Fary Khan, MBBS, MD, FAFRM (RACP)1,2,3, Bhasker Amatya, MD, MPH1, Louisa Ng, MBBS, MD, FAFRM (RACP)1,2 and Mary Galea, PhD, BAppSci (Physio), BA, GradDipPhysio, GradDipNeurosci1,2
From the 1Department of Rehabilitation Medicine, Royal Melbourne Hospital, 2Department of Medicine (Royal Melbourne Hospital), The University of Melbourne, Parkville, Victoria, 3School of Public Health and Preventive Medicine, Monash University, Victoria, Australia
OBJECTIVE: To assess the effectiveness of an interdisciplinary ambulatory rehabilitation programme for persons with spina bifida in an Australian community cohort.
METHODS: Fifty-four participants randomized to a treatment group (n = 27) for a high-intensity rehabilitation programme (with cognitive behavioural therapy) or a control group (n = 27) comprising usual care. Outcome measures include: Disability: Urogenital Distress Inventory (UDI6), Incontinence Impact Questionnaire-7 (IIQ7), American Urological Association Symptom Index (AUA), Wexner-Faecal Incontinence Score (WFIS), Neurological Disability Scale (NDS); Participation: Depression, Anxiety Stress Scale (DASS), McGill Quality of Life (MQOL), Brief COPE Scale, Generalized Self-efficacy Scale (GSE). Assessments were made at baseline and 3-months post-intervention.
RESULTS: Adjusted for baseline disease and demographic covariates, the intervention group improved significantly at 3-month follow-up for primary and secondary outcomes, with moderate to large effect sizes (r): urinary/bowel dysfunction (AUA, UDI6, IIQ7, WFIS) (p < 0.001 for all, r = 0.4–0.7); and cognitive function: NDS “cognitive” and “mood” (p < 0.01, r = 0.6 for both); DASS “depression”, “anxiety” and “stress” (p < 0.001 for all, r = 0.5–0.7); MQOL total (p = 0.013, r = 0.5), “psychological symptoms” (p < 0.001, r = 0.8); “active coping” (p = 0.035) and “self-efficacy” scores (GSE p < 0.001). No difference between groups was noted in other subscales.
CONCLUSION: Targeted rehabilitation can improve clinical outcomes in persons with spina bifida. Further research is needed for longer-term outcomes related to “ageing” and participation restriction.
Key words: spina bifida; rehabilitation; disability; participation; impairment; patient outcome.
J Rehabil Med 2015; 00: 00–00
Correspondence address: Fary Khan, Department of Rehabilitation Medicine, Royal Melbourne Hospital, 34–54 Poplar Road, Parkville, Victoria 3052, Australia. E-mail: fary.khan@mh.org.au
Accepted May 20, 2015; Epub ahead of print Jul 15, 2015
INTRODUCTION
Spina bifida (SB), a congenital neural tube defect, has an annual incidence of 1 per 1,000 live births worldwide (7 per 10,000 live births in Australia) (1). Myelomeningocele is the most common type (1, 2). The aetiology of SB is unknown, and can be heterogeneous, including chromosome abnormalities, single gene disorders, and teratogenic exposure (3). Although there is improved survival of persons with SB (pwSB) (78% survive to ≥ 17 years) due to better management of disease-related complications and medical care (4, 5), many have disease- and age-related secondary disabilities, which require interdisciplinary (ID) care over a lifetime (6). SB remains a significant cause of chronic disability worldwide, with associated financial, economic and personal costs to the pwSB, their carers and the community (6).
Based on the International Classification of Functioning, Disability and Health (ICF) framework (7), SB-related impairments (such as neuromuscular weakness, neurogenic bladder or bowel, hydrocephalus, cognitive impairment, bone or joint deformity, insensate skin) can cause limitation in “activity” (reduced mobility, self-care ability, cognitive dysfunction) and “participation” (employment, study, social reintegration) (8, 9). Numerous complications result from various childhood procedures (such as ventriculo-peritoneal shunts, urinary diversionary procedures, orthopaedic surgery) (8, 9). As the disease progresses other issues surface, such as tethered cord, syringomyelia, degenerative musculoskeletal issues, osteoporosis, cardiopulmonary disease, obesity, latex sensitivity, and others (4, 8). These disabilities have a cumulative effect in pwSB, reduce their quality of life (QoL) and can cause considerable distress.
PwSB require concurrent rehabilitation for longer-term management in conjunction with medical and surgical management (2, 3). Rehabilitation provides medically supervised patient-centred ID care delivered by various health disciplines that maximize activity and participation. The SB population is physically inactive with poor aerobic fitness, muscle strength, and flexibility (10, 11), and with mobility restrictions in 26–61% (11). Exercise training in pwSB improves aerobic and strength training and cardiorespiratory endurance (11). Although the majority of pwSB have normal intelligence, many have specific cognitive disabilities related to multiple shunt revisions and hydrocephalus, and are amenable to cognitive remediation (3, 12). These impairments may further reduce mobility and self-care ability, and lead to skin break-down, social isolation, poor QoL and low self-confidence (13). Approximately one-third of hospitalized pwSB in the USA are due to conditions that are potentially preventable with better outpatient care (urinary infection, skin wounds) (14). Despite the high prevalence of depression and anxiety in pwSB (12, 15), the impact of psychological distress is not well studied.
Although several studies in the paediatric population have demonstrated the effectiveness of a coordinated ID approach to management, this does not extend to adults, and there is lack of comprehensive ambulatory care models (16–18). The aim of this study, therefore, was to conduct a randomized controlled trial (RCT) to assess the effectiveness of a structured ID rehabilitation intervention to improve disability and participation in an adult SB population in an Australian community cohort.
METHODS
Participants and setting
The study was conducted at the Royal Melbourne Hospital (RMH), a tertiary referral centre in Victoria, Australia. It has the only state-wide ID clinic in Victoria to address disability management for pwSB. Patients are referred from public and private clinics across the state and enrolled in the SB clinic database at the RMH in conjunction with the Department of Health, Victoria. Participants were eligible if they were ≥ 18 years with a confirmed diagnosis of SB (clinical and radiological), able to communicate and understand English, and willing to give informed consent. Exclusion criteria were: those who were medically unstable, or with unstable psychiatric disorders that limited participation in rehabilitation, persons who were bed-bound and/or institutionalized. In addition, pwSB who were inpatients at RMH during the study period and those who had received inpatient care in the 6 months preceding recruitment were excluded.
The study was approved by the Royal Melbourne Hospital Ethics Committee (HREC number 2012.078) and informed consent was obtained from all participants.
Procedure
All 85 eligible patients were invited to participate in this project by an independent project officer at the RMH SB clinic. Those who met the inclusion criteria were provided with detailed information and, after providing written consent, were recruited for the study. An independent statistician randomized participants to treatment or control groups using a computer-generated sequence, with allocation concealed from the treating team. The treatment group received an individualized high-intensity ID ambulatory rehabilitation programme (see treatment schedule) with intensive education, continence and skin care programmes, and cognitive behavioural therapy (CBT). The control group received standard care (supervised by their family doctors) and, as per usual practice, were reviewed in the SB clinic at RMH at 6-monthly intervals for advice about bladder/bowel, skin care and seating.
Assessment interviews. All baseline (T1) assessments were completed by 2 independent researchers, within a 3-week period in the hospital clinic using a structured format. These assessors (a physician and a research nurse) received training in cognitive and functional ability assessments. They were not in contact with the treating team or shared information about participants or assessments. The assessors collected participant information using standardized instruments (see measures below). Each interview took approximately 45 min.
Follow-up assessments (T2) were completed at the hospital clinic or in participants’ homes 3 months after completion of the ID rehabilitation programme for the treatment group, and 3 months after initial assessment for the control group. The assessors did not have access to previous assessments, participant treatment schedules or treating team documentation.
Treatment schedules. The RMH SB ambulatory rehabilitation programme provides intensive treatment, beyond symptomatic management, and specific strategies to improve activity and participation. This includes advice and limited support for pwSB attending maintenance programmes at various community centres across the state. The RMH centre-based programme included 30-min blocks of individual therapy sessions, 2–3 times per week for 6 weeks (provided by physiotherapist (PT), occupational therapist (OT) and social worker (SW)), such as a physical reconditioning programme, wheelchair/seating evaluation, task reacquisition skills and whole-body adaptive techniques. Subsequently, participants were involved in similar maintenance programmes, either at home or in the community while not attending the treatment centre. Participants in the treatment group, in addition to the ambulatory rehabilitation programme, received individualized ID care with intensive focus on education for self-management, continence and skin care, and a cognitive behavioural programme for an additional 4–6 weeks beyond the usual programme. The study interventions followed the Template for Intervention Description and Replication (TIdieR) Check List (19). This included:
Individualized bladder management: assessment of bladder type, pattern and function, bladder re-education, behaviour management, pelvic floor exercises, strategies for timed/double voiding, catheter care and medication review.
The participants in the control group received a standard outpatient rehabilitation programme at home or at a local community rehabilitation centre, as appropriate. This included usual monitoring by their family physician and 6-monthly reviews at the RMH clinic for bladder and bowel intervention, seating/wheelchair review and limited psychology services as per usual availability in the community.
Blinding of participants was not possible; however, treating therapy teams (at RMH and community centres) were unaware of participant allocation, and the outcome assessors were not in contact with, nor part of, the treating rehabilitation teams. Compliance with the programme was defined as participant attendance in > 80% of the education/treatment sessions. Adverse effects of the rehabilitation programme were noted (injury during treatment, pain, fatigue, etc.).
Disability assessment
Guy’s Neurological Disability Scale (NDS). This is a reliable, responsive measure with 12 categories (cognition, mood, vision, speech, swallowing, upper limb, lower limb function, bladder, bowel function, sexual function, fatigue, and “others”), which assessed neurological disability (graded using a Likert scale of 0 = normal status to 5 = total loss of function/maximal help required) (20).
Urogenital Distress Inventory (UDI6). This assessed the degree to which the symptoms associated with urinary incontinence (UI) were troubling, in 3 domains (symptoms related to stress UI, detrusor over-activity and bladder outlet obstruction) using a 4-point response scale (0 = not at all, 1 = slightly, 2 = moderately and 3 = greatly) (21).
American Urological Association Symptom Index (AUA). A 7-item index assessed severity of urinary symptoms. The participants chose 1 of 6 answers (scored on 0 = no problem to 5 = severe impact). The total score ranged from 0 to 35 (asymptomatic to very symptomatic). A single question assessed QoL due to urinary symptoms, with response ranging from 0 (delighted) to 6 (terrible) (22).
Incontinence Impact Questionnaire (IIQ7). This questionnaire assessed the impact of urinary and bowel incontinence in 4 domains (physical activity, social relationships, travel and emotional health) using a 4-point response scale (0 = not at all to 3 = greatly) (23).
Wexner Faecal Incontinence Score (WFIS). The WFIS measured symptom severity, with the score derived from a rating of frequency of the type of incontinence and whether the patient’s lifestyle is altered by incontinence (0 = neither incontinence or impact, 20 = worst possible incontinence and impact) (24).
Participation measurement
Depression Anxiety Stress Scale (DASS). A 21-item instrument assessed the negative emotional states of depression, anxiety and stress (25). Participants rated the extent to which they experienced each state over the past week on a 4-point Likert rating scale.
McGill Quality of Life questionnaire (MQOL). A 16-item questionnaire with 5 domains (physical wellbeing, physical symptoms, existential wellbeing, psychological symptoms and support) assessed overall QoL (26). Each question was rated from 0 (not at all) to 10 (extremely). A single-item scale (QOL-SIS) (rated from 0 = very bad to 10 = excellent) assessed participants’ self-perceived QoL.
Brief COPE scale (B-COPE). An inventory of 14 subscales (active coping, planning, positive reframing, acceptance, humour, religion, using emotional support, using instrumental support, self-distraction, denial, venting, substance use, behavioural disengagement and self-blame) assessed effective and ineffective coping (27).
Generalized self-efficacy scale (GSE). This scale assessed a general sense of perceived self-efficacy (28). The 10 items of the scale were mixed at random into a larger pool of items that have the same response format on a 4-point scale.
Statistical analysis
Data were de-identified, entered and analysed by an independent project officer. Clinical data were presented in a descriptive manner, with additional analyses conducted for scores of all measurement tools.
The primary outcome was the impact of intervention on disability (bladder/bowel). A sample of 22 participants in each group was needed for an 80% chance to detect a 3-point difference between the intervention and control groups in UDI6 and IIQ7 from baseline to 3 months, assuming a standard deviation of 3.5 in both groups (29). Because of the distribution of scores, non-parametric tests (Mann-Whitney U tests) compared change scores (baseline minus 3 months post-treatment) on each of the outcome measures (UDI6, IIQ7,WFIS, DASS, MQOL, Brief Cope, GSE) for the control and treatment groups. Clinically important changes were estimated as effect sizes (ES, r) using Cohen’s criteria (0.1 = small, 0.3 = medium, 0.5 = large effect). Additional analyses compared change scores on NDS, AUA total and AUA QoL. The estimate was based on a 2-sided α=0.05. Analyses were on an intention to treat (ITT) basis, with participants assigned according to their initial allocation irrespective of their subsequent compliance with the protocol.
RESULTS
Of the 85 eligible patients from the RMH SB database, 54 agreed to participate and provided written consent. Three patients had received inpatient rehabilitation within 6 months prior to study recruitment and 1 person was an inpatient during the study period, and hence were not eligible. The remaining participants declined due to excessive travel distance or expense, work, study and not being able to participate for 6 weeks consecutively; and 2 patients were not contactable. Of the 54 participants, 27 each were allocated to the treatment and control groups. One participant in the treatment group and 3 in the control group dropped out at the 3-month follow-up (Fig. 1). No participants in the control group required treatment during the study period. The compliance rate of the intervention group in their rehabilitation programme was 82%.
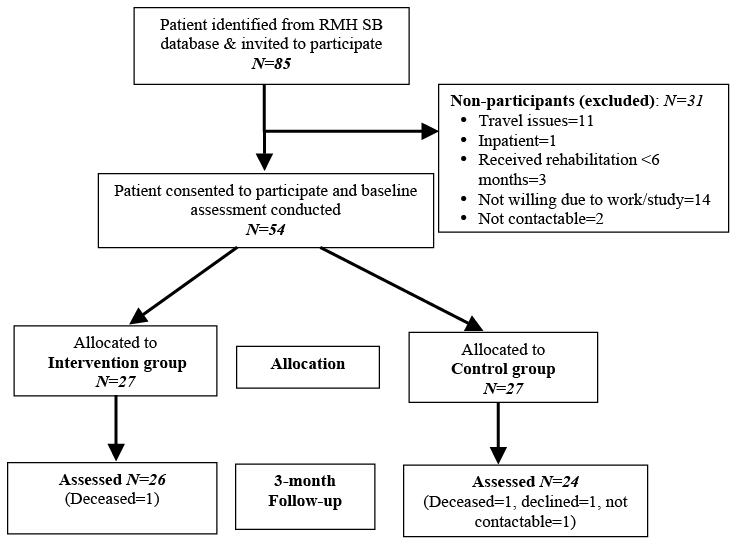
Baseline characteristics
Participants’ socio-demographic and clinical characteristics at baseline are summarized in Table I. The mean age of participants was 33.3 ± 9.3 years (range 18–49 years) and 57% were female. Although baseline demographic and medical characteristics were similar across treatment arms, participants’ in the intervention group had more myelomeningoceles (18 vs 13) and L3–L5 level of injury (16 vs 10), compared with the treatment group; this, however, was not statistically significant. Participants in both groups reported neurogenic bladder and bowel, and half had some degree of cognitive impairment assessed by the treating SB team as well as those reported by the patient and/or carer. Bowel incontinence (and overflow) was reported by 29 participants (17 in the intervention group), while severe constipation was reported by 9 participants (5 in the intervention group). The majority reported urinary incontinence (n = 35, 65%; 19 in the intervention group). The most common bladder pattern was detrusor hyporeflexia, and approximately half had bladder augmentation or other bladder surgery in childhood. Approximately n = 26 (48%) (12 in the intervention group) used urinary catheters for drainage. All participants received prophylactic cranberry capsules and antibiotics (when indicated for urinary tract infections). All study participants used some aid for their mobility: such as orthopaedic bracing, crutches or a wheelchair, of which just over half (52%, n = 26: 17 in the intervention group) used a wheelchair. No adverse events were reported in either group. There was no significant difference between participants lost to follow-up and those who provided post-treatment results in terms of demographic and medical characteristics, and median scores for measures used.
|
Table I. Socio-demographic characteristics of participants (n=54) |
||
|
Characterisitics |
Intervention group (n=27) |
Control group (n=27) |
|
Age, years, mean (SD) [range] |
32.9 (9.2) [18.4–47.5] |
29.7 (9.2) [18.5–48.8] |
|
Sex, female |
18 (66.7) |
13 (48.1) |
|
Marital status, n (%) |
||
|
Married/partner |
3 (11.1) |
4 (14.8) |
|
Single/divorced/separated/widow |
24 (88.9) |
23 (85.2) |
|
Living condition, n (%) |
||
|
Alone |
6 (22.2) |
3 (11.1) |
|
Partner/ family |
19 (70.4) |
20 (74.1) |
|
Education, n (%) |
||
|
Secondary |
17 (63.0) |
9 (33.3) |
|
Tertiary |
17 (63.0) |
8 (29.6) |
|
Employed, n (%) |
11 (40.7) |
14 (51.9) |
|
Carer, n (%) |
14 (51.9) |
13 (48.1) |
|
Smokers, n (%) |
5 (18.5) |
6 (22.2) |
|
Consumes alcohol, n (%) |
14 (51.9) |
14 (51.9) |
|
SB type (n = 46), n (%) |
||
|
Meningocoele |
7 (28.0) |
5 (23.8) |
|
Myelomeningocele |
18 (72.0) |
16 (76.2) |
|
Level of injury (n = 44), n (%) |
||
|
≈ L2 |
5 (21.7) |
3 (14.3) |
|
L3–L5 |
16 (69.6) |
10 (47.6) |
|
<S1 |
1 (4.3) |
7 (33.3) |
|
VP stunt >1 (n = 51), n (%) |
15 (57.7) |
17 (68.0) |
|
Co-morbidities, n (%) |
17 (62.9) |
9 (33.3) |
|
Currently on medications, n (%) |
22 (81.5) |
20 (74.1) |
|
Latex allergy, n (%) |
3 (11.1) |
8 (29.6) |
|
Symptoms, n (%) |
||
|
Cognitive impairment |
18 (66.7) |
16 (59.3) |
|
Visual impairment* |
8 (29.6) |
7 (25.9) |
|
Hearing impairment |
2 (7.4) |
1 (3.7) |
|
Falls |
8 (29.6) |
2 (7.4) |
|
Contracture |
9 (33.3) |
11 (40.7) |
|
Dysphasia |
2 (7.4) |
1 (3.7) |
|
Bladder dysfunction |
22 (81.5) |
20 (74.1) |
|
Bowel dysfunction |
23 (85.2) |
19 (70.4) |
|
Overweight/obese |
8 (29.6) |
6 (22.2) |
|
Mobility (wheelchair/crutches/braces) |
17 (63.0) |
11 (40.7) |
|
At high risk of pressure area |
12 (44.4) |
12 (44.4) |
|
*Significant at the 0.05 level. SB: spina bifida; SD: standard deviation; VP: ventriculoperitoneal. |
||
Outcome measurements change scores
Change scores (baseline minus post-treatment) for all outcome measures were calculated for the control and treatment groups (Table II.)
|
Table II. Summary of per protocol analysis of outcomes of multidisciplinary rehabilitation programme |
|||||||
|
Scales |
Intervention group (n = 26) |
Control group (n = 24) |
Mann-Whitney U |
Z value |
p-value |
Effect size |
|
|
Median (IQR) |
Median (IQR) |
||||||
|
AUA |
|||||||
|
Total (0–35) |
6.5 (0, 9.25) |
0 (0, 0) |
127.0 |
–3.81 |
< 0.001 |
0.54 |
|
|
QoL (0–6) |
0 (0, 1.0) |
0 (–1.75, 0) |
176.5 |
–2.84 |
0.004 |
0.40 |
|
|
IIQ-7 (0–21) |
2.0 (0, 5.5) |
0 (–1.75, 0) |
88.5 |
–4.47 |
< 0.001 |
0.63 |
|
|
UDI (6–24) |
2.0 (0, 4.0) |
0 (–1.0, 0) |
68.0 |
–5.07 |
< 0.001 |
0.72 |
|
|
WFIS (0–20) |
0.5 (0, 3.25) |
0 (–0.75, 0) |
117.0 |
–4.34 |
< 0.001 |
0.61 |
|
|
GUY’s NDS (0–5) |
|||||||
|
Cognitive disability |
1.0 (0, 2) |
0 (0, 0) |
141.0 |
–3.95 |
<0.001 |
0.56 |
|
|
Mood |
1 (0.2) |
0 (–0.75, 0) |
104.0 |
–4.41 |
< 0.001 |
0.62 |
|
|
Visual |
0 (0, 0) |
0 (0, 0) |
276.0 |
–1.70 |
0.089 |
0.24 |
|
|
Speech |
0 (0, 0) |
0 (0, 0) |
312.0 |
0.00 |
1.000 |
0.00 |
|
|
Swallowing |
0 (0, 0) |
0 (0, 0) |
310.5 |
–0.06 |
0.951 |
0.01 |
|
|
Upper limb |
0 (0, 0) |
0 (0, 0) |
299.5 |
–0.47 |
0.641 |
0.07 |
|
|
Lower limb |
0 (0, 0) |
0 (0, 0) |
270.5 |
–1.20 |
0.229 |
0.17 |
|
|
Bladder |
0 (0, 1) |
0 (–0.75, 0) |
225.0 |
–1.97 |
0.049 |
0.28 |
|
|
Bowel |
0 (0, 1.5) |
0 (0, 0) |
212.5 |
–2.29 |
0.022 |
0.32 |
|
|
Sex |
0 (0, 0.5) |
0 (0, 0) |
292.0 |
–0.52 |
0.604 |
0.07 |
|
|
Fatigue |
0 (–0.5, 0.5) |
0 (0, 0) |
290.0 |
–0.52 |
0.605 |
0.07 |
|
|
Other |
0 (0, 0) |
0 (0, 0) |
300.5 |
–0.31 |
0.758 |
0.04 |
|
|
DASS |
|||||||
|
Total (0–126) |
15.0 (2.0, 37.5) |
–2.0 (11.5, 0) |
12.0 |
–5.86 |
< 0.001 |
0.83 |
|
|
Depression (0–42) |
7.0 (1.5, 14) |
0 (–11.5, 0) |
77.5 |
–4.61 |
< 0.001 |
0.65 |
|
|
Anxiety (0–42) |
2.0 (0, 12.5) |
–3.0 (–7.5, 0) |
73.5 |
–4.72 |
< 0.001 |
0.67 |
|
|
Stress (0–42) |
3.0 (0, 12.5) |
–2.0 (–4.0, 2.0) |
121.0 |
–3.74 |
< 0.001 |
0.53 |
|
|
MQOL |
|||||||
|
Total (0–150) |
–9.0 (–23.0, 1.0) |
0.5 (–7.5, 11.25) |
184.0 |
–2.49 |
0.013 |
0.35 |
|
|
Single item scale (0–10) |
–0.5 (–2.0, 1.0) |
0 (–1.0, 0) |
270.0 |
–0.84 |
0.402 |
0.12 |
|
|
Physical symptom (0–30) |
–3.0 (–6.25, 1.25) |
0 (–4.0, 1.75) |
270.0 |
–0.93 |
0.402 |
0.13 |
|
|
Physical wellbeing (0–10) |
0 (–2.0, 2.0) |
–2.0 (–3.75, 1.0) |
229.0 |
–1.03 |
0.305 |
0.15 |
|
|
Psychological symptoms (0–30) |
–5.5 (–12.25, –1.75) |
3.0 (0.25, 5.75) |
9.0 |
–5.92 |
< 0.001 |
0.84 |
|
|
Existential wellbeing (0–60) |
–2.0 (–5.25, 4.25) |
–2.0 (–4.0, 1.75) |
304.0 |
–0.16 |
0.876 |
0.02 |
|
|
Support (0–20) |
–1.0 (–2.0, 0.25) |
0 (–1.0, 0) |
277.5 |
–0.69 |
0.492 |
0.10 |
|
|
B-COPE |
|||||||
|
Total (28–112) |
–3.0 (–13.5, 3.75) |
3.5 (–4.5, 23.7) |
231.5 |
–1.56 |
0.118 |
0.22 |
|
|
Problem-focused coping strategies (2–8) |
|||||||
|
Active coping |
–1.0 (–3.0, 0.25) |
0 (–1.0, 2.0) |
204.5 |
–2.11 |
0.035 |
0.30 |
|
|
Planning |
0 (–3.0, 1.25) |
0 (–2.5, 2.75) |
236.0 |
–1.49 |
0.136 |
0.21 |
|
|
Positive re-framing |
0 (–2.25, 1.25) |
0.5 (–1.0, 3.75) |
242.5 |
–1.36 |
0.173 |
0.19 |
|
|
Acceptance |
0.5 – (2.0, 4.0) |
0.5 (–1.75, 3.5) |
308.0 |
–0.08 |
0.938 |
0.01 |
|
|
Humour |
0 (–1.0, 1.0) |
0 (–0.75, 2.0) |
293.5 |
–0.37 |
0.712 |
0.05 |
|
|
Religion |
0 (–0.25, 1.25) |
0 (0, 1.0 |
300.5 |
–0.28 |
0.812 |
0.04 |
|
|
Using emotional support |
–1.0 (–2.25, 0.25) |
0 (–1.75, 2) |
222.5 |
–1.76 |
0.079 |
0.25 |
|
|
Using instrumental support |
–1.0 (–2.0, 1.25) |
0.5 (–2.0, 3.0) |
261.0 |
–1.00 |
0.319 |
0.14 |
|
|
Emotion-focused coping strategies (2–8) |
|||||||
|
Self-distraction |
–1.0 (–3.0, 0.25) |
–1.0 (–3.0, 2.0) |
288.5 |
–0.46 |
0.645 |
0.07 |
|
|
Denial |
0 (0, 0) |
0 (0, 0.75) |
288.5 |
–0.52 |
0.605 |
0.07 |
|
|
Venting |
0 (–0.25, 2.0) |
0 (0, 2.0) |
266.0 |
–0.93 |
0.353 |
0.13 |
|
|
Substance use |
0 (0, 0) |
0 (0, 0) |
299.5 |
–0.29 |
0.773 |
0.04 |
|
|
Behavioural disengagement |
0 (–0.25, 1.25) |
0 (0, 0) |
279.0 |
–0.71 |
0.480 |
0.10 |
|
|
Self-blame |
0 (–1.25, 2.0) |
0 (0, 1.75) |
294.0 |
–0.36 |
0.717 |
0.05 |
|
|
GSE (10–40) |
–1 (–9.25, 0) |
0.5 (0, 3.0) |
137.0 |
–3.50 |
< 0.001 |
0.49 |
|
|
p < 0.05 is given in bold. Effect size was calculated as r = z/square root of n, where n = total number of cases. Values above 0.5 represent large effect sizes. AUA: American Urological Association Symptom Index; B-COPE: Brief Coping Scale; DASS: Depression Anxiety Stress Scale; ES: effect size; GSE: Generalized Self-Efficacy Scale; IIQ7: Incontinence Impact Questionnaire; IQR: interquartile range; MQOL: McGill Quality of Life; NDS: Guy’s Neurological Disability Scale; n: total number; QoL: quality of life; UDI6: Urogenital Distress Inventory. |
|||||||
Change in subjective disability outcomes. At the 3-month post-treatment follow-up, both bowel and bladder function improved significantly in the intervention group compared with the control group. Mann-Whitney U tests revealed a significant difference between treatment and control group participants in IIQ-7, UDI, AUA and WFIS total scores (p<0.001 for all), with moderate to large ES (r = 0.4–0.7) and NDS “bladder” and “bowel” subscales (p < 0.05, r = 0.3 for both). Significant improvement in cognitive symptoms was also seen in favour of the intervention group (NDS “cognitive disability” and “mood” subscales (p < 0.01, r = 0.6 for both). There were no significant effects on other outcomes (Table II).
Change in participation and QoL outcomes. At the 3-month follow-up, compared with the control group, statistically significant improvement in the treatment group was seen in most of the participatory measures assessed: DASS “depression” (p < 0.001, r = 0.6), “anxiety” (p < 0.001, r = 0.7) and “stress” (p < 0.001, r = 0.5) subscales; MQOL total (p = 0.013, r = 0.5) and “psychological symptoms” subscale (p < 0.001, r = 0.8); Brief COPE “active coping” subscale (p = 0.035, r = 0.3) and GSE total score (p < 0.001, r = 0.5). Significant improvement in QoL in relation to current urinary symptoms was found in favour of the intervention group (single item AUA QoL scale, p = 0.004, r = 0.4). No difference between groups was noted in other subscales (Table II).
DISCUSSION
To our knowledge this is the first RCT evaluating the effectiveness of an ambulatory ID, integrated rehabilitation programme specifically designed to address symptomatology and psychological issues in a SB population in an Australian community cohort. This study demonstrates that a comprehensive, coordinated clinical approach targeting specific symptoms (such as continence), and cognitive behavioural strategies for self-management, coping and psychological adjustment, improve activity and participation in pwSB. The treatment group compared with the control group, showed a significant reduction in bladder- and bowel-related disability and psychological distress, and improved QoL (and psychosocial gains) at 3-month follow-up. Participants in this study were similar to those in other studies in terms of age, gender, disease severity and treatment (30–34). The ID rehabilitation programme provided standard treatment and management in accordance with existing care protocols and guidelines (4, 8).
Rehabilitative and supportive care needs are frequently experienced by pwSB many years after initial treatment (16, 17). In this study many participants reported ongoing transient and/or persistent physical and psychosocial morbidity, such as bowel and/or urinary dysfunction and psychosocial issues, consistent with other published reports (32, 35, 36). There is evidence that urinary and faecal incontinence not only interfere with everyday life, but are also associated with poorer self-concept, self-esteem and participation (educational achievement, employment, etc.) (32, 37, 38). Furthermore, cognitive impairments are common in this patient population, with detrimental effect on their emotional health and coping ability (39). Consistent with other reports (40), many participants (almost 50%) in this study were dependent on their carers for management and support. This information has implications for the future planning of clinical service delivery models for improved patient outcomes for pwSB.
It is difficult to compare the findings of this study with others, due to the lack of studies in a similar context. However, the positive effects on various aspects of bladder/bowel and cognitive/behavioural function in this study are consistent with other reports in different SB subgroups and settings (5, 6, 18, 19, 37, 38). The improvements in bladder/bowel dysfunction, cognitive and other outcomes (QoL, coping strategies) were independent of participants’ demographic and clinical characteristics, which suggest the need to further engage pwSB in rehabilitation activities. It is not surprising that targeted ID rehabilitation strategies for continence care, etc. in the short-term (3 months) improved self-management using task re-acquisition skills. The improvements in coping ability, psychosocial interactions, and other cognitive abilities (problem-solving, memory) may be due to participant characteristics, indicating low baseline health scores and the need for individualized education and specific interventions within the ID programme. The study participants were complex in terms of disease severity, symptoms and co-morbidities (reflective of clinical practice), which required an individualized approach. The majority presented with a range of issues, restricting the extent to which therapy could be standardized, therefore “manualization” of treatments was used (i.e. a described intervention provided by therapist X, e.g. a 30-min treatment session included a continence and cognitive educational approach). However, determining the effective intensity, components and combination of treatment modalities was beyond the scope of this study.
Rehabilitation in pwSB is challenging as they can present with various combinations of disabilities (neurological, urological, orthopaedic physical, cognitive and sensory dysfunctions) (17). In addition to the primary motor and sensory impairments, a cycle of deconditioning can result, followed by inactivity, as well as psychosocial issues that limit participation (30). With improved survival rates in pwSB, the long-term impact of these disabilities on physical and psychological function is often under-estimated (16). A comprehensive ID ambulatory care continuum model should include: prevention and treatment of common clinical issues, such as urinary tract infections (UTIs), pressure areas, neurogenic bowel, sexual dysfunction and secondary conditions resulting from “overuse” syndromes and “ageing” with a disability (17).
The ICF (7) was used as a conceptual framework in selecting the best outcomes for measurement in this study for describing the impact of SB at the level of limitation in “activity” and “participation”. This model explains differences in outcomes, such as employment, social relationships and emotional well-being, which are not possible within a pure biological model (suggesting an initial lesion and birth anatomy determining the outcomes such as motor function). Further work is needed to link various ICF categories to issues reported by pwSB, and to develop a developmental disability ICF core-set to facilitate clinical communication, assessment and management.
This study has some potential limitations. First, selection bias cannot be ruled out, as participants were a selective cohort listed on a single database held at single tertiary institution (RMH) who agreed to participate in research projects, thus potentially limiting the generalizability of the findings. However, all eligible participants on the database were contacted, irrespective of their demographic or disease status, and the study cohort came from the only state-wide ID clinic, representing a wider sample of SB in the community. Comparison and generalizability of these results is difficult, larger sample sizes in different settings are needed to confirm these findings. There was no significant difference in any of the study variables between participants who completed post-treatment assessment and those lost to follow-up. We acknowledge that other factors may have impacted bowel/bladder and psychological issues in participants and were not studied. We did not have detailed information on previous neuropsychological reports conducted, and used bedside clinical cognitive assessments to categorize patient eligibility. However, patient self-report and carer report were considered. Although the vast majority of patients had incomplete spinal cord injury, there was no tool or measure to document this precisely. A generic pressure sore grading classification was used for risk management. In this study cognitive dysfunction was not the main outcome measure for assessment, and more research is needed for impact of cognitive deficits. To reduce potential bias the treating therapists and assessors were blinded. The assessors were independent of the rehabilitation or acute hospital teams. Important outcomes, such as impact on carers and families and analysis of costs associated with care, were beyond the scope of this study. The impact of other components of ID rehabilitation modalities and interventions is unknown. There were many challenges in conducting a RCT in a rehabilitation setting. This study was conducted in the “real world” setting of a tertiary public hospital with finite resources, without additional funding. Access to transport for those residing further away from treating facility was difficult. The control group was provided with the ID rehabilitation programme as per usual practice, and were not unduly disadvantaged. Operationally, it was beyond the resources of our hospital to provide therapy for this many patients simultaneously.
Targeted ID rehabilitation care has much to offer pwSB throughout the disease continuum for maintaining activity and participation over the longer-term. This has implications for health service delivery, planning and policy. More research is needed for the effectiveness of “specific” rehabilitation interventions in this population, cost-efficacy and return to work/education; and longer-term outcomes related to ageing with disability and contextual factors associated with participation restriction.
ACKNOWLEDGEMENTS
The authors are grateful to all participants in this study; we thank the Developmental Disability Rehabilitation Clinical Team at Royal Park Campus, Royal Melbourne Hospital for their assistance; Drs Ishani Rajapaksa, Geoff Abbott and Ms Loren Oscari for participant assessments. We thank Taha Khan for data entry.
The authors declare no conflicts of interest.
REFERENCES
