Mônica de Oliveira Melo, PhD1,2, Klauber Dalcero Pompeo, MSc1, Bruno Manfredini Baroni, PhD3 and Marco Aurélio Vaz, PhD1,4
From the 1Exercise Research Laboratory, School of Physical Education, Federal University of Rio Grande do Sul, Porto Alegre, 2Research Centre of Ciências e Artes do Movimento Humano, University of Caxias do Sul, Caxias do Sul, 3Federal University of Health Sciences of Porto Alegre and 4Physique – Centre of Physical Therapy, Porto Alegre, RS, Brazil
OBJECTIVE: To determine the effects of neuromuscular electrical stimulation and low-level laser therapy on neuromuscular parameters and health status in elderly subjects with knee osteoarthritis.
DESIGN: A randomized evaluator-blinded clinical trial.
SUBJECTS: Forty-five elderly women with knee osteoarthritis.
METHODS: Subjects were randomized into 1 of the following 3 intervention groups: electrical stimulation group (18–32 min pulsed current, stimulation frequency 80 Hz, pulse duration 400 μs, stimulation intensity 40% of maximal isometric voluntary contraction), laser group (dose 4–6 J per point, 6 points at the knee joint) or combined group (electrical stimulation plus laser therapy). The outcomes included muscle thickness and anatomical cross-sectional area (ultrasonography), knee extensors’ electrical activity (electromyography), torque (dynamometry) and health status (Western Ontario and McMaster Universities Osteoarthritis Index). All groups underwent a 4-week control period (without intervention) followed by an 8-week intervention period.
RESULTS: Muscle thickness and anatomical cross-sectional area increased in the electrical stimulation and combined groups. All groups presented similar improvements in torque, electrical activity and health status.
CONCLUSION: Electrical stimulation alone or in combination with laser therapy generated positive effects on all evaluated parameters. Laser therapy increased health status and electrical activity, but had no effect on muscle mass.
Key words: osteoarthritis; low-level laser therapy; electrical stimulation; neuromuscular effects; elderly; combined modality therapy.
J Rehabil Med 2016: 48: 293–299
Correspondence address: Mônica de Oliveira Melo, Ave Willy Eugênio Fleck 1500, n. 1500, casa 187, 90150-180 Porto Alegre, Brazil. E-mail: momelo@ucs.br
Accepted Dec 11, 2015; Epub ahead of print Feb 12, 2016
INTRODUCTION
Knee osteoarthritis (OA) is the most prevalent type of osteoarthritis. The prevalence of knee OA is expected to increase in the near future due to increased life expectancy of populations worldwide (1). Quadriceps weakness, which is associated with muscle mass loss and/or reduced muscle activation, is typical in patients with knee OA (2, 3). Evidence suggests that the loss of muscle strength increases according to the disease stage, and thus indicates progression of the degenerative process (3, 4). Furthermore, it has been reported that pain and inflammation accelerate the degenerative process through a reduction in neural activation (2, 5, 6), which leads to a vicious cycle of pain-weakness-pain. This cycle can ultimately affect the functional status, independence and quality of life of patients with knee OA (7).
Low-level laser therapy (LLLT) has been proposed for OA treatment due to its analgesic (6, 8), anti-inflammatory (6, 9) and regenerative (10, 11) effects. Because of the effects of this treatment on pain modulation (12, 13) and the release of anti-inflammatory agents (7, 8), LLLT might facilitate muscle activation and improve the functioning of patients with knee OA. However, no studies have been performed on the effects of LLLT on muscle strength or muscle activation in elderly patients with knee OA.
Patients with knee OA have difficulties achieving a level of voluntary effort to maintain full neuromuscular function (i.e. greater than 60% of maximal voluntary contraction (14)). Neuromuscular electrical stimulation (NMES) is an effective therapy for quadriceps strengthening in elderly individuals with knee OA (15, 16). Due to the non-selective recruitment of motor units, muscle fibres type I and II are simultaneously recruited through NMES, even at relatively low intensities of stimulation (17–19), potentially producing structural and functional changes in the neuromuscular system. However, there have been few studies on the effects of NMES on muscle mass parameters (anatomical cross-sectional area and/or muscle thickness) or on electrical activity (surface electromyography) in elderly subjects with knee OA (20, 21).
To the best of our knowledge, there are no studies investigating the combined effects of LLLT and NMES on neuromuscular parameters in patients with knee OA. The aim of the present study was therefore to determine the individual and combined effects of LLLT and NMES on quadriceps strength, muscle mass and electrical activity, and health status (pain, stiffness and physical function) in elderly subjects with knee OA. Considering the analgesic and anti-inflammatory effects of LLLT and the strengthening effects of NMES on the neuromuscular system, the central hypothesis of the present study is that the combination of LLLT and NMES promotes greater improvements in muscle strength, muscle morphology, muscle electrical activity and health status compared with each therapy alone.
METHODS
Trial design
This study was a randomized, single-blinded, clinical trial (ClinicalTrials.gov Identifier: NCT02067871). Ethical approval was obtained from the University Ethics in Research Committee (Protocol number 20160). All patients provided written consent prior to data collection. The participants were assessed at 3 different time-points over a 12-week period at the University Exercise Research Laboratory, Federal University of Rio Grande do Sul, Porto Alegre, Brazil. The first 4 weeks were used as a control period, when no intervention was performed. The intervention period lasted 8 weeks. Evaluation was performed before the control period (pre-control), before the intervention period (pre-intervention), and after the intervention period (post-intervention).
Participants and randomization
Participants were recruited via advertisements in the local newspaper. The inclusion criteria encompassed a knee OA Grade 2 or Grade 3, diagnosed by an orthopaedist according to the criteria of Kellgren & Lawrence (22); age between 60 and 75 years; female gender; and 1 or more episodes of knee pain in the past 6 months. The following exclusion criteria were observed: body mass index (BMI) higher than 40 kg/m2; hip, ankle, or toe osteoarthritis diagnosis; the use of crutches for locomotion; participation in strength-training programmes or physiotherapy treatment for knee OA in the past 6 months; neurological or cognitive disorders; rheumatoid arthritis; the use of pacemakers; previous or upcoming surgery (within 3 months); or any cardiorespiratory, neuromuscular, or metabolic disease representing an absolute contraindication or contraindication to the performance of maximum strength tests.
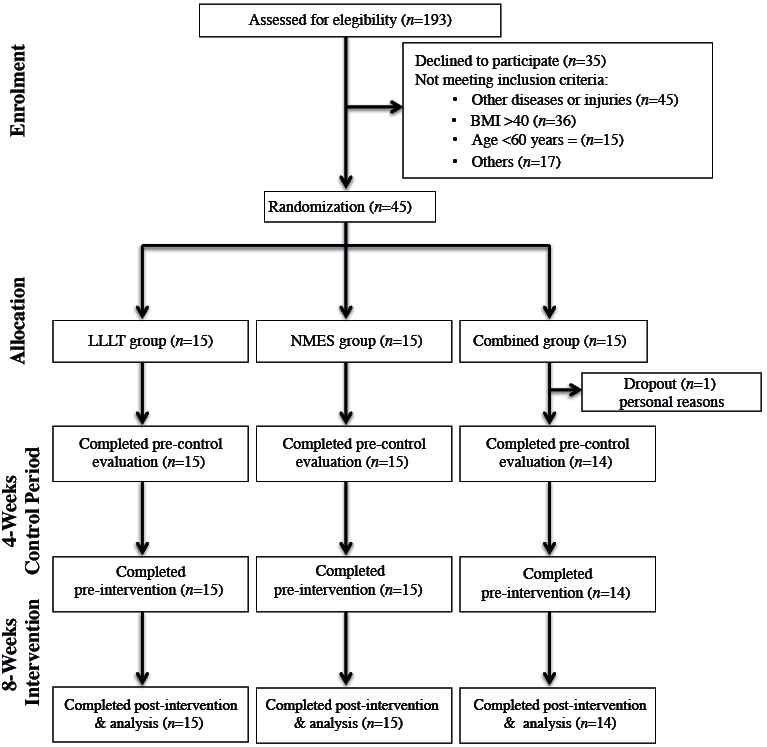
Researchers responsible for data collection and data analysis were blinded to the randomization and intervention of the patients. Forty-five participants were randomly assigned to 1 of 3 study groups: Group 1 – LLLT group; Group 2 – NMES group; and Group 3 – combined treatment group (NMES plus LLLT) (Fig. 1). Group allocation was randomized into 3 blocks of 15 sealed envelopes without external marks, which were mixed and numbered from 1 to 15, containing a piece of paper with the group allocation. The envelopes were opened, and randomization occurred only after a participant met all of the inclusion criteria and successfully completed the baseline study evaluation. All participants received treatment, and the results were included in the data analysis.
Intervention protocols
The 3 intervention protocols were applied by a researcher experienced in using the tested therapies and blinded to the data acquisition and data analysis. LLLT was administered twice a week over a period of 8 weeks, with a minimum interval of 48 h between sessions. A THOR DD2 Control Unit, comprising an infrared gallium-aluminium-arsenide (GaAlAs) diode laser probe (λ = 810 nm, continuous wave, 200 mW output power, 0.0364 cm2 spot size area, and 0.218 J/cm2 power density) (THOR®, London, UK), was used for the laser application. The laser was applied while the probe was held stationary and perpendicular to the skin, and light pressure was applied to 3 anteromedial and 3 anterolateral points over the intercondylar notch (8, 13).
The LLLT programme was based on the World Association for Laser Therapy (WALT) recommendations (23) and on studies obtaining positive results for the relief of osteoarthritic symptoms (8, 13). During the first 4 intervention weeks, laser therapy was administered for 30 s per point, at a dose of 6 J per point (total 36 J), to optimize the analgesic (9) and anti-inflammatory (3) effects of the laser. In the remaining 4 weeks, the treatment focused on cartilage regeneration (10, 11), for which an approximately 30% lower energy dose was used, i.e. 20 s per point, resulting in a dose of 4 J per point (total 24 J).
In the NMES group, the participants underwent supervised NMES sessions twice a week, at 48 h intervals, over an 8-week period with a progressive increase at the intensity and volume. Electrical stimulation was administered using portable, constant-voltage electrical stimulation equipment developed specifically for use in the present trial. NMES was applied for 18–32 min and was performed at the same time of day, with participants seated on a conventional chair, knees flexed to 90° (0° = full extension) and the treated lower-limb strapped to the chair using a band.
Before starting the NMES programme, the quadriceps motor point was determined using an electrical stimulator pen (KLD Biosystems, Brazil) with a faradic current, a maximum frequency of 30 Hz and sufficient intensity to produce a tetanic contraction. During the NMES protocol, 2 electrodes (5 × 13 cm) were placed anteriorly on the participants’ thighs. The proximal electrode was positioned over the quadriceps motor point, and the distal electrode was placed perpendicular to the longitudinal thigh axis just above the patellar border (21, 24). A pulsed symmetric biphasic rectangular current, with a pulse frequency of 80 Hz, pulse duration of 400 μs, and an intensity adjusted to the maximum tolerance level, was used during electrical stimulation (21, 24). The stimulator recorded the individual intensity and treatment volume during all sessions, and the values were stored on a computer after each intervention. At the end of the 4-week treatment period, maximal isometric knee extensor torque was measured to identify the training intensity (i.e. the level of torque evoked through NMES, expressed as a fraction of the maximal voluntary contraction (MVC)). The mean percentage torque evoked through electrical stimulation was 40% of the pre-intervention maximal torque. To increase the treatment volume, the total stimulation time was gradually increased and the between-contractions rest time was decreased (24).
The combined treatment was administered twice a week with at least 48 h between each session over an 8-week period. The participants received LLLT prior to electrical stimulation using the same parameters used for the isolated NMES and LLLT groups.
Assessment protocol
The outcome measures were muscle strength, muscle morphology (muscle thickness and anatomical cross-sectional area), muscle electrical activity and health status (Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain, stiffness, physical function scales).
Maximal isometric knee extensor torque was measured using a Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Shirley, New York, USA) to assess the effects of the interventions on muscle strength. The participants were positioned on the dynamometer according to the manufacturer’s recommendations for knee evaluations, with a fixed hip angle of 85°. The trunk, hips and thighs of each patient were firmly strapped to the apparatus. The subjects performed a warm-up protocol comprising 3 sets of 15 knee extension/flexion concentric repetitions at an angular velocity of 90°/s with a submaximal effort level. After warm-up, each subject executed 3 maximal isometric knee extensor contractions at a fixed 90° knee flexion angle (0°= full extension). Each contraction lasted 5 s, and a 2 min interval was observed between consecutive contractions. The peak torque values from each contraction were assessed during data collection, and an additional maximal knee extensor contraction was performed when torque variation was higher than 10% between the first 3 tests.
Muscle morphologic parameters were assessed using an ultrasound system (SSD 4000, 51 Hz, ALOKA Inc., Japan) with a linear array probe (60 mm, 7.5 MHz). The subjects were evaluated at rest in the supine position with the knees and thighs fully extended (25, 26). A researcher highly experienced in ultrasound measurements, blinded to the data analysis, performed all ultrasound measurements.
The distance between the deep and superficial aponeuroses was measured at 5 different points in each longitudinal ultrasound image, and the mean value was used as the mean muscle thickness (MT) of that ultrasound image. Three ultrasound images were captured using a probe positioned parallel to the direction of the muscle fibres (25, 27, 28). The midway point between the greater trochanter and lateral femur condyle was used as a reference point for rectus femoris (RF) and vastus lateralis (VL) assessment, whereas vastus medialis (VM) measurements were obtained at 25–30% of this distance according to the characteristics of the subject. The mean values were obtained from 3 ultrasound images as the MT for each muscle. The MT values from the VL, RF and VM muscles were summed (ΣMT), representing the quadriceps femoris muscle mass (27).
The RF anatomical cross-sectional area (ACSARF) was also obtained using an ultrasound technique highly correlated with magnetic resonance imaging measurements (28). Three transversal images were obtained at 50% of the distance between the greater trochanter and lateral femur condyle. Five measures of the RF area were obtained in each transversal ultrasound image, and the mean value between the 3 ultrasound images was determined as the ACSARF. A single researcher, blinded to group allocation and data acquisition, analysed the muscle morphological parameters using Image-J software (National Institute of Health, USA) according to previously described validated procedures (25, 26). These 2 parameters (ΣMT and ACSARF) were used to assess the morphological effects of the interventions.
The electrical activity of the RF, VL and VM muscles was registered during maximal isometric knee extensor tests using an 8-channel electromyography (EMG) system (AMT-8, Bortec Biomedical Ltd, Calgary, Alberta, Canada) connected to a Windaq data acquisition system (Dataq Instruments Inc., Akron, Ohio, USA) and synchronized using a dynamometer. Skin preparation and electrode positioning for EMG evaluation were performed according to standard procedures (29). Raw EMG signals were digitized using a sampling frequency of 2,000 Hz per channel with a DI-720 16-bits analogue-to-digital board (Dataq Instruments Inc., Akron, Ohio, USA) and stored for subsequent analysis. Passive electrodes (Meditrace 100, Kendall, Florida, USA) were positioned in bipolar configuration (inter-electrode distance: 2.2 cm) on the RF (50% on the line from the anterior superior iliac spine to the superior part of the patella), VL (66% on the line from the anterior superior iliac spine to the lateral side of the patella) and VM (80% on the line from the anterior superior iliac spine and the joint space in front of the anterior border of the medial ligament) muscles. Maps on overhead transparency films were developed using anatomical reference points (i.e. border of patella) and skin marks (i.e. freckles and scars) to ensure similar electrode positioning in all evaluations (27). A reference electrode was fixed onto the medial surface of the tibia. The data were exported to SAD32 software (SAD32; version 2.61.07, 2002) and filtered using a Butterworth band-pass filter with cut-off frequencies of 20 and 500 Hz. The root mean square (RMS) values were calculated from 1 s segments of the EMG signals synchronized with the knee extensor peak torque. The sum of the highest RMS values for the RF, VL and VM in each isometric test (ΣRMS) was used for statistical analysis, representing a large portion of the quadriceps femoris muscle activation (25).
The WOMAC was used to determine the health status of the OA participants both before and after interventions. The WOMAC comprises 24 items that are divided into 3 subscales (pain, stiffness, and physical function) and were measured according to the Likert scale; the scores ranged from 0 = none to 4 = extreme (30). Higher WOMAC scores indicate worse pain, stiffness, and functional limitations.
Statistics
Using torque as the main outcome variable and estimating the minimum difference equivalent to a standard deviation (SD) of 30 Nm and α = 0.05, a sample size of 14 subjects per group achieved a calculated power of 0.80 (WinPepi 1.45 for Windows) and was used in the study. The t-test for independent samples was used to verify the differences between the pre-control and pre-intervention tests.
To determine the effects of the interventions on the neuromuscular variables, 2-way (group × time points) repeated measure analyses of covariance (ANCOVA) were conducted using the initial values as covariates. Significant effects and interactions were further examined using Bonferroni’s post-hoc analysis. The percentage variation between pre- and post-intervention (the difference between the pre- and post-intervention scores divided by the pre-intervention score) was compared between the groups using 1-way ANOVA, followed by Bonferroni’s post-hoc test.
To determine the effects of the interventions on health status, the Friedman test was used to verify differences between the pre- and post-intervention tests. The Kruskal-Wallis test was used to verify the between-groups differences after 8 weeks of intervention. The significance level was set to α < 0.05 for all statistical analyses. Neuromuscular results are expressed as the means and SD, and health status is expressed as the median and interquartile range (IQR) in the tables. The percentage changes for all variables are expressed as means ± standard error (SE).
RESULTS
Anthropometric characteristics
Forty-four elderly women completed the full protocol (NMES group = mean age 69.3 years (SD 5.5), mean height 1.52 m (SD 0.10), mean total body mass 77.5 kg (SD 13.7); LLLT group = mean age 67.7 years (SD 4.7), mean height 1.59 m (SD 0.10), mean total body mass 74.7 kg (SD 11.7); and combined group = mean age 69.6 years (SD 4.7), mean height 1.55 m (SD 0.15), mean total body mass 70.9 kg (SD 8.9)). No significant between-groups differences were observed after the pre-control anthropometric assessment (p > 0.05).
Muscle torque
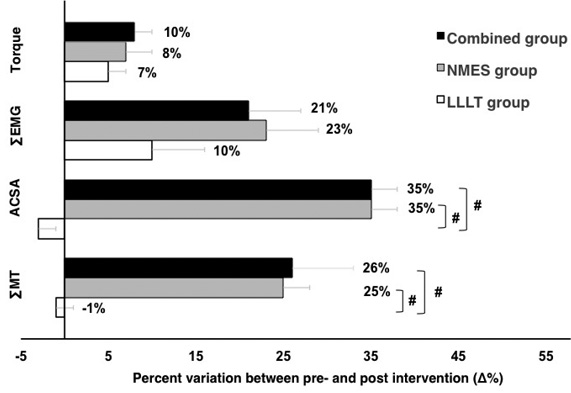
There were no significant differences between the pre-control and pre-intervention tests for all experimental groups (NMES group: p = 0.70; combined group: p = 0.70; and LLLT group: p = 0.48). All experimental groups showed increases in torque after 8 weeks of intervention (p = 0.02, Table I). No between-groups differences were observed for the torque percentage change values after 8 weeks of intervention (p = 0.65) (Fig. 2).
Muscle electrical activity. There were no significant differences for the maximal muscle activation between the pre-control and pre-intervention tests for all experimental groups (NMES group: p = 0.18; LLLT group: p = 0.92; and combined group: p = 0.13, Table I). All experimental groups showed increased EMG activity after 8 weeks of intervention (p = 0.01, Table I). No between-groups differences were observed for the ΣRMS percentage values after 8 weeks of intervention (p = 0.14) (Fig. 2).
|
Table I. Electrical activation, muscle thickness, cross-sectional anatomical area and torque during the study evaluation times |
||||
|
|
|
Pre-control Mean (SD) |
Pre-intervention Mean (SD) |
Post-intervention Mean (SD) |
|
∑RMS (mV) |
LLLT |
0.36 (0.17) |
0.39 (0.18) |
0.42 (0.18)* |
|
NMES |
0.35 (0.12) |
0.35 (0.12) |
0.43 (0.13)* |
|
|
Combined |
0.40 (0.11) |
0.41 (0.15) |
0.50 (0.17)* |
|
|
ACSARF (cm2) |
LLLT |
2.18 (0.87) |
2.25 (0.94) |
2.19 (0.75) |
|
NMES |
2.84 (1.00) |
2.78 (1.00) |
3.37 (1.30)*,# |
|
|
Combined |
2.30 (0.74) |
2.21 (0.76) |
2.99 (1.00)*,# |
|
|
∑MT (cm) |
LLLT |
3.71 (0.89) |
3.94 (0.87) |
3.89 (0.88) |
|
NMES |
3.89 (0.81) |
3.92 (0.70) |
4.90 (0.93)*,# |
|
|
Combined |
3.89 (0.82) |
3.87 (0.86) |
4.75 (0.71)*,# |
|
|
Torque (Nm) |
LLLT |
97.33 (33.07) |
94.90 (28.73) |
99.52 (29.64)* |
|
NMES |
102.76 (21.87) |
102.61 (24.23) |
109.77 (22.55)* |
|
|
Combined |
88.62 (24.11) |
90.16 (24.03) |
97.05 (22.73)* |
|
|
*Significant difference between pre- and post-intervention. #Significant difference compared with LLLT group in post-intervention (p < 0.05). NMES: neuromuscular electrical stimulation; LLLT: low-level laser therapy; Combined: NMES plus LLLT; ACSARF: rectus femoris anatomical cross-sectional area; ΣMT: sum of muscle thickness; ΣRMS: sum of root mean square values. |
||||
Muscle thickness and anatomical cross-sectional area. No significant differences were observed for the morphological parameters between the pre-control and pre-intervention tests for the NMES group (ACSARF: p = 0.25; ∑MT: p = 0.85), for the combined group (ACSARF: p = 0.68; ∑MT: p = 0.80) or for the LLLT group (ACSARF: p = 0.62; ∑MT: p = 0.60) (Table I).
A significant time×group interaction was observed for the morphological parameters (ACSARF: p = 0.001; ∑MT: p = 0.001). Only the NMES (ACSARF: p < 0.001; ∑MT: p = 0.05) and combined (ACSARF: p = 0.001; ∑MT: p = 0.03) groups showed a muscle mass increase after 8 weeks of intervention (Table I). When the groups were compared post-intervention, the NMES (ACSARF: p = 001; ∑MT: p < 0.001) and combined (ACSARF: p = 0.011; ∑MT: p < 0.001) groups showed higher morphological values compared with the LLLT group. The NMES and combined groups showed the highest increases in the percentage values for the morphological parameters after 8 weeks of intervention compared with the LLLT group (p < 0.001, Fig. 2).
Health status
All groups showed improvements in WOMAC scores following the intervention period (NMES group (pain: p < 0.001; stiffness: p = 0.004; physical function: p = 0.02); LLLT group (pain: p < 0.001; stiffness: p = 0.02; physical function: p = 0.001) and combined group (pain: p = 0.013; stiffness: p = 0.003; physical function: p = 0.001)). No between-groups differences for health status scores were observed during the post-intervention period (p > 0.05) (Table II).
|
Table II. Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores (pain, stiffness and functional limitation) pre- and post-intervention (median (interquartile range; IQR)) |
||||
|
|
Pain Median (IQR) |
Stiffness Median (IQR) |
Functional limitation Median (IQR) |
|
|
LLLT |
Pre |
9 (6–12) |
4 (3–6) |
32 (15–39) |
|
Post |
4 (3–7)* |
2 (1–3)** |
19 (11–25)*** |
|
|
Δ% |
49 |
53 |
26 |
|
|
NMES |
Pre |
9 (7–10) |
4 (2–5) |
26 (18–38) |
|
Post |
3 (2–5)** |
2 (0–3)*** |
11 (9–15)** |
|
|
Δ% |
52 |
53 |
49 |
|
|
Combined |
Pre |
8 (6–11) |
4 (2–5) |
34 (25–36) |
|
Post |
3 (2–5)*** |
2 (1–2)** |
14 (6–21)** |
|
|
Δ% |
51 |
46 |
53 |
|
|
Significant differences between pre- and post-intervention: *p < 0.001, **p < 0.01, ***p < 0.05. Δ%: difference between pre- and post-intervention scores divided by the pre-intervention score; NMES: neuromuscular electrical stimulation; LLLT: low-level laser therapy; Combined: NMES plus LLLT; IQR: interquartile range. |
||||
DISCUSSION
The main findings of this study were: (i) NMES, LLLT and combined treatments improved the strength, and electrical activity of the quadriceps and health status of patients with OA; (ii) only treatments involving NMES (alone or in combination with LLLT) resulted in increased muscle mass; and (iii) the combination treatment did not increase the effects on the evaluated parameters compared with those of the NMES treatment alone.
The percentage increase in torque obtained through high-intensity NMES is consistent with previous findings of studies using similar stimulation intensities (21, 31). However, a lack of increase in knee extensor torque after 6 weeks of NMES treatment, with a stimulation intensity of approximately 35% MVC in elderly patients with grade 3–4 knee OA (K-L scores), has also been reported (20). The relationship between the weakness level and NMES dosage (intensity and volume of stimulation) might explain these contradictory results. Similar to the present study, Talbot et al. (31) and Vaz et al. (21) reported increases in the torque of 9% and 8%, respectively, in elderly patients with grade 2–3 knee OA (K-L scores). Talbot et al. (31) used progressive increases in NMES intensity (10–40% of MVC) for 12 weeks, and Vaz et al. (21) adopted progressive increases in not only the stimulation intensity but also the treatment stimulation volume for 8 weeks. In the present study, we used a NMES protocol similar to that of Vaz et al. (31), with a progressive increase in the stimulation volume and intensity equal to or greater than 40% of the MVC, and the results showed torque increases of approximately 7%. In practical terms, these data indicate that when the objective of the treatment is promoting muscle strength, the protocol duration must be equal to or greater than 8 weeks, the stimulation intensity should be equal or greater than 40% of the MVC, and the mechanical load should be increased progressively through an increase in the stimulus intensity and treatment volume. The second clinically relevant result obtained after comparing the different methodologies between these studies is that patients with grade 3–4 knee OA might not benefit from NMES, potentially reflecting the high levels of pain (and likely muscle inhibition) that these patients experience with high degrees of the degenerative disease.
Review studies have reported that increases in muscular strength during the NMES treatment reflect neural mechanisms without consistent evidence for hypertrophy (32). The findings of the present study confirm the results of other recent studies showing that NMES promotes quadriceps muscular mass increments in elderly patients with symptomatic OA (20, 21). Furthermore, the present study showed that NMES increased the electrical activity of the quadriceps muscle. Studies performed with healthy adults reported increases in the electrical activity registered through EMG during MVC tests after 4 (33) and 8 weeks (34) of NMES treatment. The increases in the EMG signal analysed during these experimental periods have been associated with increased motor unit recruitment (33, 34) and can be considered indicative of neural adaptation. Although the exact mechanisms for this EMG signal increase (or for this neural adaptation) are not completely understood, a few hypotheses have been developed. Muscle hypertrophy might increase the fibre ACSA, thereby increasing the EMG signal amplitude (and the force) produced from these fibres. NMES could also facilitate neural transmission in both afferent and efferent directions, potentially changing the neural drive to these muscles and increasing EMG activity. In addition to the force increase, NMES also increases the daily life activities of these patients, which might in turn decrease the amount of subcutaneous fat tissue and result in an increase in the EMG signal amplitude through a decrease in the distance between the muscle fibres and the EMG electrodes at the skin surface. However, these hypotheses require further examination.
The percentage change in the morphological parameters (ΣMT = 35%; ACSARF = 25–26%) was higher than that reported in previous studies (20, 21). Bruce-Brand et al. (20) observed a 5.4% increase in the muscle cross-sectional area evaluated through magnetic resonance imaging (MRI) using a biphasic symmetrical pulsed current with a 50 Hz stimulation frequency, 100–400 µs pulse duration and the maximal tolerance intensity. Using NMES parameters (pulse duration and stimulation frequency) identical to the those of the present study, but with stimulation intensity not expressed as a percentage of the MVC (subject’s maximally tolerated stimulation intensity), Vaz et al. (21) reported a 13% increase in the vastus lateralis MT, measured using ultrasonography, after 8 weeks of NMES treatment. The superior increments in the hypertrophy observed in participants of this study probably reflect the NMES dosage (volume × intensity). Although the volume used in the present study was the same as that used in the Vaz study, the higher intensity used in the present study (40% MVC) likely contributed to the main differences between these 2 studies. These findings suggest that when the aim of the treatment is to reduce muscle atrophy, clinicians should use a progressive increase in the NMES treatment volume to achieve force levels of at least 40% MVC.
Improvements in the WOMAC scores following intervention confirm the results reported from other studies with LLLT (8, 13) or NMES (20, 21, 31) treatments. However, the present study shows a new finding: LLLT increased the torque and electrical activation of the knee extensor muscles. Considering the well-known analgesic effect of LLLT (8, 9), pain relief probably leads to a more complete muscle activation through a reduction in efferent information from nociceptors (6, 8, 9). This observation is supported by improvements in functioning (Table I) and increases in knee extensor EMG activity (Table I, Fig. 2) in the LLLT group. However, considering that the signal cancellation observed before and after evaluation could substantially undermine the interpretation of the changes in EMG activity after intervention (19), the effects of LLLT on muscle activation should be evaluated using the twitch interpolation technique to confirm or refute this hypothesis.
Although placebo effects could have occurred, the positive findings about the LLLT effects are clinically important, as these data show evidence that LLLT postpones the degenerative process via muscle inhibition through a reduction in pain and inflammation at the initial stages of the disease (2, 3). Further studies should evaluate the effects of LLLT on cartilage structure and investigate the potential correlation of these effects with the clinical findings of increased muscular activation and improved functioning.
The hypothesis that LLLT associated with NMES could generate additional effects on neuromuscular and functional parameters was not confirmed in the present study. It is notable that additional clinical effects (regenerative and anti-inflammatory effects), which were not evaluated in this study, might have been exclusively experienced in the combined group. However, this hypothesis also requires further evaluation.
The sample size used in the present study might have been insufficient to determine differences in the variables, as the observed power was determined a priori based on a large effect reported in previous studies evaluating the NMES effects alone on the selected primary variable. Moreover, participants were not prevented from using analgesic or anti-inflammatory drugs, and these drugs might have masked the actual effects of LLLT. Therefore, future studies should increase the sample size and control the use of drugs.
Moreover, the absence of a control or sham group might be considered as a further study limitation. To minimize this limitation, we used a control period, during which no intervention was used, to evaluate the natural variance expected without intervention. In this sense, during the control period, when no intervention was performed, no effect was observed within the experimental groups, showing that the structural and functional effects observed post-intervention reflected the interventions used and were not due to simple coincidence.
In conclusion, NMES with progressive volume and intensity results in positive effects on health status and increased quadriceps strength and muscle mass. The combination of NMES and LLLT does not have any additional effects on functioning or neuromuscular parameters.
Acknowledgements
This work was supported by the Financiadora deEstudos e Projetos Ministério da Ciência e Tecnologia – FINEP [105074400], Fundação de Amparo e Ensino. Pesquisa – PAPERGS [1015272] and ConselhoNacional de Desenvolvimento Científico e Tecnológico – CNPq [304039/2012-8].
REFERENCES
