Marjorie Kerzoncuf, MD1, Laurent Bensoussan, MD, PhD1,2, Alain Delarque, MD, PhD1,2, Jacques Durand, PhD2, Jean-Michel Viton, MD, PhD1,2 and Christiane Rossi-Durand, PhD2
From the 1APHM, Hôpital de la Timone, Pôle de Médecine Physique et de Réadaptation and 2Aix Marseille Université, CNRS, INT UMR 7289, Marseille, France
OBJECTIVE: The therapeutic effects of intramuscular injections of botulinum toxin-type A on spasticity can largely be explained by its blocking action at the neuromuscular junction. Botulinum toxin-type A is also thought to have a central action on the functional organization of the central nervous system. This study assessed the action of botulinum toxin-type A on spinal motor networks by investigating post-activation depression of the soleus H-reflex in post-stroke patients. Post-activation depression, a presynaptic mechanism controlling the synaptic efficacy of Ia-motoneuron transmission, is involved in the pathophysiology of spasticity.
Patients: Eight patients with chronic hemiplegia post-stroke presenting with lower limb spasticity and requiring botulinum toxin-type A injection in the ankle extensor muscle.
METHODS: Post-activation depression of soleus H-reflex assessed as frequency-related depression of H-reflex was investigated before and 3, 6 and 12 weeks after botulinum toxin-type A injections in the triceps surae. Post-activation depression was quantified as the ratio between H-reflex amplitude at 0.5 and 0.1 Hz.
RESULTS: Post-activation depression of soleus H-reflex, which is reduced on the paretic leg, was affected 3 weeks after botulinum toxin-type A injection. Depending on the residual motor capacity of the post-stroke patients, post-activation depression was either restored in patients with preserved voluntary motor control or further reduced in patients with no residual voluntary control.
CONCLUSION: Botulinum toxin treatment induces synaptic plasticity at the Ia-motoneuron synapse in post-stroke paretic patients, which suggests that the effectiveness of botulinum toxin-type A in post-stroke rehabilitation might be partly due to its central effects.
Key words: H-reflex; botulinum toxin; post-activation depression; stroke; lower limb spasticity.
J Rehabil Med 2015; 00: 00–00
Correspondence address: Marjorie Kerzoncuf, APHM, Hôpital de la Timone, Pôle de Médecine Physique et de Réadaptation, FR-13385, Marseille, France. E-mail : marjorie.kerzoncuf@ap-hm.fr
Accepted Jul 31, 2015; Epub ahead of print Oct 1, 2015
INTRODUCTION
Local injection of botulinum toxin-type A (BTx-A) is the standard treatment for focal spasticity, particularly in post-stroke patients. BTx-A acts by blocking the release of acetylcholine from motoneuron terminals at the neuromuscular junction, relieving excessive muscle contraction for up to several months. In addition to its peripheral local action, there is evidence for a central effect of BTx-A (1–6). Through blocking the endplates of intrafusal muscle fibres (7–10), BTx-A may reduce discharge firing from muscle spindles, which may, in turn, alter the functioning of the central motor networks fed by spindle afferents at both spinal and supraspinal levels. Furthermore, animal experiments have shown that BTx-A can be transported retrogradely in the motor axon to motoneuron soma and possibly trans-synaptically (6, 11), hence inhibiting cholinergic synaptic transmission in the spinal cord, as recently suggested for humans (12).
The central effects of BTx-A on different spinal inhibitory mechanisms of patients with spasticity have been investigated. These mechanisms include presynaptic inhibition (13) reciprocal inhibition (14, 15), recurrent inhibition (12) and, in the case of spasticity, secondary to various motor disorders: stroke (10, 12, 14), cerebral palsy (16), multiple sclerosis (17) and dystonia (9, 13, 18). Thus far, electrophysiological findings are controversial, depending on the spinal motor network investigated and the physiopathology of spasticity in different motor disorders.
To our knowledge the effects of BTx-A on the mechanism of post-activation depression (post-AD) of H-reflex have not yet been examined. In healthy conditions the amplitude of the electrically elicited reflex declines following previous activation of the monosynaptic pathways (19). A related phenomenon of low-frequency depression has also been reported (20, 21). The H-reflex is depressed at stimulation rates greater than 0.1 Hz and usually completely abolished at 10 Hz. This reflex depression is sustained by a mechanism of homosynaptic depression acting at the Ia-motoneuron synapse and results from a decrease in transmitter release due to repetitive activation of the synapse (20, 21). The post-AD of H-reflex is therefore a presynaptic inhibitory mechanism that acts on Ia terminals and controls the efficacy of the synaptic transmission in physiological conditions according to the discharge frequency of primary spindle afferents. The post-AD of H-reflex would prevent excessive gain for the stretch reflex during voluntary contraction in healthy conditions. In spasticity, the post-AD is less pronounced; namely H-reflex amplitude is less depressed when the inter-stimulation interval is shortened (22, 23). Reduced post-AD of H-reflex was reported for patients with spasticity of different causes; multiple sclerosis, spinal cord injury (24), cerebral palsy (25) and stroke (23, 26). The reduction in the post-AD at the Ia-motoneuron synapse may provide the neural substrate to the stretch reflex exaggeration and is assumed to be one of the main mechanisms underlying spasticity (23). It therefore appears appropriate to address the question of whether this spinal mechanism is affected by treatment with BTx-A.
The aim of this study was to determine whether injections of BTx-A into the calf muscles affect the post-AD of soleus H-reflex in post-stroke spasticity. For this purpose, post-AD of soleus H-reflex, assessed as frequency-related depression of H-reflex (19, 21, 23, 26), was explored before and after BTx-A injections in the triceps surae muscles. We hypothesize that BTx-A treatment would modulate the post-AD in patients, probably by inducing synaptic plasticity at Ia-motoneuron synapse, mediated by changes in the muscle afferent inflow. If so, the effectiveness of BTx-A in post-stroke rehabilitation would also depend on its central effect.
MATERIAL AND METHODS
The study was approved by the local ethics committee (Sud-Mediterranee-Marseille) and Aix-Marseille University (approval number EudraCt 2013-000600-42) and received the agreement of the Agence Nationale de Sécurité du Médicament (ANSM). Patient recruitment and therapeutic assessment pre- and post-treatment were performed in the Department of Physical Medicine and Rehabilitation (University Hospital of Marseille). Patients were included in the protocol after providing written informed consent as required by the Declaration of Helsinki (1975).
Patients
Patients with chronic hemiplegia following stroke, presenting with lower limb spasticity and requiring BTx-A injection in the calf muscles, were tested. Inclusion criteria were: a minimum 12-month interval since stroke, lower limb spasticity with a Modified Ashworth Scale (MAS) score greater than or equal to 2 for the triceps surae, minimum 6-month interval since a previous BTx-A injection, and age over 18 years. Exclusion criteria were: previous treatment of spasticity with phenol or alcohol injection and surgery on the paretic side. Any ongoing anti-spasmodic treatment and/or physical therapy remained unchanged for the duration of the experimental protocol.
Botulinum toxin injections protocol
BTx-A (Botox®, Allergan®, 2.5 physiological serum dilution) was injected into the triceps surae in all patients. Doses were adapted to the patient and the intensity of spasticity. The goals of BTx-A injections in the triceps surae muscles were defined with the patients before injection and were to reduce foot drag and cloni and to improve ankle stability.
Injections were performed with the patient in the supine position, with their feet beyond end of bed, so that the dorsiflexion was not large enough to trigger cloni during the procedure. The injection site was guided by electrical stimulation.
Clinical parameters
Clinical examination was performed by the same physician (among the authors) for all the patients and all the experimental sessions. The degree of spasticity in the triceps surae was measured with the MAS from 0 to 5, the occurrence of cloni on soleus or gastrocnemius, passive range of ankle dorsiflexion, and residual voluntary motor control. The Functional Independence Measure (FIM) and the Functional Ambulation Classification (FAC) evaluated the limitation of activities.
Electrophysiological recordings
The patient was positioned supine in a semi-sitting position, hip flexed (80°), knee flexed (30°) (maintained by foam positioned under the thigh), and small talo-crural extension. Neurophysiological investigations were performed on both treated (paretic) and non-treated (non-paretic) legs.
Surface electromyography (EMG) was recorded from the soleus muscle by pairs of Ag-AgCl surface electrodes placed 2 cm apart over the muscle belly, with the proximal electrode 3 cm below the insertion of the gastrocnemii on the Achilles tendon. The EMG signal was amplified, filtered with a bandpass of 30 Hz to1 kHz, and then digitized online at a sampling rate of 5 kHz using a CED 1401 interface and Spike 2 software (CED, UK).
H-reflexes were elicited in the soleus with bipolar stimulation of the posterior tibial nerve (1 ms pulse) at the popliteal fossa. By increasing the stimulus intensity (1 ms duration), we determined the maximum amplitude of H-reflex (Hmax) and of M wave (Mmax). When possible, a soleus H-M recruitment curve was built up. Stimulus strength was then adjusted to elicit a motor response with a mean amplitude of approximately 25% of Mmax at rest or a H-reflex amplitude of 30–50% of Hmax when the Mmax was not obtained.
The post-AD of soleus H-reflex was evaluated by testing the effects of 2 frequencies of stimulation on H-reflex amplitude (23, 26, 27). H-reflex depression is absent at a stimulation frequency of 0.1 Hz and present at frequencies higher than 0.33 Hz (20). Soleus reflex amplitude was analysed in response to electrical stimulation applied to the tibial nerve at 0.1 and 0.5 Hz. Ten stimuli were applied at both stimulation rates. The first 2 stimuli were ignored and the responses to the remaining 8 stimuli were averaged. The mean reflex amplitude was measured peak-to-peak. Post-AD of H-reflex was assessed from the ratio H0.5Hz/H0.1Hz; the greater the ratio, the smaller the post-AD. Fig. 1 is an example of a recording.
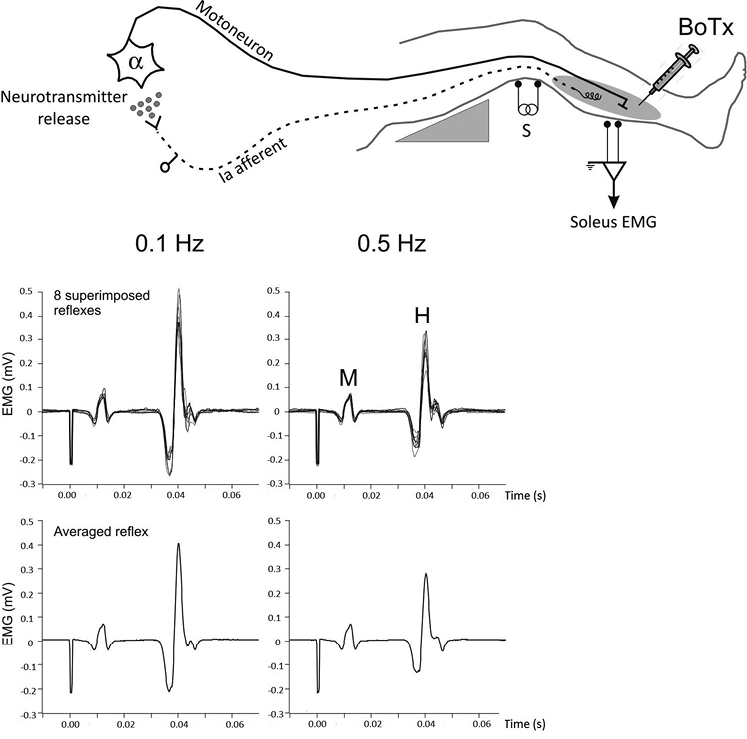
Fig. 1. Experimental set-up, with the patient lying in a supine position with the leg resting on a support that holds the knee slightly flexed. Electrical stimulation was applied to the tibial nerve at the popliteal fossa to elicit soleus H-reflex. Surface electromyographic activity (EMG) was recorded in the soleus muscle receiving the botulinum toxin-type A (BTx-A) injection. Stimulation intensity was adjusted to evoke M- and H-response. Recordings illustrate data for 1 patient. Superimposed H-reflexes in response to 8 stimuli are shown at 0.1 and 0.5 Hz (upper histograms) together with the corresponding averaged reflex (lower histograms). Post-activation depression of H-reflex is given by the ratio H0.5Hz/H0.1Hz. The higher the ratio, the smaller the post-activation depression.
Experimental design
Patients were clinically and neurophysiologically evaluated before and after BTx-A treatment. The control measures, performed on both spastic and non-spastic sides, were recorded on the day of treatment, just before the intramuscular injections (pre-injection, PI). The test measures, performed on the affected side only, were repeated 3, 6 and 12 weeks after BTx-A injection. The timing was determined on the basis of the estimated duration of the clinical benefit of the toxin, which is classically assessed 1–6 weeks after the injection and disappears at 12 weeks. A 12-week delay was the minimum post-injection delay required for a second injection (28).
Statistical analysis
The data averaged across the population of patients were expressed as mean ± standard deviation (SD). The non-parametric permutation test for 2 related samples (STATXACT 7.0; Cytel Inc., Cambridge, MA, USA) was used to determine significant differences in post-AD of H-reflex between the paretic and non-paretic leg before BTx-A injection and to compare the pre-injection values with the post-injection values in the treated leg. The statistical significance level was set at p < 0.05 throughout.
RESULTS
Patients’ initial characteristics
Eight patients with chronic hemiplegia following stroke (7 men, 1 woman; mean age 57.4 years (SD 9.3 years)) presenting with lower limb spasticity and requiring BTx-A injection in the calf muscles were tested. The clinical features of the patients are shown in Table I. The lesion was haemorrhagic for 2 patients and ischaemic for 6 patients. The mean time since stroke was 60 months (SD 45) (range 29–168 months). The MAS was 3 for 6 patients; patients 5 and 7 had a stronger spasticity, rated at 4. Four of the 8 patients (1–4) were able to voluntarily contract their calf muscles, whereas the other 4 (patients 5–8) had no residual voluntary motor control. The population of patients with no residual voluntary motor control also tended to have the highest functional disability, with a lower rate of the Functional Independence Measure (FIM) and Functional Ambulation Classification (FAC).
|
Table I. Patients’ initial clinical characteristics |
||||||||||
|
Rank |
Age/Sex |
Stroke mechanism |
Paretic side |
Delaya |
Anti-spastic drug |
Previous BTx injections, n |
Muscle tone (MAS) |
FIM /126 |
FAC /6 |
Residual VMC |
|
1 |
40/M |
Haemorrhage |
Left |
53 |
Baclofen 30 mg/day |
1 |
3 |
114 |
5 |
Yes |
|
2 |
49/F |
Haemorrhage |
Right |
29 |
None |
4 |
3 |
111 |
5 |
Yes |
|
3 |
64/M |
Ischaemia |
Right |
168 |
Baclofen 60 mg/day |
0 |
3 |
112 |
5 |
Yes |
|
4 |
59/M |
Ischaemia |
Left |
43 |
None |
1 |
3 |
97 |
5 |
Yes |
|
5 |
55/M |
Ischaemia |
Left |
72 |
None |
1 |
4 |
56 |
3 |
No |
|
6 |
64/M |
Ischaemia |
Left |
33 |
Baclofen 60 mg/day |
2 |
3 |
108 |
3 |
No |
|
7 |
59/M |
Ischaemia |
Left |
42 |
Baclofen 30 mg/day Dantrolen 200mg/day |
5 |
4 |
107 |
5 |
No |
|
8 |
69/M |
Ischaemia |
Left |
38 |
None |
2 |
3 |
106 |
3 |
No |
|
aDelay between stroke and botulinum toxin-type A (BTx-A) injection (months). M: male; F: female; MAS: modified Ashworth score; FIM: Functional Independence Measure; FAC: Functional Ambulation Classification; VMC: Voluntary Motor Control. |
||||||||||
Only 1 patient (patient 3) had not received any BTx-A injection prior to the study. The BTx-A doses were 100–150 Units Botox® Allergan© and were injected in 2 sites in the soleus. The doses in the gastrocnemius were between 75 and 50 U Allergan©. The maximal total dose injected per patient was 250–300 U Allergan©.
Clinical parameters after injection
The degree of spasticity, as given by the MAS, decreased following BTx-A injection in the triceps surae. For all patients tested, the MAS at 3 weeks was lower by 1 point compared with pre-injection MAS (Table II). The MAS score returned to its initial value after 12 weeks for 4 patients and remained stable for the 4 other patients. In addition, an increase in passive range of motion of ankle dorsiflexion was noted following BTx-A injection for 6 patients and a lack of improvement for 2 patients (patients 6 and 8).
|
Table II. Clinical parameters of the patients at the pre-injection (PI) session and at 3 post-injection times (3, 6 and 12 weeks) |
||||||||
|
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
Patient 7 |
Patient 8 |
|
|
MAS |
||||||||
|
PI |
3 |
3 |
3 |
3 |
4 |
3 |
4 |
3 |
|
3 weeks |
2 |
2 |
2 |
2 |
3 |
2 |
2 |
2 |
|
6 weeks |
2 |
2 |
2 |
2 |
3 |
2 |
2 |
2 |
|
12 weeks |
3 |
2 |
2 |
2 |
3 |
3 |
3 |
3 |
|
ROM |
||||||||
|
PI |
+15 |
+15 |
+10 |
+15 |
–25 |
+10 |
+15 |
+10 |
|
3 weeks |
+20 |
+20 |
+20 |
+20 |
–15 |
+10 |
+10 |
+10 |
|
6 weeks |
+20 |
+25 |
+20 |
+20 |
–15 |
+10 |
+10 |
+10 |
|
12 weeks |
+15 |
+25 |
+20 |
+15 |
–15 |
+10 |
+15 |
+10 |
|
MAS: Modified Ashworth Scale; ROM: range of motion of the ankle dorsiflexion with the knee flexed; W: weeks. |
||||||||
Post-activation depression of soleus H-reflex
Prior to BTx-A treatment, post-AD of H-reflex was significantly lower in the paretic leg than in the non-paretic side (p = 0.01). H-reflex amplitude in response to 0.5 Hz was less depressed in the paretic leg than in the non-paretic leg in 7 of the 8 patients. The H0.5Hz/H0.1Hz ratios obtained on both sides for each patient are shown in Fig. 2A, along with the mean value. The mean value of the ratio was 0.76 (SD 0.20) for the paretic side and 0.55 (SD 0.11) for the non-paretic side. This reduced post-AD on the paretic side is in line with previous studies (23, 26).
Changes in post-AD were observed in the paretic side after intramuscular BTx-A injection. The value of the ratio obtained in the paretic leg before and 3 weeks after injection is shown for each patient in Fig. 2B. In 4 patients (1–4), the ratio decreased and tended towards the non-paretic leg value, whereas in the other 4 patients (5–8) the ratio increased after injection. Thus, due to the opposite effect on the 2 halves of the patient population, for the whole population of patients there was no change in the mean value of the ratio before and 3 weeks after toxin injection.
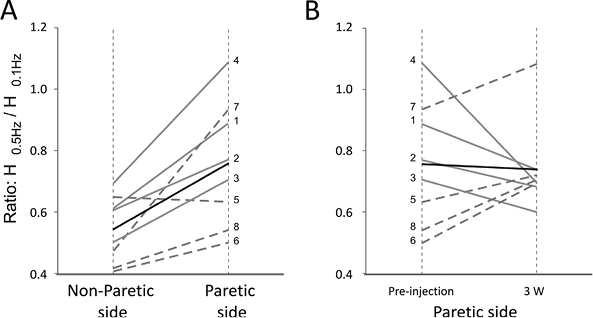
Fig. 2. Changes in post-activation depression of soleus H-reflex. (A) Paretic vs non-paretic sides. (B) Pre- vs post-injection in the paretic leg. The ratio value is shown by thin grey lines for patients 1–4 and by dotted lines for patients 5–8; the mean ratio by a thick black line. (A) In the pre-injection session, the post-activation depression (AD) of soleus H-reflex was reduced in the paretic leg compared with the non-paretic leg. (B) Three weeks’ post-injection, the ratio H0.5Hz/H0.1Hz decreased in the paretic leg of patients 1–4 and tended towards the non-paretic value. For patients 5–8, the ratio increased, which means that post-AD was reduced.
Patients 1–4 and 5–8 also differed in their abilities to perform voluntary muscle contraction, which supports the view that the direction of the change in post-AD of H-reflex might depend on the patients and calls for separating the patients into 2 groups (a group with residual voluntary motor control and a group with no voluntary motor control) and for analysing the data independently.
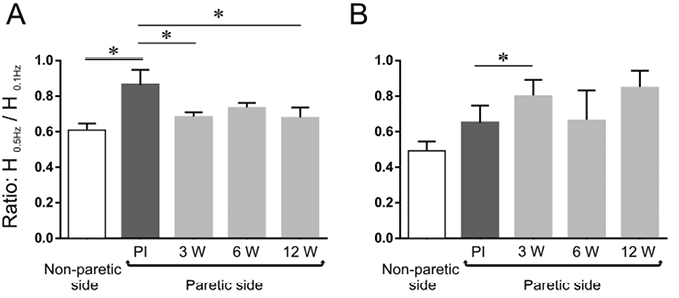
The mean values of the H0.5Hz/H0.1Hz ratio obtained over time in the paretic leg, along with that in the non-paretic leg, are shown for the 2 groups in Fig. 3. In the group of patients (1–4) with residual voluntary motor control (Fig. 3A), the ratio on the paretic side was significantly smaller 3 weeks after injection (0.68 ± 0.06) than before injection (0.86 ± 0.17), which means that the post-AD increased on the paretic side 3 weeks after BTx-A injection (p = 0.04). The values at 6 and 12 weeks after injection (0.73 ± 0.06 and 0.68 ± 0.12, respectively) did not significantly differ from those at 3 weeks; the value at 12 weeks remaining significantly lower than the value in the pre-injection condition. In the group of patients (5–8) with no residual voluntary motor control (Fig. 3B), the ratio on the paretic side was significantly higher 3 weeks after injection (0.80 ± 0.19) than before injection (0.65 ± 0.19), which means that the post-AD decreased on the paretic side 3 weeks after BTx-A injection (p = 0.02). The values at 6 and 12 weeks after injection (0.66 ± 0.34 and 0.85 ± 0.20, respectively) did not differ significantly from those at 3 weeks and in the pre-injection condition.
DISCUSSION
Spasticity, when developed in the ankle extensor muscles of chronic stroke patients, leads to an equinus varus foot, which is one of the main causes of disorders in balance and gait (29). Reduction in hypertonia in the ankle extensor muscles is therefore crucial in order to improve activities of daily living. Botulinum toxin injection, in particular in both gastrocnemius and soleus, is the main pharmacological treatment proposed to stroke survivors in rehabilitation medicine. The aim of the present study was therefore to further explore the central action of BTx-A for better understanding its therapeutic effects.
We examined the effects of BTx-A on the mechanism of post-activation depression (post-AD) of H-reflex, as assessed as the soleus H-reflex depression at low rate of nerve stimulation. Post-AD of the soleus H-reflex was analysed in the paretic leg of 8 hemiplegic spastic post-stroke patients after intramuscular injection of BTx-A in the soleus. Taking the data as a whole, we found no change in the post-AD of the soleus H-reflex after intramuscular injection of BTx-A in the soleus of the paretic leg. However, depending on the patient’s residual motor capacity, post-AD of soleus H-reflex was either increased or decreased 3 weeks after BTx-A injection, which suggests that intramuscular injection of BTx-A does affect the excitability of this spinal inhibitory mechanism.
Central action of BTx-A treatment on post-activation depression of H-reflex
In humans, several studies have explored the neurophysiological consequences of BTx-A treatment in different spinal inhibitory mechanisms and, so far, electrophysiological findings are controversial, probably due to the various motor disorders investigated, the physiological mechanisms tested, and the different toxin injection protocols used (13–18). For example, changes in transmission in the pathway mediating reciprocal Ia inhibition between forearm muscles have been reported after BTx-A injection in spastic dystonia (15), but not in patients with essential tremor (13) or in stroke patients (14). In contrast, the pathways of reciprocal inhibition and recurrent inhibition were affected at the lumbar level in stroke after BTx-A injection in the ankle plantar flexors (12, 26). The present study shows for the first time that the post-AD of soleus H-reflex is also affected.
The post-AD of H-reflex is associated with the mechanism of homosynaptic depression; that is, with a decrease in efficacy of synaptic transmission at the synapse of Ia spindle afferents on motoneurons, due to transmitter depletion in Ia sensory terminals after repetitive activation (21, 30). It can therefore be assumed that synaptic plasticity occurred at the Ia/motoneuron synapse after BTx-A treatment.
Possible mechanisms underlying changes in post-activation depression of H-reflex after BTx-A treatment
The post-AD of H-reflex is related to the frequency of discharge of sensory endings, i.e. when the Ia firing rate is high, the subsequent dynamics of turnover and release of neurotransmitter are slowed and the mechanism of homosynaptic depression in Ia afferent terminals is reduced. Thus, homosynaptic depression is reduced during voluntary contraction as α-γ coactivation makes Ia afferents fire at higher rates (30). It has been also suggested that activity in proprioceptive pathways contributes to changes in post-AD after motor training (27).
The present study, showing changes in the post-AD in spastic patients following BTx-A treatment, suggests that intramuscular injection of the toxin induced changes in the muscle afferent inflow, probably by blocking the intrafusal muscles endplates. Cholinergic blockage of intrafusal muscle fibres by the toxin would lower the mechanical sensitivity of muscle spindle and thereby reduce the Ia afferent resting discharge. BTx-A action on γ-motoneuron endings has been evidenced in rat preparations (7) and strongly suggested in humans, in particular by the suppression of the tonic vibration reflex (TVR) following BTx-A treatment in dystonia and stroke (9, 10). By blocking γ-neuromuscular transmission, BTx-A is thus likely to reduce spindle afferent outflow, which may in turn induce plastic changes in Ia afferent transmission.
Why then does BTx-A treatment lead to an improvement in post-AD of soleus reflex in the 4 patients with a residual motor voluntary control and not in the 4 others with the most severe sequelae? The answer may lie in the mechanism of BTx-A entry into motor nerve terminals (6, 8). The binding and internalization of BTx-A into motor endings is assumed to use the synaptic vesicle cycle and therefore to be activity-dependent. Thus, the fact that BTx-A is more effective in blocking active neuromuscular junctions could explain why BTx-A is likely to block γ-neuromuscular transmission and subsequently to reduce spindle afferent outflow in patients with residual motor activity only. In a study of the possible effects of BTx-A on fusimotor synapses, Trompetto and colleagues (10) tested the TVR in upper limbs of hemiplegic spastic subjects after stroke and found that TVR was affected after BTx-A injection only in patients who had residual motor capacity before the treatment. These results suggest that the alpha-gamma coupling, which could be compromised in patients with severe impairment and no residual motor skills, is necessary for the action of BTx-A on intrafusal fibres. The reduction in spindle inflow following intramuscular injection of BTx-A may therefore contribute to the normalization of the post-AD of soleus H-reflex observed here in the 4 patients with preserved voluntary motor control. Thus, BTx-A could affect post-AD of H-reflex indirectly through peripheral actions on intrafusal muscle fibres.
The more severe motor impairment and the restricted voluntary movements displayed by the 4 patients with no residual voluntary motor control (patients 5–8) might therefore explain the lack of post-AD improvement, but not the increased abnormality of the post-AD of H-reflex observed in this group. It has been suggested that BTx-A also uses an alternative pathway of cell entry, independent of the vesicle cycle, which contributes to BTx-A internalization under resting conditions (6, 8). Following such internalization, BTx-A is likely to be transported retrogradely to motoneuron soma and possibly trans-synaptically to afferent terminals impinging onto motoneurons as demonstrated in animal experiments (11, 31). Marchand-Pauvert and colleagues (12), investigating the effects of BTx-A on spinal recurrent Renshaw inhibition in post-stroke patients, claimed that retrograde transport is the only possible mechanism for the decrease in inhibition after BTx-A injection. It is tempting to attribute the changes in post-AD observed in our group of patients with more severe motor impairment to BTx-A retrograde transport and transcytosis to afferent terminals. As discussed above, BTx-induced blockade of γ-neuromuscular junctions is less likely to occur in this group, allowing the long-distance effects of BTx-A to be manifest. Finally, post-AD of H-reflex being under descending control (32, 33), the supraspinal influences could be suppressed in case of severe motor impairment secondary to stroke, which may also impact the synaptic plasticity at the Ia/motoneuron synapses and lead to the post-AD changes observed in the group of patients with higher functional disabilities and no residual voluntary motor control.
Clinical implications
Studies in spastic patients have revealed that many spinal inhibitory mechanisms controlling motoneuron discharge are reduced, which may contribute to the hyperexcitability of the monosynaptic reflex pathways that characterizes spasticity. However, their effective involvement in spasticity is debated because of the complexity of the pathophysiological mechanisms. The only mechanism whose dysfunction seems actually to contribute to spasticity development is the post-AD of H-reflex (23). Indeed, it is the only mechanism to be consistently reduced in spasticity regardless of its cause, whether multiple sclerosis, spinal cord injury (24), cerebral palsy (25) or stroke (23, 26). In agreement with the literature (23, 26), the post-AD in the paretic side of the whole post-stroke patient population was lower than in non-paretic side.
As expected, BTx-A injection in the soleus muscles decreased spasticity. For all the patients, the MAS was reduced 3 weeks after injection, which is the delay reported for maximal clinical benefit (34). Spasticity improvement tends to persist in the group of patients with residual voluntary motor control, but not in the group of patients without voluntary motor control. The score is still reduced 12 weeks after injection for 3 out of 4 patients in the first group, but only for 1 subject out of 4 in the second group. Similarly, passive ankle dorsiflexion was improved after BTx-A injection in all patients with residual voluntary motor control, but for only 1 patient in the other group. These results suggest that motor recovery following BTx-A intramuscular injection was overall better for patients with residual voluntary motor control. In line with this assumption, post-AD was restored 3 weeks after intramuscular injection of BTx-A in patients who retained some degree of active movements and this effect persisted over a period of 12 weeks. Conversely, the reduction in post-AD was transiently reinforced for 3 weeks after injection in patients whose voluntary movements were initially restricted. These findings support previous observations that the central action of BTx-A depends on the residual motor capacity of the chronic post-stroke patients (10).
Taking into account that physical exercise tends to normalize post-activation depression in post-stroke patients (27), it is suggested that the effects of BTx-A intramuscular injection may constitute a priming of the effects of physical therapy in post-stroke patients with preserved voluntary motor control. However, a study in a large group of patients will be necessary to determine whether BTx-induced changes in frequency-related depression of H-reflex amplitude may be a useful predictor of the efficacy of rehabilitation therapy in post-stroke patients.
Conclusion
Post-activation depression of soleus H-reflex, which is reduced on the affected leg of hemiplegic spastic patients, is affected 3 weeks after intramuscular injection of BTx-A into the ankle extensor muscle, suggesting that BTx-A modulates Ia synaptic transmission onto motoneurons. Depending on the residual motor capacity of the chronic spastic post-stroke patients, post-AD is found to be either restored or further reduced. It can be assumed that the effectiveness of BTx-A treatment in post-stroke patients, although mainly due to its peripheral action, might also depend on its central effects, which induce synaptic plasticity at the Ia-motoneuron synapse.
REFERENCES
