Anne Hokstad, MD1,2, Bent Indredavik, MD, PhD1,2, Julie Bernhardt, PhD3,4, Birgitta Langhammer, PhD1,5, Mari Gunnes MSc1, Christine Lundemo MSc1, Martina Reiten Bovim1 and Torunn Askim, PhD1,6
From the 1Department of Neuroscience, Faculty of Medicine, Norwegian University of Science and Technology, 2The Stroke Unit, Department of Medicine, St Olavs Hospital, University Hospital of Trondheim, Trondheim, Norway, 3Stroke Division, Florey Institute of Neuroscience and Mental Health, 4Faculty of Health Sciences, La Trobe University, Melbourne, Victoria, Australia, 5Physiotherapy Programme, Faculty of Health Science, Oslo and Akershus University College of Applied Sciences, Oslo and 6Department of Physiotherapy, Faculty of Health Education and Social Work, Sør-Trøndelag University Collage, Trondheim, Norway
OBJECTIVE: To assess the amount of early upright activity of patients managed in Norwegian stroke units and its association with functional outcome and health-related quality of life 3 months later.
DESIGN: A prospective observational multi-centre study.
SUBJECTS: A total of 390 acute stroke patients, mean age 76.8 years, 48.1% men, less than 14 days post-stroke, recruited from 11 Norwegian stroke units.
METHODS: Time spent in different activity categories (in bed, sitting out of bed, upright) was observed with a standard method. Outcome was assessed by modified Rankin Scale (mRS), and health-related quality of life by EuroQol-5 Dimension 5 level (EQ-5D-5L) 3 months later. Ordinal logistic and linear regression analyses were used to examine the association between activity categories and mRS and EQ-5D-5L, respectively. Age, National Institute of Health Stroke Scale (NIHSS) score, premorbid mRS, sex, and hospital-site were added as covariates.
RESULTS: The odds ratio (OR) (95% confidence interval (CI)) for poorer functional outcome (higher mRS) decreased as time spent in upright activities increased (OR 0.97 (95% CI 0.94–1.00)). There was also a significant positive association between time in upright activity and higher EQ-5D-5L, Beta 0.184 (95% CI 0.001– 0.008) 3 months later.
CONCLUSION: This study confirms the beneficial effect of upright activity applied during hospital stay in Norwegian stroke units.
Key words: stroke; rehabilitation; physical activity; outcome assessment; health-related quality of life.
J Rehabil Med 2016; 48: 280–286
Correspondence address: Torunn Askim, Department of Neuroscience, NTNU, Faculty of Medicine, Postbox 8905, NO-7491 Trondheim, Norway. E-mail: torunn.askim@ntnu.no
Accepted Nov 10, 2015; Epub ahead of print Feb 4, 2016
INTRODUCTION
Stroke is the second most frequent cause of death and a major cause of disability in adults worldwide. Up to half of stroke survivors are dependent in activities of daily living 3 months post-stroke (1, 2). Stroke patients also rate their health-related quality of life (HRQoL) lower than healthy people of the same age and people with other medical diseases (3).
Stroke unit care has shown to be the most powerful, broadly applicable treatment after acute stroke, reducing death and dependency (4). Early mobilization with out-of-bed activities, such as sitting, standing and walking, has been regarded as an important contribution to the short- and long-term effects of stroke unit care (5, 6), and is now recommended in most national guidelines for stroke care across Western Europe, Australia and North America (7). However, recently, a worldwide study of early mobilization (AVERT) demonstrated that too much out-of-bed activity within the first few days after onset of stroke may impair the recovery process (8).
Even though most guidelines recommend mobilization within 24 h, only in the Australian and Norwegian guidelines has mobilization been defined as out-of-bed activity (7, 9). Despite these recommendations, several observational studies in these countries have shown that less than 60% of patients are mobilized out of bed within 24 h after stroke onset (10, 11), indicating that guidelines alone do not change practice and that clinical practice reflects the healthcare providers’ expertise, the patients’ values and expectations, as well as process and pragmatic factors.
It is becoming increasingly apparent that the timing of first mobilization may be less important than the total amount and frequency of early out-of-bed activity during hospital stay (12); however, timing of first mobilization also probably acts as a proxy for the organization of post-stroke rehabilitation care in the acute setting (7). The first 2 weeks after stroke continues to be a period of great interest in recovery research, as pre-clinical studies suggest it may be a critical time-window for promoting recovery (13). Recent studies examining time spent in upright activity (defined as standing, walking, climbing stairs and all other activities, including transfer, with the feet on floor), measured on a single day within the first 2 weeks after onset of stroke have shown significant variation between hospitals (14, 15). To more fully understand the impact of this variation in clinical practice, the association between the amount of early upright activity after stroke and outcome should be more thoroughly assessed.
The overall aim of the present study was to assess the association between the timing and amount of upright activity applied in clinical practice in patients admitted to multiple Norwegian stroke units and degree of disability and HRQoL 3 months later.
We hypothesized that a higher amount of early upright activity and shorter time to first mobilization would be associated with increased probability of good functional outcome and improved HRQoL at the 3-month follow-up.
MATERIAL AND METHODS
Study design and setting
This was a prospective cohort study recruiting patients from 11 Norwegian stroke units. Motor activity was registered within the first 2 weeks of hospital stay and functional outcome was measured 3 months later.
The participating hospitals were located in Central Norway (n = 8), in Northern Norway (n = 1) and in South-East Norway (n = 2). Two of the hospitals were university Hospitals, 2 were small, treating fewer than 100 patients per year, and 7 middle-sized treating between 100 and 400 stroke patients per year.
Participants
Patients were eligible if they were diagnosed with acute stroke within the previous 14 days, age > 18 years, Norwegian speaking, and not receiving palliative care. Patients were excluded if they were likely to be discharged from hospital with less than 5 h of observation.
Informed consent was obtained from those who were able to agree, and for those not able to consent the next of kin gave verbal consent to participate. This is in keeping with Norwegian consent procedures for patients who are unable to consent.
The Regional Committee for Medical and Health Research Ethics in Central Norway approved the study and storage of data on behalf of all participating hospitals (REC number 2011/1428).
Observation
Every second week each hospital was visited if the hospitals had 2 or more eligible patients. Four well-trained observers travelled and performed all observations in the study. The training of the observers included assessment of agreement and the training continued until agreement was excellent.
For observation, the behavioural mapping method was used (16). Observations were conducted every 10 min from 08.00 h to 17.00 h on a single day. However, due to long travelling distances, some of the observations were undertaken across 2 consecutive days, but covering the same hours. At each time-point, the observer recorded patient activity, who was attending the patient, and the patient’s location. When patients were out of view (e.g. in the bathroom or off-ward), activity was acquired retrospectively, by questioning the patient or the caregiver, or from a separate activity form completed by the physiotherapist or the occupational therapist during off-ward treatment. They were marked as not observed if it was not possible to retrieve the data. The patients were observed for approximately 1 min at each time-point.
Categories of motor activity
At each observation, 12 prescribed activities were recorded: (i) no active motor supine; (ii) no active motor on left side; (iii) no active motor on right side; (iv) sit support in bed; (v) sit support out of bed; (vi) transfer with hoist; (vii) roll and sit up; (viii) sit with NO support; (ix) transfer with feet on floor; (x) standing; (xi) walking; and (xii) stairs. For analyses, 3 main activity categories were explored: in bed (activities 1–4), sitting out of bed (activities 5–8), and all other activities with the feet on the floor were defined as upright activity (activities 9–12) (10).
Commencement of mobilization
The time to the first mobilization out of bed from hospital admission was registered prospectively.
Baseline assessment
Demographic information, including age, sex premorbid function by modified Rankin Scale (mRS) (17), premorbid living conditions, stroke severity obtained by National Institute of Health Stroke Scale (NIHSS) (18), stroke type (infarction or haemorrhage), and mRS at inclusion were recorded.
Outcome assessment 3 months post-stroke
Degree of disability was obtained by mRS, with scores ranging from 0 (no sign or symptoms) to 6 (death). The assessment was performed as a structured interview, either face to face or by phone, with a trained assessor. Phone assessment is shown to be a reliable method to determine mRS (19, 20). For those not able to answer, healthcare providers were used as proxies or data were derived from the hospital records.
HRQoL was assessed by the European Quality of Life-5 Dimension-5 Level (EQ-5D-5L) instrument (21). EQ-5D-5L is a generic HRQoL measure consisting of 5 specific questions regarding mobility, self-care, pain/discomfort, usual activities and anxiety/depression and a visual analogue scale (EQ-VAS) where the patients demonstrate their general health state, with the worst imaginable health scored as 0 and the best imaginable health as 100. The 5 levels of answer categories, range from no problem in the given dimension (level 1: e.g. “I have no problems in walking about”) to worse outcome (level 5: e.g. “I am unable to walk about”). The 5 dimensions constitute a health profile, which can be transformed into an index value, with range from –0.6 (worse health outcome) to 1.0 (best outcome). To obtain the EQ-index values we used the Danish interim EQ-5D-5L value set. EQ-5D-5L is available for telephone interview and has been shown to have better measurement properties in different chronic conditions including stroke, than the previous EQ-5D-3L (22, 23).
Data processing and analysis
The highest level of activity in every 10-min interval was recorded in the database (Microsoft Access 2007). The recorded activity levels were put into 1 of the 3 pre-defined activity categories, and the proportion of time spent in each category was calculated.
Statistical analyses were conducted using IBM SPSS version 21 and the gologit2 program in Stata version 12.
Descriptive statistics were used to report the mean and proportion of baseline variables, mean time in motor activity and the distribution of the mRS score and the EQ-5D-5L at 3 months follow-up. t-test statistics and Mann-Whitney U test were used to compare mean and median between the subgroups answering and not answering EQ-5D-5L at follow-up.
Missing activity data was imputed as sitting out of bed if 1–2 observations were missing because the patient was in the bathroom. If more than 2 observations were missing it was maintained as not observed. Missing activity data because of computed tomography (CT)/ magnetic resonance (MR) scan or ultrasound of heart and blood-vessels were also imputed as in bed activity. All other “not observed” were categorized as missing. For patients not mobilized at all, time to first mobilization was imputed as the time from admission to the time at the end of the observation.
To determine which variable was the strongest predictor for functional outcome (mRS score at 3 months) among a set of possibly correlated variables (the motor activity data) the proportional odds model, recommended by the OAST collaboration was used (24). In the proportional odds model the odds ratios (ORs) are assumed to be equivalent across all mRS-cut-points (e.g. 0 vs 1–6, then 0–1 vs 2–6, and so on). This is a straight-forward generalization of the logistic regression model. The “Brant test” was used to analyse whether this assumption was fulfilled (24).
To determine the strongest predictor for good HRQoL (EQ-5D-5L), a linear regression model was used because the standardized residuals of EQ-VAS and EQ-index were normally distributed except for a few outliers of EQ index-value.
The independent variables of interest were: (i) time spent in bed, (ii) time spent sitting out of bed, (iii) time spent in upright activity, and (iv) time from admission to first mobilization. In addition, a set of important predictors were added as covariates. Age was added because younger patients are shown to have better outcomes (25), NIHSS score was added because severe initial neurological impairment is shown to be associated with death and disability (26), pre-stroke mRS was added because pre-stroke disability is shown to be associated with poorer outcomes (1), and sex was added, even though the association with outcome is unclear (1). Finally, hospital site was added as a covariate to adjust for any possible hospital effects. The independent variables were tested in both a simple and a comprehensive multivariable model. In the simple model each independent variable was evaluated 1 at a time. In the comprehensive model time in bed and time upright were entered simultaneously and the third category (time sitting out of bed) was kept out of the analysis because it is co-dependent on the other 2 activity categories. This means that changes in 1 activity category keeping the second category constant was at the expense of sitting out of bed, which was not added to the model. Time to first mobilization was also entered in the comprehensive model.
RESULTS
The study was performed between December 2011 and September 2013. A total of 547 patients were screened for inclusion. Fig. 1 shows the flow of patients through the study. A total of 390 patients were available for the analysis of mRS at 3 months, while 262 patients were available for analysis of EQ-5D-5L or EQ-VAS at 3 months. Out of these patients 261 answered the EQ-5D-5L and 247 answered the EQ-VAS. The main reasons for missing EQ-5D-5L scores were death (n = 39) or severe cognitive impairments or illness (n = 73), while 16 patients were lost to follow-up. The 14 patients who responded to the EQ-5D-5L, but not the EQ-VAS, reported problems in dealing with the VAS scale.

Fig. 1. Patients screened for inclusion and reason for drop out. EQ-5D-5L: European Quality of Life–5 Dimensions–5 Levels; EQ-VAS: European Quality of Life – 5 Dimensions – 5 Levels VAS scale score.
Seven patients were not mobilized out of bed because of severe strokes and unstable clinical condition.
The NIHSS score and, age at inclusion, in addition to median (interquartile range; IQR) mRS score at 3 months, differed significantly between those responding to the EQ-5D-5L (n = 262) and the stroke survivors not responding (n = 89). The mean (SD) differences between the 2 groups were 5.0 (5.0) points vs 12.3 (8.5) points, p < 0.000, on NIHSS, 74.6 (11.5) years vs 79.3 (9.0) years, p < 0.0003, on age and median (IQR) 3.0 (2.0–3.0) points vs 5.0 (4.0–5.0) points, p < 0.000, for mRS, respectively.
Table I shows the baseline characteristics of the included patients, while the mean (SD) and median (IQR) percentage of daytime spent in different motor activity levels are presented in Table II. The results showed that 266 (76.7%) of all patients were mobilized within 24 h of admission.
|
Table I. Baseline characteristics of patients n = 390 |
|
|
Patients’ characteristics |
|
|
Age, years, mean (SD), median (range) |
76.8 (11.3) 79.0 (30–100) |
|
Male, n (%) |
189 (48.1) |
|
First-ever stroke, n (%) |
284 (72.3) |
|
Time since stroke, days, mean (SD), median (range) |
5.1 (2.8) 5 (1–14) |
|
NIHSS score, mean (SD), median (range) |
7.9 (7.7) 5 (0–34) |
|
Severity groups, n (%) |
|
|
Mild stroke (NIHSS < 8) |
249 (63.8) |
|
Moderate stroke (NIHSS 8–16) |
76 (19.5) |
|
Severe stroke (NIHSS > 16) |
65 (16.7) |
|
Stroke classification, n (%) |
|
|
Infarction |
334 (85.6) |
|
Haemorrhage |
56 (14.4) |
|
SD: standard deviation; NIHSS: National Institute of Health Stroke Scale. |
|
|
Table II. Time spent in different motor activities as a percentage of the day and time from admission to first mobilization (n = 390) |
||
|
Motor activity category |
Mean (SD) |
Median (IQR) |
|
Time spent in upright, % of day |
8.3 (8.8) |
5.5 (1.8–12.7) |
|
Time spent sitting out of bed, % of day |
43.3 (22.0) |
44.5 (27.3–58.6) |
|
Time spent in bed, % of day |
44.1 (26.7) |
41.8 (23.6–61.8) |
|
Not observed, % of day |
4.3 (7.4) |
0.0 (0.0–5.5) |
|
Time from admission to first mobilization, h |
21.0 (31.9) |
9.0 (2.5–22.3) |
|
SD: standard deviation; IQR: interquartile range. |
||
Fig. 2 shows that the number (%) of patients classified with mRS ≤ 2 (independent) increased from 76 (19.4%) at inclusion to 138 (35.4%) at 3 months follow-up. A total of 39 (10.0%) patients died during follow-up. Table III shows the distribution of EQ-5D-5L dimension responses at the 3-month follow-up. The number (%) of patients reporting moderate to extreme problems within the different domain was 77 (29.5%) for mobility, 50 (19.2%) for self-care, while 91 (34.9%) patients reported moderate to extreme problems within the domain of usual activities. For the domains pain/discomfort and anxiety/depression the corresponding numbers were 59 (22.6%) and 45 (17.2%), respectively, while the mean (SD) EQ-index and EQ-VAS score were 0.72 (0.25) and 60.0 (20.8), respectively.
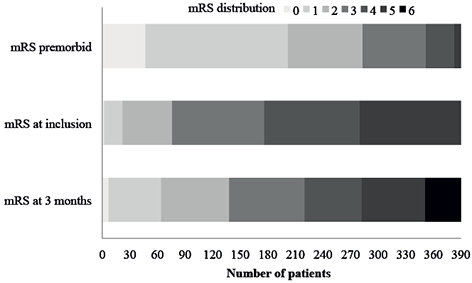
Fig. 2. Distribution of modified Rankin Scale (mRS) at different time-points.
|
Table III. Distribution of EQ-5D-5L dimension responses at 3-month follow-up (n = 261) |
|||||
|
Level |
Mobility n (%) |
Self-care n (%) |
Usual activities n (%) |
Pain/discomfort n (%) |
Anxiety/depression n (%) |
|
1 |
107 (41.0) |
164 (62.8) |
106 (40.6) |
140 (53.6) |
161 (61.7) |
|
2 |
77 (29.5) |
47 (18.0) |
64 (24.5) |
61 (23.4) |
55 (21.1) |
|
3 |
33 (12.6) |
18 (6.9) |
39 (14.9) |
31 (11.9) |
32 (12.3) |
|
4 |
30 (11.5) |
24 (9.2) |
27 (10.3) |
26 (10.0) |
9 (3.4) |
|
5 |
14 (5.4) |
8 (3.1) |
25 (9.6) |
2 (0.8) |
4 (1.5) |
|
EQ-5D-5L: European Quality of Life–5 Dimensions–5 Levels; Level 1: indicating no problem; Level 2: indicating slight problems; Level 3: indicating moderate problems; Level 4: indicating severe problems; Level 5: indicating extreme problems. |
|||||
Associations with outcome at 3-month follow-up
The partial proportional odds assumption was fulfilled for all independent variables, as the Brant’s test was not significant.
In the simple model, assessing one independent variable at a time adjusted for the covariates (NIHSS score, age, sex, pre-stroke mRS and hospital-site) the OR for poorer functional outcome (e.g. higher mRS score) was 0.96 (95% confidence interval (95% CI) 0.94–0.99, p = 0.010) as time in upright activity increased. The linear regression analysis for EQ-5D-5L showed that more time in upright activity was associated with an increase in EQ-index score, Beta 0.178 (95% CI 0.067–0.289, p = 0.002) and EQ-VAS score Beta 0.185 (95% CI 0.060–0.307, p = 0.004). Despite a significant association between increased time in bed and a decline in EQ-VAS, Beta –0.140 (95% CI –0.261 to – 0.018, p = 0.024), there were no other significant associations between time sitting out of bed, time in bed or time to first mobilization and outcome (Table IV).
In the comprehensive model, which included 2 activity categories and time to first mobilization, adjusted for the covariates, the odds for poorer functional outcome decreased as time spent upright increased, OR 0.97 (95% CI: 0.94–1.00, p = 0.048). The comprehensive linear regression model also showed a significant positive association between time spent upright and EQ-index, Beta 0.184 (95% CI 0.055–0.312, p = 0.005) and EQ-VAS, Beta 0.153 (95% CI 0.008–0.296, p = 0.038) after adjusting for all covariates. The association between time to first mobilization and outcome was not significant in any analyses. The analysis included only those patients completing at 3 months (Table IV).
|
Table IV. Partial proportional odds model and linear regression analysis for the association between motor activity and outcome at 3-month follow-up |
|||||||||
|
Independent variables |
mRSc |
|
EQ-Indexd |
|
EQ-VASd |
||||
|
OR (95% CI) (n = 390) |
p-value |
|
Beta (95% CI) (n = 261) |
p-value |
|
Beta (95% CI) (n = 247) |
p-value |
||
|
Simple multivariate modela |
|||||||||
|
|
Time upright |
0.96 (0.94 to 0.99) |
0.010 |
|
0.178 (0.067 to 0.289) |
0.002 |
|
0.185 (0.060 to 0.307) |
0.004 |
|
|
Time sitting out of bed |
0.99 (0.98 to 1.00) |
0.221 |
|
–0.010 (–0.118 to 0.097) |
0.848 |
|
0.074 (–0.047 to 0.195) |
0.232 |
|
|
Time in bed |
1.01 (1.00 to 1.02) |
0.064 |
|
–0.075 (–0.183 to 0.034) |
0.176 |
|
–0.140 (–0.261 to –0.018) |
0.024 |
|
|
Time to first mobilization |
1.00 (0.99 to 1.01) |
0.985 |
|
–0.045 (–0.151 to 0.062) |
0.411 |
|
0.006 (–0.116 to 0.128) |
0.921 |
|
Complex multivariate modelb |
|||||||||
|
|
Time upright |
0.97 (0.94 to 1.00) |
0.048 |
|
0.184 (0.055 to 0.312) |
0.005 |
|
0.153 (0.008 to 0.294) |
0.038 |
|
|
Time in bed |
1.00 (0.99 to 1.01) |
0.480 |
|
0.018 (–0.107 to 0.142) |
0.778 |
|
–0.074 (–0.215 to 0.066) |
0.299 |
|
|
Time to first mobilization |
1.00 (0.99 to 1.01) |
0.898 |
|
–0.023 (–0.130 to 0.085) |
0.678 |
|
0.040 (–0.082 to 0.163) |
0.516 |
|
aIn the simple multivariable model each independent variable was evaluated 1 at a time. The analyses were adjusted for age, sex, pre-stroke function obtained by mRS, stroke severity obtained by National Institutes of Stroke Scale and hospital site. bIn the comprehensive multivariable model 2 independent variables were entered simultaneously. The analyses were adjusted for age, sex, pre-stroke function obtained by mRS, stroke severity obtained by National Institutes of Stroke Scale and hospital site. cPartial proportional odds model. dLinear regression analyses. OR: odds ratio; mRS: modified Rankin Scale; EQ-Index: European Quality of Life – 5 Dimensions – 5 Levels index score; EQ-VAS: European Quality of Life – 5 Dimensions – 5 Levels VAS scale score. |
|||||||||
DISCUSSION
This multi-site study of 390 acute stroke patients admitted to 11 Norwegian stroke units is currently the largest observational study assessing the association between upright activity measured on a single day during post-stroke hospital stay and outcome 3 months later. The main finding was a significant association between higher amount of early upright activity and good outcome, but no association was found between time to first mobilization and outcome 3 months later after adjusting for important predictors of activity and outcomes such as stroke severity, age, sex and pre-stroke function.
In the present study, patients were mobilized, in mean, 21 h after admission, 76.7% of the patients were mobilized within 24 h of admission, and 44% had little or no disability (mRS 0–2) 3 months post-stroke. Given the broad inclusion criteria for this study (all patients not receiving palliative care) this pattern of mobilization commencement probably reflects adaptations for the severely affected and unstable patients in usual care.
The comprehensive multivariate model applied in this study included time to first mobilization and 2 activity categories as independent variables. Because time spent in bed, sitting out of bed and time in upright activity always add up to almost 100% (will add up to 100% if “time not observed” is included), the effect of the variable of interest, holding the second variable constant, will be at the cost of the third variable not included in the model, which was sitting out of bed. This means that for every % increase in time in upright at day-time between 08.00 h and 17.00 h (which translates into 5.4 min) we expect a 3% decrease in the risk of poorer outcome (higher mRS score), holding time in bed and time to first mobilization constant.
Our results suggest that a linear relationship exists between the amount of upright activity and good outcome (the more the better), which has also been proposed in earlier research (27). However, results from the recent AVERT trial indicate that caution needs to be applied in the early post-stroke period (i.e. too much training may be harmful) (8). This new knowledge needs to be balanced against our current understanding that too much bed rest and delaying mobilization can also be harmful (5, 6, 12). Whether the period for greatest caution is the first day or several days post-stroke is currently unknown.
The present study also showed a strong association between early activity and HRQoL, confirming the positive association between increased motor activity and HRQoL shown in other studies (28, 29). This finding was not unexpected, as the EQ-5D-5L is shown to be strongly correlated with the mRS (30). The EQ-5D-5L scores reported among the participating patients were mainly in line with previous studies assessing HRQoL in stroke survivors (31, 32). This was evident even though our population was more dependent compared with the other studies (31, 32). Although stroke patients rate their self-perceived health a little lower than the general age-matched population (33), their quality of life is generally good. In Norway, most hospitals offer an early supported discharge service, which has been shown to improve HRQoL in both rural and urban areas (34, 35).
This study had a number of limitations. First, the observational design increased the risk of confounding factors associated with outcome. Secondly, there was a lack of observation of patients from 17.00 h to 08.00 h the next morning. However, the time from 08.00 h to 17.00 h is regarded as the most active time of the day, with the highest number of nurses and therapists present on the ward. A further limitation is the high proportion of patients (n = 73) who did not respond to EQ-5D-5L because of cognitive problems or severely illness. Although proxies rate HRQoL lower than the patients themselves, a recent evaluation of EQ-5D-5L found that a proxy respondent could be used for patients not able to respond because of aphasia or dementia (23). Hence, proxies should be considered for use in future studies within this field.
The major strengths of the present study were the large sample size, including almost 400 patients from 11 Norwegian stroke units, and the naturalistic study design investigating clinical practice as usual. The study sample appears to be slightly older, with more severe strokes compared with the average Norwegian stroke population (36). The follow-up procedure, whereby all patients were contacted in person or by phone if possible, and the use of proxies ensured a high response rate, particularly for mRS. Another strength was the use of behavioural mapping to measure the amount of motor activity. This is a well-documented method, which has shown good correlation with accelerometer device (37). However, a body-worn sensor system might be an alternative method to investigate how the activity pattern changes across multiple days during hospital stay in future research.
Despite the current unknowns, this study supports previous work, including the results from the AVERT trial, showing good outcome associated with early out of bed activity in usual care (8), and shows that activity applied within the first week after stroke is associated with functional independence 3 months later. However, future research should focus on exploring the pathophysiological mechanisms associated with early upright activity and on determining the optimal dosages of activity and rest during the early phase after stroke.
In conclusion, this study confirms the beneficial effect of upright activity applied during hospital stay in Norwegian stroke units on global function and HRQoL 3 months later. There was no association between timing of mobilization and outcome. However, the optimal timing, frequency and dosage of early activity needs to be determined. There is also a need for a more thorough understanding of the pathophysiological mechanisms associated with early upright activity after stroke.
ACKNOWLEDGEMENTS
The authors would like to thank Jan Chamberlain and Li Chun Quang for preparing the data.
This study was supported by Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology and The Research Council of Norway (grant number NRC no 205309).
The authors declare no conflicts of interest.
REFERENCES
