Yen-Chang Huang, MD1, Wei-Te Wang, MD2, Tsan-Hon Liou, MD, PhD1,3,4, Chun-De Liao, PT, MS1,5, Li-Fong Lin, PT, PhD1,6* and Shih-Wei Huang, MD1,3*
From the 1Department of Physical Medicine and Rehabilitation, Shuang Ho Hospital, Taipei Medical University, Taipei, 2Department of Physical Medicine and Rehabilitation, Changhua Christian Hospital, Changhua, 3Department of Physical Medicine and Rehabilitation, School of Medicine, College of Medicine, 4Graduate Institute of Injury Prevention, Taipei Medical University, 5School and Graduate Institute of Physical Therapy, College of Medicine, National Taiwan University and 6School of Gerontology and Health Management, Taipei Medical University, Taipei, Taiwan. *These authors contributed equally to this study.
OBJECTIVE: The Postural Assessment Scale for Stroke Patients (PASS) is used to assess static and dynamic balance of stroke patients. PASS has demonstrated good measurement properties for reliability and validity, but its predictive effect for ambulation in stroke patients has not been investigated. The aim of this study was to investigate the predictive value of PASS for ambulation in patients with stroke after inpatient rehabilitation.
METHODS: In this retrospective study, a total of 341 stroke patients were recruited from a rehabilitation ward of a medical university hospital. Patients were assessed at baseline using PASS and observation of rolling ability, and divided into 2 groups at discharge: independently ambulatory (n = 246) and non-ambulatory (n = 95). Receiver operating characteristic curve and adjusted bivariate logistic regression was applied to analyse the predictive value of baseline PASS scores, variables of demographic data, and rolling ability at admission to inpatient rehabilitation.
RESULTS: For all stroke patients, mean admission to the rehabilitation ward was 34.40 days after stroke and mean length of hospitalization 18.12 days. The receiver operating characteristic curve was obtained with a cut-off score of 3.5 points for static PASS, 8.5 points for dynamic PASS, and 12.5 points for total PASS, demonstrating the highest percentage of accurately predicted ability of independently walking at discharge. Adjusted bivariate logistic regression found rolling ability, static PASS and dynamic PASS to be predictors for ambulation of stroke patients at discharge.
CONCLUSION: Initial static PASS score, dynamic PASS score and rolling can be predictors for independent ambulation of stroke patients after a course of inpatient rehabilitation.
Key words: PASS; rehabilitation; stroke; walking ability.
J Rehabil Med 2016; 00: 00–00
Correspondence address: Shih-Wei Huang, Department of Physical Medicine and Rehabilitation, Shuang Ho Hospital, Taipei Medical University, 291 Jhongjheng Rd, Jhonghe, New Taipei City 235, Taiwan. E-mail: 13001@s.tmu.edu.tw
Accepted Oct 23, 2015; Epub ahead of print Dec 14, 2015
INTRODUCTION
Stroke is the most common cause of disability among elderly people, often resulting in dependence in activities of daily living (ADLs) (1). Pound et al. reported that the loss of independent ambulation is perceived as the most disabling consequence of stroke, affecting almost every aspect of daily life (2). Hence, determining how to reduce disability after stroke through effective rehabilitation is critical to improving patients’ quality of life.
The goal of intensive rehabilitation during stroke recovery is to improve basic mobility functions and the ability to perform ADLs. Approximately 60–70% of subacute stroke patients regain their ability to walk independently at discharge (3). Early prediction of stroke patient ambulation is critical for facilitating discharge planning and anticipating the need for home adjustments and community support (4).
A previous study found that age, severity of paresis, size of lesion, presence of hemianopia, and type of stroke were predictors of mobility 30 days after a stroke; however, few other factors have been investigated (5). Other possible factors affecting mobility outcomes should therefore be considered. Recent studies have found that within 72 h after a stroke, the ability to sit and the strength of the hemiplegic leg could predict walking ability at 6 months post-stroke (5). Scrivener et al.(6) found that exercise intensity within the first week after admission for stroke is a crucial indicator of walking speed at discharge and the time used to achieve independent ambulation. However, there is a lack of quantitative evaluation tools for prediction of independent ambulation among stroke patients when discharging from the rehabilitation ward.
The Postural Assessment Scale for Stroke Patients (PASS) was developed specifically for assessing balance in stroke patients. PASS demonstrates high inter- and intra-rater reliability (7), favourable individual item agreement (8), and high test-retest reliability (9, 10). PASS is used to examine a patient’s ability to maintain or change a given lying, sitting, or standing posture. It is easy to administer in a clinic, and is suitable for all patients, even those with poor postural performance (7). However, there has been no research focusing on the prediction of PASS scores with ambulation after rehabilitation. The aim of this study was to investigate whether initial PASS scores can predict the independent ambulation ability of acute stroke patients at discharge from rehabilitation.
METHODS
Participants
In this retrospective study, 625 stroke patients were recruited from the rehabilitation ward of a medical university hospital in Taiwan between January 2012 and December 2013. Inclusion criteria were: (i) unilateral ischaemic stroke lesion confirmed by computerized tomography or magnetic resonance imaging; (ii) first incidence of stroke, with the onset occurring less than 6 months prior to discharge; (iii) the patient required and underwent the rehabilitation programme intervention during admission in the stroke care unit; (iv) on admission to the rehabilitation ward, the patient had motor function impairment and the inability to walk with or without assistive devices. Exclusion criteria were: (i) ability to ambulate before rehabilitation; (ii) bilateral limb weakness; (iii) admission period of less than 14 days; (iv) unstable vital signs and vital organ decompensation status (such as heart failure); (v) inability to pay for the rehabilitation programme during admission; (vi) previous history of other neurological or orthopaedic problems known to affect balance or walking; and (vii) a recurrent stroke or other complications that could have interfered with rehabilitation during hospitalization. After inclusion and exclusion processing, a total of 341 patients were enrolled in the study.
Study design
This was a retrospective study involving patients with stroke who were non-ambulatory at baseline and were divided into 2 groups at discharge: an ambulation group composed of patients who could ambulate independently for more than 10 m at discharge, and a non-ambulatory group comprising those who could not. Patients in both groups underwent a standard physical therapy programme. A physical therapist recorded each patient’s demographic data and functional status during the first session of physical therapy. These data were analysed to investigate the predictors of walking ability in stroke patients after rehabilitation.
Rehabilitation programme
Both groups underwent the same conventional rehabilitation programme, including physical therapy and occupational therapy, each lasting 60 min per day for 5 consecutive days a week. Speech therapy was administered according to individual need. The programme for all patients was conducted by the same physical therapist and occupational therapist, each with more than 3 years of clinical experience in our hospital. When the patients received their rehabilitation, this retrospective study had not been proposed; therefore the therapists who assessed and treated the patients were blinded to the study.
Measurements
PASS was used as our primary balance measurement. Designed specifically for stroke patients (7), PASS contains 12 items for evaluating balance: 5 items (sitting without support; standing with support; standing without support; standing on non-paretic leg; standing on paretic leg) to assess the maintenance of posture (static PASS) and 7 items (supine to affected side lateral; supine to non-affected side lateral; supine to sitting up on the edge of the mat; sitting on the edge of the mat to supine; sitting to standing up; standing up to sitting down; standing, picking up a pencil from the floor) to evaluate changes in posture (dynamic PASS). PASS can therefore be used to assess functional equilibrium, which requires both static and dynamic balance. Each PASS item is rated on a scale from 0 to 3, for a maximum total score of 36: on this scale, the higher the score is, the more favourable the balance in stroke patients. Static PASS (from 0 to 15), dynamic PASS (from 0 to 21), and total PASS (from 0 to 36) were measured in this study. PASS is highly correlated with the Functional Independence Measure (FIM), and has good internal consistence (Cronbach’s alpha = 0.95), intra-rater reliability (0.72) and inter-rater reliability (0.88) (7). Rolling ability (the motor skill of moving on a bed from prone to supine and vice versa in a coordinated manner) at admission was recorded as an independent predictor.
Physical therapists performed each patient’s PASS assessment before the start of rehabilitation, and the outcome measure was completed at discharge. Assessments of each patient at admission and discharge were performed by the same physical therapists from our department. These data were obtained from chart records by a physiatrist. Other demographic data were obtained from each patient’s medical records: age, sex, body weight, height, history of diabetes mellitus, hypertension, coronary artery disease, nasogastric tube, Foley catheter, stroke onset duration (the number of days from stroke onset to admission to rehabilitation ward), urinary tract infection, pneumonia, aphasia, and acupuncture. Independent rolling status at admission was chosen as one of the variables and recorded separately because it is easy and convenient to evaluate independent rolling at bedside. At discharge, the patients were divided into 2 groups: those who could walk independently for more than 10 m at discharge were assigned to the ambulation group and the others to the non-ambulatory group. If necessary, they could walk with an assistive device, which was used in the regular physical therapy session. Static PASS scores, dynamic PASS scores, total PASS scores, and other factors were evaluated for both groups.
Statistical analysis
Differences in demographic data, medical comorbidity, and functional aspect (independent rolling, static PASS, dynamic PASS, total PASS) between the ambulation and the non-ambulatory groups were calculated. Comparisons between continuous variables were analysed using Student’s t-test, and comparisons between categorical variables were performed using χ2 test. It was assumed that static PASS, dynamic PASS, and total PASS scores can be predicting tools, and receiver operating characteristics (ROC) curves for independent ambulation of stroke patients were generated by plotting the sensitivity against 1 minus the specificity. The area under the curve (AUC) was calculated with a 95% confidence interval (CI). The area under the ROC curve was used to assess the accuracy of the prediction model for a high likelihood of walking ability after rehabilitation. In a ROC curve, the true-positive rate (sensitivity) is plotted against the false-positive rate (1-specificity); an AUC of 0.5 indicates no discrimination above chance, whereas an area under the curve of 1 indicates perfect discrimination. Analysis was performed using the Youden Index to determine the optimal cut-off value. Kappa symmetry was analysed to determine the consistency of PASS prediction of independent ambulation at discharge and positive predictive value (PPV) of these cut-off values by PASS was also analysed in this study.
Adjusted bivariate logistic regression analysis was then used to identify predictors and the odds ratio with 95% CI for predicting independent ambulation of stroke patients after rehabilitation. All data analyses were performed using SPSS software, version 20.0 (IBM, Chicago, IL, USA); p < 0.05 was considered to represent a statistically significant difference.
RESULTS
A total of 341 patients (209 men and 132 women) met the necessary criteria and were included in the study. Demographic data are shown in Table I.
|
Table I. Demographic characteristics, co-morbidity, medical disorders of all participants |
|||
|
Variables |
Ambulatory (n = 95) |
Non-ambulatory (n = 246) |
p-value |
|
Age, years, mean (SD) |
63.22 (13.28) |
68.93 (13.83) |
< 0.001** |
|
Sex (M/F), n (%) |
60/35 |
149/97 |
0.661 |
|
BMI, mean (SD) |
31.88 (6.90) |
29.25 (6.50) |
0.031* |
|
Stroke onset duration, days, mean (SD) |
26.22 (30.30) |
31.05 (39.42) |
0.362 |
|
Admission duration, days, mean (SD) |
18.38 (8.02) |
15.10 (9.60) |
0.602 |
|
Pneumonia, n (%) |
19 (5.6) |
61 (17.9) |
0.351 |
|
UTI, n (%) |
13 (3.83) |
64 (18.8) |
< 0.001** |
|
NG, n (%) |
13 (3.82) |
88 (25.9) |
< 0.001** |
|
Foley, n (%) |
15 (4.4) |
80 (28.5) |
< 0.001** |
|
Diabetes mellitus, n (%) |
44 (12.9) |
104 (30.7) |
0.476 |
|
Hypertension, n (%) |
78 (22.9) |
207 (60.7) |
0.654 |
|
CAD, n (%) |
21 (6.2) |
30 (8.8) |
0.023* |
|
Aphasia, n (%) |
22 (6.5) |
94 (28.0) |
0.022* |
|
Acupuncture, n (%) |
37 (10.8) |
64 (18.7) |
0.023* |
|
Rolling, n (%) |
54 (15.8) |
49 (14.3) |
< 0.001** |
|
Static PASS, mean (SD) |
5.80 (2.98) |
1.94 (2.30) |
< 0.001** |
|
Dynamic PASS, mean (SD) |
11.69 (4.40) |
4.41 (4.32) |
< 0.001** |
|
Total PASS, mean (SD) |
17.49 (6.91) |
6.35 (6.31) |
< 0.001** |
|
*p < 0.05, **p < 0.001. p-values were calculated using independent t-tests for the continuous variables and with a χ2 test for the categorical variables. PASS: Postural Assessment Scale for Stroke Patients; SD: standard deviation; M: male; F: female; BMI: body mass index; UTI: urinary tract infection; NG: nasal-gastric tube; CAD: coronary artery disease. |
|||
According to the outcome, patients were divided into 2 groups: an ambulation group consisting of 246 patients (72%), and a non-ambulatory group of 95 patients (28%). The differences between the ambulation and the non-ambulatory group (Table I) were highly significant (p < 0.01) for the following variables: static PASS score, dynamic PASS score, total PASS score, rolling ability, urinary tract infection, nasogastric tube and Foley catheter. There were no statistically significant differences between the groups regarding acupuncture, pneumonia, aphasia, diabetes mellitus, hypertension, or coronary artery disease.
We used the ROC curve and Youden Index to determine the most appropriate cut-off point. The optimal ROC curve (Table II and Fig. 1) was obtained with a cut-off score of 3.5 points for static PASS (sensitivity 77.9%; specificity 82.1%), 8.5 points for dynamic PASS (sensitivity 77.9%; specificity 82.5%), and 12.5 points for total PASS (sensitivity 78.9%; specificity 83.7%) (Table III). Fig. 1 illustrates the ROC curve for static PASS (AUC = 0.860), dynamic PASS (AUC = 0.876), and total PASS (AUC = 0.884). All cut-off points have good discrimination.
|
Table II. Receiver operation characteristic curve (ROC) analysis for predicting independent ambulation of stroke patients at discharge |
||||
|
Variables |
AUC |
SE |
95% CI |
p-value |
|
Static PASS |
0.860 |
0.022 |
0.816–0.904 |
< 0.001 |
|
Dynamic PASS |
0.876 |
0.020 |
0.837–0.915 |
< 0.001 |
|
Total PASS |
0.884 |
0.020 |
0.846–0.923 |
< 0.001 |
|
PASS: Postural Assessment Scale for Stroke Patients; AUC: area under the curve; SE: standard error; 95% CI: 95% confidence interval. |
||||
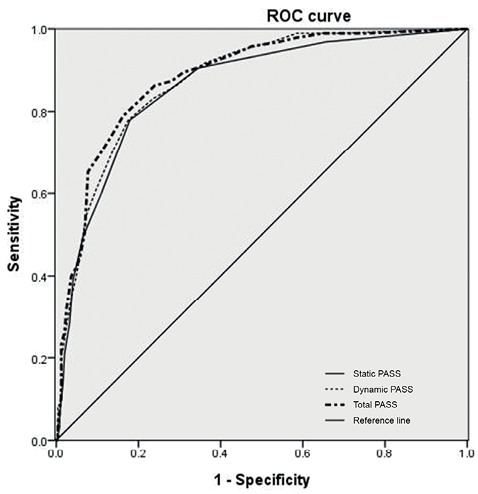
Fig. 1. Receiver operating characteristic (ROC) curve analyses of assessed independent ambulation of stroke patients with static, dynamic, and total Postural Assessment Scale for Stroke Patients (PASS) scores.
|
Table III. Sensitivity and specificity of ambulation prediction by Postural Assessment Scale for Stroke Patients (PASS) scores |
|||||||||||
|
Variables |
PASS scores |
Non-ambulatory n |
% |
Ambulatory n |
% |
p-valuea |
Kappa |
p-valueb |
Sensitivity |
Specificity |
PPV |
|
Static PASS |
< 3.5 |
202 |
59.2 |
21 |
6.2 |
< 0.001 |
|||||
|
≥ 3.5 |
44 |
12.9 |
74 |
21.7 |
0.559 |
< 0.001 |
0.779 |
0.821 |
0.627 |
||
|
Dynamic PASS |
< 8.5 |
203 |
59.5 |
21 |
6.2 |
< 0.001 |
|||||
|
≥ 8.5 |
43 |
12.6 |
74 |
21.7 |
0.564 |
< 0.001 |
0.779 |
0.825 |
0.632 |
||
|
Total PASS |
< 12.5 |
206 |
60.4 |
20 |
5.9 |
< 0.001 |
|||||
|
≥ 12.5 |
40 |
11.7 |
75 |
22 |
0.589 |
< 0.001 |
0.789 |
0.837 |
0.652 |
||
|
ap-value by χ2 analysis; bp-value by Kappa symmetry analysis. PPV: positive predictive value. |
|||||||||||
Logistic regression analysis found rolling ability (p = 0.05), static PASS score (p = 0.03), and dynamic PASS score (p = 0.04) were predictors for independent ambulation at discharge after adjusting for other variables in this study (Table IV).
|
Table IV. Adjusted binary logistic regression analysis for predictors of independent ambulation at discharge from stroke rehabilitation ward |
|||
|
Variables |
OR |
95%CI |
p-value |
|
Age (Ref: ≤ 57) |
|||
|
58–78 years |
0.77 |
0.34–1.69 |
0.512 |
|
≥ 79 years |
0.93 |
0.34–2.49 |
0.881 |
|
Sex (Ref: Female) |
|||
|
Male |
1.11 |
0.87–1.37 |
0.660 |
|
BMI (Ref: ≤ 24.84) |
|||
|
24.9–34.12 |
0.91 |
0.38–2.13 |
0.824 |
|
≥34.13 |
2.13 |
0.81–5.54 |
0.124 |
|
Stroke onset duration (Ref: ≤ 5) |
|||
|
6–41 days |
1.49 |
0.67–3.29 |
0.333 |
|
≥ 42 days |
1.35 |
0.52–3.47 |
0.544 |
|
Urinary tract infection |
0.98 |
0.42–2.27 |
0.961 |
|
Nasogastric tube |
0.52 |
0.21–1.23 |
0.142 |
|
Foley |
0.51 |
0.20–1.26 |
0.154 |
|
Coronary artery disease |
1.34 |
0.57–3.09 |
0.502 |
|
Aphasia |
0.61 |
0.29–1.29 |
0.204 |
|
Acupuncture |
1.41 |
0.67–2.93 |
0.363 |
|
Rolling |
1.91 |
0.94–3.82 |
0.044* |
|
Static PASS (Ref: 3.5) |
|||
|
> 3.5 |
2.95 |
1.09–7.94 |
0.034* |
|
Dynamic PASS (Ref: 8.5) |
|||
|
> 8.5 |
3.00 |
1.03–8.69 |
0.042* |
|
Total PASS (Ref: 12.5) |
|||
|
> 12.5 |
2.30 |
0.61–8.66 |
0.221 |
|
*p-value < 0.05. Age, BMI, stroke onset duration were dichotomized by quartile distribution (25%, 75%); PASS scores were dichotomized by Youden Index. PASS: Postural Assessment Scale for Stroke Patients; BMI: body mass index. |
|||
Our results showed that the odds of walking at discharge for patients with a static PASS score greater than 3.5 points were 2.95-fold higher than for patients scoring less than 3.5. Furthermore, patients with a dynamic PASS score greater than 8.5 points had 3.0-fold higher odds of walking at discharge compared with those scoring less than 8.5.
DISCUSSION
In this study the predictive value of PASS was found to be good regarding independent ambulation ability at discharge. According to previous reports, PASS scores at baseline significantly predicted with the FIM score at discharge, the change in FIM score during rehabilitation, and the destination at discharge (11). Based on our research, this is the first study to assess the relationship between PASS at baseline and the ambulation ability of patients with stroke at discharge.
The ability to walk successfully is a critical factor for community mobility and reintegration. A higher PASS score at admission in patients with acute stroke predicted a more likely walking ability at discharge. PASS could be useful for establishing a prognosis for recovery of walking ability, which helps in decision-making about objectives and rehabilitative treatment for individual patients.
Our results show that static and dynamic PASS scores were both accurate predictors of walking ability. This is consistent with a previous study, which found that sitting balance was predictive of independence in ADLs, including transfers, dressing and toileting (12). Another study showed that improving standing balance was more crucial than improving strength in enhancing post-stroke gait dysfunction, and that static balance was a critical factor for walking ability (13). Our study revealed that PASS used at admission to a rehabilitation ward can objectively predict the ability to ambulate at discharge.
Our results also showed that rolling was an accurate predictor of walking ability. This finding is compatible with those of previous studies indicating that rolling ability at admission could be a predictor of walking ability at discharge. One review assessed prognostic factors within one week of stroke onset and concluded that the initial grade of paresis was the most crucial predictor of mobility recovery at least 3 months post-stroke (14, 15). One review found that the severity of paresis and reduced leg power were predictive of, or associated with, walking within 30 days post-stroke (16). Another study revealed that walking ability is closely related to severity of paresis and leg power (17). In addition, the Trunk Control Test (TCT), administered 6 weeks after a cardiovascular accident, has been found to be a predictor of walking ability at 18 weeks (18). Both the trunk control test and PASS encompass items such as rolling to one’s strong and weak side. Rolling to both sides after stroke is dependent on paretic and non-paretic limb’s muscle power. Rolling ability was easy to assess by history taken from patient or caregiver, and therefore deemed suitable for clinical physicians to use as a prognostic indicator.
Several studies have evaluated predictors of mobility or physical functioning. Predictors of walking include severity of paresis, age, reduced leg power, global aphasia, unilateral spatial neglect, male sex, vocational status, presence of hemianopia, size of brain lesion, and type of stroke; these were all shown to be predictive of, or associated with, walking within 30 days post-stroke (16, 19–22). Among these predictors of walking ability, categorical variables were found to be unsuitable in presenting the level of severity of walking ability. PASS would therefore seem to be a more clinically useful tool than other factors for predicting walking ability after stroke.
There are 2 scales that typically are used in stroke studies: the Berg Balance Scale (BBS) and PASS. While these scales share some common items for maintaining-posture, there are additional items with regard to the ability of changing-posture in PASS that are not included in the BBS. Both scales are easy to use and demonstrate high validity (23). Previous studies have found a strong correlation between PASS and BBS at admission to an inpatient rehabilitation unit, suggesting that PASS and BBS may be measuring similar constructs (8, 24). However, some studies have shown that the BBS had a significant floor or ceiling effect (25). Mao et al. (8) reported a significant floor effect of BBS at 14 days post-stroke. Chou et al. (26) observed a large floor effect when the BBS was administered 14 days after stroke onset. Salbach et al. (27) found a large ceiling effect of BBS by 38 days post-stroke. Thus, we chose PASS as our measurement tool. PASS further has the advantage of possessing dynamic and functional items that are appropriate for low-functioning patients.
Chien et al. (28) developed a short form of PASS, and the study provides strong evidence that the short form of PASS has sound psychometric properties in people with stroke. The short form of PASS is simple and fast to administer. However, the floor effect may affect this measure’s ability to discriminate some patients with severe stroke. The notable floor effect of the short form of PASS may have resulted from the removal of 3 lying and sitting items, which appeared to be the easiest tasks among the 12 original items. Removing these items from the original PASS could reduce the ability of the short form scale to detect changes in lying and sitting function and lead to a floor effect. In our study, some people experienced a severe stroke episode and had low PASS at an early stage. Therefore, we preferred to use the original PASS to gain better discrimination ability.
The predictive value of PASS-TC (trunk control) at an early stage after stroke on comprehensive ADL function in patients surviving for 6 months was well supported (29). PASS-TC had 5 items (i.e. sitting without support, supine to affected side lateral, supine to non-affected side lateral, supine to sitting up on the edge of the table, and sitting on the edge of the table to supine) to measure trunk control. However, the discriminative and evaluative abilities are limited over the first 6 months after a stroke (30). Thus, the original PASS was more suitable to use in this study.
It is important for stroke patients to achieve walking ability by the time they are discharged from rehabilitation, both for minimizing activity limitations and for maximizing quality of life. PASS score was found to be a useful predictor of walking ability, and thus has the potential to assist clinicians in determining the likely walking outcomes for individual stroke survivors. It may also be useful to explain this scale to stroke patients, their families, and the stroke unit team for discharge planning. Further research is necessary to explore other factors that may affect the walking ability of stroke patients in varying degrees of recovery.
There are several limitations to this study. First, selection bias and confounding errors are inherent to the retrospective design of this study. Many factors, such as disease and trauma, could potentially influence walking ability. However, we applied strict inclusion and exclusion criteria to exclude internal medical disease and environmental factors. Secondly, according to a previous study, the highest validity of PASS is within the first 90 days (7). Because our study exhibited a wide distribution of time since stroke onset, this might result in errors. Finally, this study was limited to patients with ischaemic stroke requiring inpatient stroke unit care and physical therapy intervention. Future studies could investigate predictors of walking ability in all stroke survivors. We also recommend further study of additional walking factors, such as walking endurance and gait speed, which are crucial for ambulation ability.
In conclusion, this study provides key information on predicting the future ambulation ability of selected stroke patients admitted for rehabilitation. Patients with a static PASS score greater than 3.5 points had 2.95-fold the odds of walking at discharge compared with those scoring less than 3.5 points. Patients with a dynamic PASS score greater than 8.5 had 3.0-fold the odds of walking at discharge compared with those scoring less than 8.5. Our results found PASS to be an accurate predictor of ambulation ability in ischaemic stroke patients after rehabilitation. As a quantitative factor, PASS is an effective predictive tool for clinical application.
REFERENCES
