from Thielman (17) |
16 EG: 4 left, 4 right CG: 4 left, 4 right EG: 31.5 (34.7) m CG: 34 (2.3) m |
Task-specific reaching EG: TR using a harness securing the trunk to the back of the chair CG: auditory feedback on trunk movements 12 sessions, 40–45 min each, 2–3 × weekly |
Reaching kinematics: RPS - near and far target Clinical measures: FMA/ULS, WMFT, MAL, Goniometric aROM Pre-test: ≤ 5 days before intervention (15) Post-test: ≤ 2 days after intervention (15) Retention test: 1 year after intervention (17) |
Wu et al. (5) |
45 EG: 10 left, 5 right CG-I: 7 left, 8 right CG-II: 9 left, 6 right EG: 14.9 (13.6) m CG-I 15.0 (10.2) m CG-II: 16.8 (12.7) m |
EG: dCIMT with TR using a harness securing the trunk to the back of the chair CG-I: dCIMT, no TR CG-II: neuro-developmental treatment techniques, no TR 15 sessions, 2 h each, 5 × weekly, 3 weeks |
Reaching kinematics (all measures at 90% and at 125% of arm ‘s length): maximum grip aperture, time to maximum grip aperture, shoulder flexion, elbow extension, trunk displacement (slope) Clinical measures: FMA/ULS, MAL/AOU, MAL/QOM Pre-test & Post-test: exact timing not specified |
Wu et al. (6) |
57 EG: 12 left, 8 right CG-I: 7 left, 12 right CG-II: 5 left, 13 right EG: 15.7 (13.5) m CG-I: 13.7 (7.3) m CG-II: 17.7 (13.4) m |
EG: dCIMT with TR using a harness securing the trunk to the back of the chair CG-I: dCIMT, no TR CG-II: usual care based on neurodevelopmental principles, no TR 15 sessions, 2 h each, 5×weekly, 3 weeks |
Reaching kinematics: trunk displacement (slope), shoulder flexion, elbow flexion Clinical measures: MAL/AOU, MAL/QOM, ARAT, FAI, SIS Pre-test & Post-test: exact timing not specified |
Table I. Contd. |
|||
Authors/Reference |
Sample size Brain lesion side Time poststroke (mean and SD) |
Intervention Duration and intensity |
Outcome measures Testing schedule |
Lima et al. (23) |
22 EG: 5 left, 6 right CG: 3 left, 8 right EG: 86 (64.3) m CG: 75.6 (29.4) m |
mCIMT EG: TR with an 8-shaped clavicle immobilizer CG: no TR 10 sessions, 3 h each, 5×weekly, 2 weeks |
Reaching kinematics: trajectory straightness and smoothness, peak tangential wrist velocity, time to peak velocity of wrist, trunk displacement, elbow extension, ratio of elbow motion and sternum Clinical measures: MAL/AOU, MAL/QOM, WMFT, BAAS, muscle strength, SSQOL Pre-test Post-test: immediately after intervention Retention: 1 and 3 month after intervention |
de Oliveira Cacho et al. (22) |
20 EG: 6 left, 4 right CG: 5 left, 5 right EG: 4.3 (4.0) years CG: 3.4 (3.1) years |
Repetitive, task–specific training of grasping a cone and fitting it to random targets EG: TR with a harness securing the trunk to the back of the chair CG: no TR 20 sessions, 45 min each, 2 × weekly |
Reaching kinematics: Index of curvature of wrist marker, peak tangential wrist velocity, time to peak velocity, movement time, number of peaks, trunk displacement, elbow extension, shoulder flexion, shoulder adduction Clinical measures: Modified Ashworth scale, FM/ULS, Barthel index Pre-test Post-test Retention: 3 months after intervention |
EG: experimental group; CG: control group; CG-I: control group; CG-II: control group; TR: trunk restraint; SD: standard deviation; m: months; y: years; dCIMT: distributed constraint induced movement therapy; mCIMT: modified constraint induced movement therapy; ARAT: Action Research Arm Test; aROM: active range of motion; BAAS, Bilateral activity assessment scale; FAI: Frenchay Activities Index; BBT: Box and Block Test; FMA/ULS: Fugl-Meyer Assessment/Upper Limb Section; MAL: Motor Activity Log; AOU: Amount of Use; QOM: Quality of Movement; RPS: Reaching Performance Scale; SIS: Stroke Impact Scale; SSQOL: Stroke Specific Quality of Life; TEMPA: Test Evaluant la Performance des Membres supérieurs de Personnes Agées; WMFT: Wolf Motor Function Test. |
|||
Data synthesis
A systematic synthesis was performed. We reported only between-group differences concerning our primary and secondary research question. Furthermore, ESs for the reported significant between-group differences were calculated using Hedges’ g*, where data were available. Hedges’ g* adjusts for small sample sizes and is preferable to Cohen’s d because it uses the unbiased least squares estimate of the pooled standard deviation (21). In addition 95% CIs were calculated. Since Michaelsen et al. (4) reported standardized response means (SRM) (and 95% CI) as ESs, and raw scores were not available, we used the reported SRM (Table II).
Table II. Results of between-group differences |
|||||||
ICF domain |
Outcome measure |
Study |
Group |
Pre-test Mean (SD) |
Post-test/retention Mean (SD) |
Post-test/retention ES (95% CI) |
|
Body function/structure |
Trunk displacement |
Michaelsen & Levin (24) |
EG |
166 (101) |
114 (68)/133 (104) |
–0.54 (–1.29;0.21)/ –0.45 (–1.2;0.3) |
|
CG |
190 (124) |
171 (128)/188 (134) |
|||||
Woodbury et al. (16) |
EG |
0.12 (0.01) |
0.09 (0.01) |
–4.57 (–6.82;–2.32) |
|||
CG |
0.11 (0.01) |
0.14 (0.01) |
|||||
Wu et al. (5) |
EG |
3.6 (3.4) |
6.3 (3.01) |
0.70 (–0.18;1.58)b 0.76 (–0.13;1.65)c |
|||
|
CG-I |
4.1 (2.1) |
4.2 (2.9) |
||||
|
CG-II |
3.7 (2.5) |
4.01 (2.8) |
||||
Wu et al. (6) |
EG |
0.7 (3.3) |
5.5 (8.9) |
0.72 (0.06;1.38) |
|||
|
CG-II |
2.5 (6.2) |
0.2 (4.6) |
||||
Elbow extension |
Michaelsen & Levin (24) |
EG |
96 (24) |
n.s./109 (24) |
n.s. / 0.52 (–0.23;1.27) |
||
|
CG |
91 (26) |
n.s./95 (28) |
||||
|
Michelsen et al. (4) |
EG |
Pre/post/retention scores not reported |
0.98 (0.23;1.73)/ 1.40 (0.61;2.19) |
|||
|
|
CG |
|||||
Shoulder flexion |
Wu et al. (5)
|
EG CG-I CG-II |
0.13 (0.04) 0.13 (0.05) 0.14 (0.04) |
0.16 (0.06) 0.13 (0.03) 0.13 (0.03) |
0.60 (–0.28;1.47)b,c |
||
|
Wu et al. (6) |
EG CG-I |
0.14 (0.07) 0.14 (0.05) |
0.19 (0.07) 0.14 (0.06) |
0.75 (0.1;1.4)b |
||
RPS near target |
Thielman (15, 17)a |
EG |
11.6 (4) |
12.5 (4) |
–0.58 (–1.58;0.42) |
||
CG |
11.8 (3.9) |
14.9 (3.8) |
|||||
FMA/ULS |
Michaelsen et al. (4) |
EG |
Pre/post/retention scores not reported |
0.09 (–0.62;0.8)/ 0.35 (–0.36;1.06) |
|||
CG |
|||||||
Wu et al. (5) |
EG |
46.9 (5.9) |
54 (5.4) |
0.81 (0.06;1.55)c |
|||
CG-II |
45.9 (9.6) |
48.7 (7.2) |
|||||
Activity/Participation |
TEMPA |
Michaelsen et al (4) |
EG |
Pre/post/retention scores not reported |
0.35 (–0.36;1.06)/ 0.26 (–0.45;0.97) |
||
CG |
|||||||
|
|||||||
ARAT |
Wu et al. (6) |
EG |
35.9 (16.7) |
43.4 (13.9) |
0.54 (–0.11;1.19)c |
||
CG-II |
30.1 (19.8) |
33.9 (20.3) |
|
||||
FAI |
Wu et al. (6) |
EG |
15.8 (7.2) |
18.3 (6) |
0.72 (0.07;1.38)c |
||
CG-II |
12.8 (9.3) |
12.7 (9) |
|
||||
SIS/hand function |
Wu et al. (6) |
EG |
2.4 (0.9) |
2.9 (1) |
0.72 (0.06;1.38)c |
||
CG-II |
2.1 (0.8) |
2.2 (0.9) |
|
||||
MAL/QOM |
Wu et al. (5) |
EG |
1 (0.7) |
1.9 (0.8) |
0.55 (–0.18;1.23)c |
||
CG-II |
1 (0.9) |
1.4 (1.1) |
|
||||
Wu et al. (6) |
EG |
0.9 (0.8) |
1.8 (1) |
0.72 (0.06;1.38)c |
|||
CG-II |
0.7 (0.6) |
1.1 (0.9) |
|
||||
MAL/AOU |
Wu et al. (5) |
EG |
1 (0.6) |
2 (0.9) |
0.65 (–0.09;1.38)c |
||
CG-II |
0.9 (0.8) |
1.3 (1.1) |
|
||||
aFavouring CG, all others favouring TR, bES post-test between EG and CG-I, cES post-test between EG and CG-II. EG: experimental group; CG: control group; CG-I: dCIMT group no trunk restraint; CG-II: neuro-developmental treatment group and no trunk restraint; FMA/ULS: Fugl-Meyer Assessment/Upper Limb Section; RPS: Reaching Performance Scale; TEMPA: Test Evaluant la Performance des Membres supérieurs de Personnes Agées; ARAT: Action Research Arm Test; FAI: Frenchay Activities Index; SIS: Stroke Impact Scale; MAL: Motor Activity Log; AOU: Amount of Use; QOM: Quality of Movement; n.s.: not significant; ES: effect size; CI: confidence interval. Calculations of kinematic data in individual studies see Appendix IIa. |
|||||||
The results were discussed concentrating on immediate and long-term effects of TR on movement patterns (body function/structure) and functional abilities (activity/participation) while considering the studies’ potential risk of bias and methodological quality. The results were interpreted in the context of MDC and MCID when available. MDC and MCID can be reported as absolute or as relative values (normalized to the individual pre-test score). Therefore, we calculated the % change of significant between-group differences when applicable/necessary (Table II).
Results
Data retrieval, quality of studies and risk of bias
Eight studies (4–6, 15, 16, 22–24) met the inclusion criteria (Fig. 1). The risk of bias assessment is shown in Table III. The PEDro scores ranged from 4 to 7. Interpreting these scores according to Foley et al. (20) 1 fair and 7 good quality studies were included in this review (Table IV).

Fig. 1. Study selection. TR: trunk restraint.
Table III. Risk of bias assessment of included studies |
||||||
|
Random sequence generation (selection bias) |
Allocation concealment (selection bias) |
Blinding of participants and personnel (performance bias) |
Blinding of outcome assessment (detection bias) |
Incomplete outcome data (attrition bias) |
Selective reporting (reporting bias) |
Michaelsen & Levin (24) |
? |
? |
– |
? |
+ |
+ |
Michaelsen et al. (4) |
? |
? |
– |
+ |
+ |
– |
Woodbury et al. (16) |
? |
? |
– |
– |
+ |
+ |
Thielman (15) |
? |
+ |
– |
– |
+ |
+ |
Wu et al. (5) |
? |
+ |
– |
+ |
+ |
+ |
Wu et al. (6) |
? |
? |
– |
+ |
+ |
+ |
Lima et al. (23) |
? |
+ |
– |
+ |
+ |
– |
de Oliveira Cacho et al. (22) |
? |
+ |
– |
+ |
? |
+ |
+ low risk; – high risk; ? unclear risk. |
||||||
Table IV. Physiotherapy Evidence Database (PEDro) scores and Foley classification of included studies |
||||||||||||
|
Random assignment of the subjects |
Concealed allocation |
Baseline comparability |
Blinding of the subjects |
Blinding |
Blinding |
Adequate follow-up |
Intention-to-treat analysis |
Between-group comparisons |
Point estimates & variability |
Total PEDro score |
Foley classification Range in PEDro scores |
Michaelsen & Levin (24) |
Yes |
No |
Yes |
No |
No |
No |
Yes |
Yes |
Yes |
Yes |
6/10 |
Good 6–8 |
Michaelsen et al. (4) |
Yes |
No |
Yes |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
7/10 |
Good 6–8 |
Woodbury et al. (16) |
Yes |
No |
No |
No |
No |
No |
Yes |
No |
Yes |
Yes |
4/10 |
Fair 4–5 |
Thielman (15) |
Yes |
Yes |
Yes |
No |
No |
No |
Yes |
Yes |
Yes |
Yes |
7/10 |
Good 6–8 |
Wu et al. (5) |
Yes |
Yes |
Yes |
No |
No |
Yes |
Yes |
No |
Yes |
Yes |
7/10 |
Good 6–8 |
Wu et al. (6) |
Yes |
No |
Yes |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
7/10 |
Good 6–8 |
Lima et al. (23) |
Yes |
Yes |
Yes |
No |
No |
Yes |
No |
Yes |
Yes |
Yes |
7/10 |
Good 6–8 |
de Oliveira Cacho et al. (22) |
Yes |
Yes |
No |
No |
No |
Yes |
Yes |
No |
Yes |
Yes |
6/10 |
Good 6–8 |
Publication bias
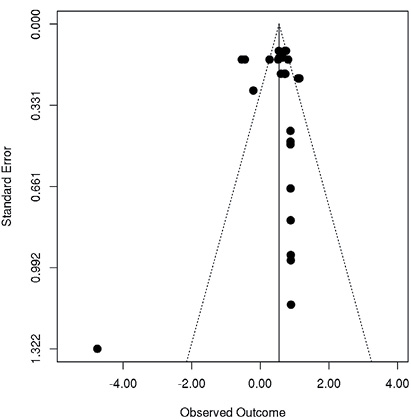
To analyse publication bias a total of 28 Hedge’s g ESs and variances from relevant outcome measures were computed from all 8 studies. The respective funnel plot is shown in Fig. 2. There was one extreme ES in Woodbury et al. (16) (Hedge’s g = –4.57). Visual inspection of the funnel plot (25) suggests missing studies on the left-hand side, particularly in the area of non-significance.
Participants
A total of 229 individuals (72% male, 28% female) with hemiparesis participated in the selected studies. Studies had sample sizes ranging from 11 (16) to 57 (6) participants. The participants sustained strokes on the left (120 participants) and the right side of the brain (109 participants) between 6 (4–6) to 101 (16) months previously. Only 2 studies (22, 23) (total 42 participants) reported stroke type: 31% haemorrhage, 64% ischaemic and 5% undefined. The mean age of the participants of the TR groups in the included studies were between 47.4 years (standard deviation (SD) 11.5) (22) and 69.4 years (SD 10.8) (4) and between 54.3 years (SD 13) (5) and 69.4 years (SD 10.8) (4) in the control groups. Mean scores on the FMA/ULS ranged from 30.4 (SD 6.7) (15) to 50.0 (SD 11.0) (24) in the TR groups and from 34.1 (SD 7.2) (15) to 49.0 (SD 13.0) (24) in the control groups. One participant in the study by Woodbury et al. (16) (due to changes in his/her job schedule), 7 in the study by Lima et al. (23) (due to refusal, health problems and difficulty with transport) and 2 in the study by De Oliveira Cacho et al. (22) (no reasons described) did not complete all study phases. Wu et al. (5) were not able to record kinematic data from 4–5 participants in each group, since those participants were unable to perform the required grasping.
Intervention
Details of the setup for restraining the trunk of each study are shown in Table I.
Thielman (15) compared mechanical TR with auditory feedback on trunk movements (i.e. an auditory signal triggered by a release of pressure on the back of the chair). In our review we considered his “stabilizer group” (with mechanical TR) as the experimental group and his “sensor group” (with auditory feedback) as the control group.
Mechanical TR was combined with repetitive reach-to-grasp training (24), task-specific reach-to-grasp training (4, 15, 22), and different versions of constraint-induced movement therapy (CIMT) (5, 6, 16, 23). A short description of the methodology used in each study is given in Table I.
Outcome measures
To answer our primary review question we concentrated on measures evaluating movement patterns (body function/structure): kinematic measures (26), the RPS (27), and the FMA/ULS (28). Among kinematic measures we focused on trunk displacement, elbow extension, and shoulder flexion. These are well-accepted measures to describe reaching performance (1, 29, 30). The calculation of these measures differed among studies (Appendix IIa). During testing participants reached objects at arm’s length (22) or at 80% (4, 16), 90% (5, 6, 23, 24) or 125% (5) of their arm’s length. We did not include data from the testing condition with 125% of arm’s length, because reaching beyond one’s arm’s length changes movement kinematics of the trunk (2) and is less suitable to explain motor function of the arm compared with testing conditions within arm’s length (31). In the kinematic analysis both reach-to-grasp (4, 5, 22–24) and reach-to-point movements (6, 16) were evaluated. For our secondary question we included performance-based and self-reported measures evaluating functional abilities (activity/participation).
Post-tests were scheduled immediately after (4, 24), within 1 week (16) after the intervention, or not specified (5, 6, 22). Additional follow-up testing was performed in 4 studies (4, 22–24). We considered retention data by Michaelsen & Levin (24) as post-test data, as they lay within the described post-test time-span of 1 week in the other studies. Finally, Thielman published 1-year follow-up data in a separate paper (17).
Immediate effects of TR on recovery of movement patterns (body function/structure)
Trunk displacement. Three of 7 studies measuring trunk displacement reported a decrease in trunk displacement in the TR groups compared with the control groups receiving the same training without TR (5, 16, 24). Both studies comparing TR with control groups receiving usual care/NDT showed a decrease in trunk displacement in the TR groups compared with the control groups (5, 6) (Table II).
Elbow extension. Two of 7 studies measuring elbow extension reported an increase in elbow extension during reaching in the TR groups compared with the control groups receiving the same training without TR (4, 24). Both studies comparing TR with control groups receiving usual care/NDT reported no differences between the groups (5, 6) (Table II).
Shoulder flexion. An increase in shoulder flexion in the TR groups compared with the control groups, receiving the same training without TR was reported in 2 of 6 studies measuring shoulder flexion (5, 6). One study of 2 studies comparing TR with usual care/NDT showed an increase in shoulder flexion in the TR group compared with NDT (5) (Table II).
FMA/ULS. Two of 5 studies using the FMA/ULS reported greater improvements in the TR group compared with the control group receiving the same training without TR (4) and to the control group receiving NDT (5) (Table II).
RPS. One study used the RPS and showed an improvement in movement patterns when reaching for near targets in the control group compared with the TR group (15) (Table II).
Summarizing the effects of TR on movement patterns 4 good studies (4–6, 24) and one fair study (16) suggest a greater benefit of training with TR compared with training without TR or usual care/NDT. However, there is evidence from 1 good study (15) that mechanical TR is less effective than training with auditory feedback.
Immediate effects of TR on functional abilities (activity/participation)
We considered the Test Evaluant la Performance des Membres supérieurs de Personnes Agées (TEMPA), the Action Research Arm Test (ARAT), the Stroke Impact Scale (SIS), and the Motor Activity Log (MAL) as measures assessing activity, and the Frenchay Activities Index (FAI) as a measure assessing participation.
Seven studies investigated the effect of TR on functional abilities with different outcome measures. The immediate effects on functional abilities were observed mostly in comparison with usual care/NDT (5, 6). However, in the study by Michaelsen et al. (4) more participants in the TR group (6/15) improved TEMPA scores over 5 points than in the control group (4/15). Comparing the TR groups with the control groups receiving usual care/NDT between-group differences in the MAL/AOU (5), MAL/QOM (5, 6), ARAT (6), FAI (6), and SIS/hand function (6) were observed (Table II).
Summarizing, there is evidence from 3 good trials (4–6) for a positive immediate impact of TR on activity compared with training without TR or usual care/NDT. Furthermore, there is evidence from one good-quality trial (6) that TR has an immediate positive effect on participation compared with usual care/NDT.
Long-term effects of TR on movement patterns (body function/structure) and functional abilities (activity/participation)
Three studies investigated the long-term effects of TR after 1 (4, 23) and 3 months (22, 23). Furthermore, we analysed follow-up data (after 1 year) by Thielman (17). Lima et al. (23) and de Oliveira Cacho et al. (22) did not reveal any long-term effects of TR. Our analysis of the data by Thielman (17) did not show any long-term between-group differences in the RPS (p > 0.05) or in the WMFT (p > 0.05). Michaelsen et al. (4) observed improvements after 4 weeks in elbow extension (available data: 6/15 participants in the TR group improved > 5° compared with 1/15 participants in the control group; mean change of 2.9° in TR group vs –9.1° in control group), FMA/ULS (available data: 12/15 participants in the TR group improved > 2 points compared with 8/15 participants in the control group) and TEMPA (available data: 10/15 participants in the TR group improved > 5 points compared with 6/15 in the control group). The study’s analysis of the severe subgroup (FMA/ULS < 50) showed that TR had an effect on the retention data of the FMA/ULS, whereas the mild subgroup (FMA/ULS ≥ 50) did not show significant between-group difference for retention. For detailed results see Table II.
MDC and MCID for between-group differences
MDC values for trunk displacement (32), elbow and shoulder movement (33), FMA/ULS (33), SIS/hand function (34), and MAL (35) were found in the literature. All of them were calculated using test-retest methodology. Anchor-based MCID values were found for SIS/hand function (34) and MAL/QOM (36) using patients’ perception of change (retrospectively) and for FMA/ULS (37) applying therapists global ratings of change (retrospectively) as an anchor. Van der Lee et al. (38) set the MCID of the ARAT at 10% of the maximum value of the score and analysed if this is beyond the threshold of a measurement error based on inter- and intra-rater reliability. The studies’ participants were in the chronic (> 6 months) (32–34, 38), in the subacute (> 3 months) (35, 37) or acute phase (36) after stroke. The mathematical procedure for calculating MDC and MCID values in the cited studies is described in Appendix IIb. For the other outcome measures (RPS, FAI, and TEMPA) neither established MDC nor MCID values were identified.
Trunk displacement. The MDC90 for trunk displacement is 35.7 mm (32). The calculations are based on a sample of n = 18 (> 6 months since stroke, age 67.6 years (SD 8.1)). The TR group in Michaelsen & Levin (24) improved more than this value. The MDC95 for trunk displacement is 59.9 mm (32) and the TR group in Michaelsen & Levin (24) changed less than this value. The other studies (5, 6, 16) used calculations that do not allow comparison (Table V).
Table V. Minimal detectable change (MDC) and minimal clinically important difference (MCID) for relevant outcome measures and interpretation of the immediate treatment effect |
||
Outcome measure |
Established in the literature |
Comparison with included studies |
MDC |
||
Trunk displacement |
MDC90 35.66mm (32) |
# (24) ? (5, 6, 16) |
Elbow extension |
aMDC95% 30.5%–38.8% (33) |
$ (24) ? (4) |
Shoulder flexion |
aMDC95% 24.4%–33.1% (33) |
# (6) $ (5) |
FMA/ULS |
MDC95 5.2 (33) |
# (5) ? (4) |
SIS/hand function |
MDC95 25.9 (34) |
$ (6) |
MAL/QOM |
MDC90 0.8 (35) |
# (5, 6) |
MAL/AOU |
MDC90 0.8 (35) |
# (5) |
MCID |
||
FMA/ULS |
5.3 (37) |
# (5) ? (4) |
ARAT |
5.7 (38) |
# (6) |
SIS/hand function |
17.8 (34) |
$ (6) |
MAL/QOM |
1 (36) |
$ (5, 6) |
aNormalized to pre-test score. ? not comparable; # passing MDC/MCID; $ not passing MDC/MCID. FMA/ULS: Fugl-Meyer Assessment/Upper Limb Section; ARAT: Action Research Arm Test; SIS, Stroke Impact Scale; MAL: Motor Activity Log; AOU: Amount of Use; QOM: Quality of Movement. |
||
Elbow extension. The MDC95% (normalized to the individuals pre-test value) for elbow extension is 30.5–38.8% (33). The calculations are based on a sample of n = 14 (mean time poststroke 14 months (SD 6.5), age 59.9 years (SD 14.6)). The change in the TR group (13.5%) and in the control group (4.4%) by Michaelsen & Levin (24) did not exceed the reported MDC95% (Table V). Michaelsen et al. (4) did not report sufficient data (pre/post/retention data missing) to allow a comparison (available data: 6/15 participants/TR group improved > 5° compared with 1/15 participants/control group at post-test and retention; mean change of 5.9° in the TR group vs –3.6° in the control group at post-test and 2.9° in the TR group vs –9.1° in the control group at retention).
Shoulder flexion. Similar to elbow extension the MDC95% for shoulder flexion is normalized to the individual’s pre-test value, and lies between 24.4% and 33.1% (33). The calculations are based on a sample of n = 14 (mean time poststroke 14 months (SD 6.5), age 59.9 years (SD 14.6)). The change in the TR group exceeded the MDC95% in 1 study (35.7% vs 0% in the control group) (6). The second study by Wu et al. (5) reported 23.1% change in the TR group and a change of 0% and –7.1% for the 2 control groups receiving mCIMT without TR or NDT/usual care. Therefore these changes did not exceed the established MDC95% (Table V).
FMA/ULS. The MDC95 for the FMA/ULS is 5.2 points (33) (based on n = 14, mean time poststroke 14 months, age 59.9 years (SD 14.6)) and the MCID is 5.3 (37) (based on n = 143, at least 4 months poststroke, age 57.1 years (SD 10.96)). In one study (5) the change in the TR group exceeded MDC95 and MCID (Table V). The study by Michaelsen et al. (4) does not provide sufficient data on the whole group for comparison (available data: 9/15 participants/TR group improved > 2 points compared with 7/15 participants/control group at post-test; 12/15 participants/TR group improved > 2 points compared with 8/15 participants/control group). However, the severe subgroup (FMA/ULS < 50) improved at retention test by 9 points, which clearly exceeds MDC95 and MCID.
MAL. The MDC90 of the MAL/AOU and the MAL/QOM is 0.8 (35). The calculations are based on a sample of n = 116 (3–9 months poststroke, mean age 63.26 years (SD 12.56)). The TR groups in 2 studies exceeded these values (Table V). The MCID for the MAL/QOM of 1 (36) (based on n = 52, at least 4 months poststroke, mean age 57.1 years (SD 10.96)) is not reached by any group.
ARAT. The MCID for the ARAT is 5.7 points (38) (based on n = 20; median time poststroke 3.6 years, interquartile range (IQR) 2.5–4.9; median age 62 years, IQR 52.5–71.8) and is reached by the TR group in one study (6) (Table V).
SIS/hand function. The MDC95 (25.9) and MCID (17.8) of the SIS/hand function (34) were not reached by the TR groups (5, 6) (Table V). The calculations for the MDC95 and MCID are based on a sample of n = 74 (> 6 months poststroke; mean age 54.1 years (SD 1.4)).
Discussion
Reach-to-grasp training involving TR provides greater recovery of arm movement patterns (body function/structure) and functional abilities (activity/participation) immediately after training (4–6, 16, 24) and at retention after 4 weeks (4) than the same training without TR or usual care/NDT. However, these effects are not consistently clinically relevant. In addition, 2 (22, 23) of the 8 included studies did not show any effect of TR after training and at retention on movement patterns or functional abilities. Moreover, there is evidence from one study (15) that mechanical TR is less effective than training with auditory feedback on movement patterns immediately after training.
Clinical relevance of effects of trunk restraint
The kinematic variables considered in this review can be understood as measures of movement quality (26). Overall, these variables improved after training with TR, although none of the studies showed significant changes in all 3 kinematic variables.
Four studies showed a significant difference in trunk displacement between groups, only in one study (24) the MDC90 is reached by the TR group. The MDC90 value is based on patients in the chronic phase poststroke, who are of similar age to the participants in the studies included in our systematic review. None of the 2 studies reporting significant differences in elbow extension showed changes exceeding the MDC95%, and while 2 studies showed a between-group difference in shoulder flexion, the change in the TR group exceeded the MDC95% only in 1 study (6). However, the MDC95% for elbow and shoulder movement was established using a reaching task while the trunk was fixed to the back of a chair, and with an object placed at 110% of arm’s length. It could be hypothesized that in the testing positions of the included studies (without trunk fixation and object placed within arm’s length) the MDC could be smaller. However, the sample used for MDC95% calculation (33) has similar age and impairment level as the studies included in our systematic review. In conclusion, the clinical relevance of the effect of TR on kinematic variables, indicating movement quality, has yet to be determined.
Significant between-group changes were also reported in FMA/ULS scores in 2 studies, but in only one study (5) were these changes found to lie above the MDC95 (33) and the MCID (37). It has to be noted that the established MCID value is based on a perceived improvement of more than 50%. There is no clear evidence for which cut-off score should be applied for clinical relevance (39). Compared with the anchor used to establish the MCID for SIS (perceived change of 10–15% (34)), a cut-off score of 50% seems to be high, resulting in a solid MCID for FMA/ULS.
The recent meta-analysis by Wee et al. (7) who included 6 studies in their review ((23) and (22) not included), reported a moderate significant effect of TR on shoulder flexion and on FMA/ULS, and a non-significant small effect on elbow extension and a large effect on trunk displacement. Our analysis suggests that these observed changes are not consistently important and detectable.
Interpreting the results within the domains of activity/participation the MCID for the ARAT (38) and the MDC90 for the MAL/AOU and MAL/QOM (35) were achieved by the TR groups, but not by usual care/NDT (5, 6) (Table V). However, the MCID for the MAL/QOM (36) and the MDC95 and MCID of the SIS/hand function (34) were not reached by the TR groups (5, 6). We would like to point out that the MCID for the MAL/QOM is based on calculations with a sample in the acute phase poststroke and with little functional ability. It is possible that an MCID value established for this population is higher than for persons in the chronic phase poststroke. Nevertheless, the impairment level of the sample for MDC/MCID of the ARAT is similar to the studies included in our systematic review. For the SIS/hand function a change of 10–15 points could also be used to indicate clinically meaningful change (40, 41). However, even these lower values are not reached by the TR group.
The meta-analysis (7) revealed a small and not significant effect of TR on functional abilities (MAL/AOU: ES –0.12; MAL/QOM: ES –0.15). Furthermore, Pain et al. (11) conclude, that training with TR does not have an effect on functional abilities when compared with the same training without TR. In contrast to the other 2 reviews (7, 11) we also included usual care/NDT as control condition; our comparison suggests that if training (dCIMT) with TR is compared with usual care/NDT; this training might be superior and can lead to detectable improvements in functional abilities.
Of the 3 studies investigating the long-term effects (4, 22, 23) the severe TR group in Michaelsen et al. (4) improved by 9 points in FMA/ULS at retention test, which is clearly exceeding MDC95 (33) and MCID (37). De Oliveira Cacho et al. (22) and Lima et al. (23) did not detect any long-term effects. Furthermore, our calculations of the data by Thielman do not show between-group differences at retention. Although scores from post-test were maintained at retention one year later (17). Therefore, the clinical relevance of long-term changes cannot be evaluated based on current data.
Quality of evidence
We chose to assess the quality of evidence with both the PEDro scale and the Cochrane Collaboration risk of bias tool. The PEDro scale is a well-known and broadly used instrument among physiotherapists. However, as presenting a composite score for risk of bias should be avoided (18), we complemented the assessment with the Cochrane Collaboration risk of bias tool.
We agree with Wee et al. (7) that, based on the Cochrane Collaboration risk of bias tool, there is only a moderate degree of confidence in the results of this review, since there is an unclear risk of selection bias among most of the included studies, due to unclear description of exact randomization procedure and/or allocation concealment and due to stratification during randomization. Furthermore, we found a high risk of performance bias in all of the included studies. However, it has to be taken into consideration that blinding of participants and therapists in the context of exercise studies is not possible.
Limitations and open questions
Although a comprehensive literature search was conducted and 2 researchers independently screened the literature the exclusion of non-English articles introduces potential selection bias into this review. Furthermore, the relatively small number of studies considered and the possible risk of bias in these studies may threaten the validity of this review. Furthermore, the asymmetric funnel plot could indicate potential publication bias. However, there are several sources of funnel plot asymmetry, e.g. reporting biases, or true heterogeneity between studies (see Sterne et al. (25) for a discussion). Here we assume that funnel plot asymmetry may be caused by selective outcome reporting and methodological heterogeneity in the studies included in our systematic review.
By registering our review in advance we reduced the potential risk of selective reporting within our review. However, our interpretation of movement patterns is based only on kinematic measures of trunk, elbow and shoulder movement and on the RPS, and FMA/ULS and on statistical calculations in the original studies. The included studies mostly used parametric statistics with ordinal scale data (except for (16)). This procedure, although commonly used, has been recently criticized (42).
Our interpretation of the clinical relevance of the findings had to rely on published MDC and MCID values, partly based on data obtained from persons in the acute (36) and subacute phase (35, 37). Furthermore, we used MCID values for our interpretation, which were calculated with anchor-based methods, even though there is no clear evidence for which cut-off score should be applied for clinical relevance (39).
The effect of other types of TR (e.g. auditory cues), as suggested by Thielman (15) should be addressed in future research. In addition, based on data by Michaelsen et al. (4) and the discussion by Lima et al. (23) the influence of severity of the impairment on effects of TR deserves more attention. It can be hypothesized that individuals with moderate to severe impairment might benefit from reach-to-grasp training with TR. Based on our research question we excluded studies with patients in the acute stage after a stroke. However, a recent study by Bang et al. (43) suggests, that mCIMT plus TR for patients 1–6 months poststroke has a larger effect on upper-extremity function than mCIMT alone. The TR group in this study improves by a mean of 15.6 (SD 5.1) in the ARAT, by 12.3 (SD 3.9) in the FMA/ULS, by 1.8 (SD 0.7) in the MAL/AOU, by 1.7 (SD 0.5) in the MAL/QOM, and by 32.8 (SD 13.6) in active elbow extension during reaching. Although these findings have to be confirmed in future research, they are promising as they clearly exceed the average improvements seen in the chronic phase after a stroke.
Conclusion
Due to risk of bias in the included studies there is a moderate degree of confidence in the results of our review. In contrast to the 2 previously published reviews (including 5 and 6 studies), we propose that despite TR-induced changes within the domains of body function/structure and activity/participation, immediately after training and at retention test after 4 weeks, the magnitude of change is not consistently clinically relevant. Moreover, the long-term effect of TR has not been conclusively demonstrated.
AcknowledgEments
The authors would like to thank Dr Tara McIsaac for reviewing the manuscript and Dr Emalie Hurkmanns for her suggestions and comments on the search strategy. The idea for this work originates from the master thesis of 1 of the authors (HA), “Therapeutic relevance of restraining compensatory trunk movements during reach-to-grasp in adults with chronic hemiparesis following stroke – a literature review”.
The authors declare no conflicts of interest.
REFERENCES
Appendix I. Search strategy for PubMed |
|
1. |
hemip*[tiab] |
2. |
stroke[tiab] |
3. |
CVA[tiab] |
4. |
brain injur*[tiab] |
5. |
cerebrovasc*[tiab] |
6. |
cerebral vascular[tiab] |
7. |
cerebral[tiab] |
8. |
brain[tiab] |
9. |
subarachnoidal[tiab] |
10. |
haemorrhage[tiab] |
11. |
hemorrhage[tiab] |
12. |
haematoma[tiab] |
13. |
hematoma[tiab] |
14. |
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 |
15. |
Rehabilitation |
16. |
physical therapy |
17. |
occupational therapy |
18. |
physiotherap* |
19. |
movement therapy |
20. |
training therapy |
21. |
exercise |
22. |
15 or 16 or 17 or 18 or 19 or 20 or 21 |
23. |
reach* |
24. |
arm |
25. |
upper extremity |
26. |
upper limb |
27. |
trunk |
28. |
24 or 25 or 26 or 27 |
29. |
14 and 22 and 23 and 28 |
Filters: Publication date from 1985/01/01 to 2015/03/30; Humans; English; Adult: 19+ years. |
|
Appendix II. Calculations |
|
(a) Calculations of kinematic variables |
|
Trunk displacement |
|
Michaelsen & Levin (24) Michaelsen et al. (4) Lima et al. (23) de Oliveira Cacho et al. (22) |
Movement of the sternal marker in the sagittal plane, measured in mm |
Woodbury et al. (16) |
Three-dimensional displacement of the marker on 10th thoracic vertebrae, calculated as a percentage of actual distance from fingertip to target in start position |
Wu et al. (5, 6) |
Calculated as ratio of trunk to upper extremity displacement in sagittal plane, calculated separately for start, middle and end phase of the reach-to-point movement) |

Appendix II. Contd. |
|
Elbow extension |
|
Michaelsen & Levin (24) Michaelsen et al. (4) Lima et al. (23) de Oliveira Cacho et al. (22) |
Angle between the forearm and the upper arm, full extension equalled 180° |
Woodbury et al. (16) |
Joint angular excursion calculated as the angle at the conclusion minus the angle at the start of the movement; full shoulder flexion defined as 90° and anatomically neutral position defined as –90° |
Wu et al. (5, 6) |
Angular change of elbow joint (difference from beginning to endpoint), normalized to task distance measured in mm |
Shoulder flexion |
|
Michaelsen & Levin (24) Michaelsen et al. (4) de Oliveira Cacho et al. (22) |
Angle between the upper arm and the sagittal plane, arm alongside the body equalled 0° |
Woodbury et al. (16) |
Joint angular excursion calculated as the angle at the conclusion minus the angle at the start of the movement; full elbow extension equalled 180° |
Wu et al. (5, 6) |
Angular change of shoulder joint (difference from beginning to endpoint), normalized to task distance measured in mm |
(b) Calculations of minimal detectable change and minimal clinically important difference values |
|
Calculation of MDC values |
|
Trunk displacement (32) |
MDC95 = SEM × 1.96 sw × √2 sw = residual mean square value SEM = square root of the mean square residual error |
Elbow and shoulder movement (33) FMA/ULS (33) |
MDC95% = (MDC95/mean)×100 mean for all observations MDC95 = SEM × 1.96 × √2 SEM = SDx × √(1–Rx) SDx = SD for all observations Rx = test-retest reliability coefficient (ICC) |
SIS/hand function (34) |
MDC95 = SEM × 1.96 × √2 SEM = SDpooled × √(1–r) SDpooled = SD for all observations R = ICC |
MAL/AOU and MAL/QOM (35) |
MDC90% = (MDC95/maximum value of score) × 100 MDC90 = SEM × 1.64 × √2a SEM = SDbaseline × √(1–ICC) |
Calculation of MCID values |
|
FMA/ULS |
GROC Scale for therapists based on GROC rating –2 groups of patients (GROC < 5; GROC=5) ROC curves y-axis with true-positive rate (sensitivity values) x-axis with 1 minus false-positive rate (specificity values) MCID = point on the ROC curve nearest the upper left corner |
ARAT |
Limits of agreement according to Bland and Altman ∆–2SD and ∆+2SD ∆ = mean of the differences between 2 ratings of the same subject SD = SD of this differences MCID = 10% of maximum value of the score A test is considered to be capable of detecting a difference of at least the magnitude of the limits of agreement |
SIS/hand function |
Patient‘s perceived overall recovery 0 indicating no recovery 100 indicating full recovery MCID = change of 10–15% perceived overall recovery |
MAL/QOM |
Patient‘s global rating of perceived change „how well is your arm doing“a MCID: mean change score for the smallest meaningful change (score 2 “little change, meaningful“) |
a7-point Likert scale: 1 – much better; 2 – little better, meaningful; 3 – little better, not meaningful; 4 – about the same; 5 – little worse, not meaningful; 6 – little worse, meaningful; 7 – much worse. MDC: minimal detectable change; MCID: minimal clinically important difference; SEM: standard error of measurement; SD: standard deviation; ICC: intraclass correlation coefficient; GROC: Global Rating of Change Scale; ROC, receiver operating characteristic curves; FMA/ULS: Fugl-Meyer Assessment/Upper Limb Section; ARAT: Action Research Arm Test; SIS: Stroke Impact Scale; MAL: Motor Activity Log; AOU: Amount of Use; QOM: Quality of Movement. |
|
