Henrik Pettersson, RPT, MSc1,2, Gun Faager, RPT, PhD1,2 and Elisabeth Westerdahl, RPT, PhD3
From the 1Department of Neurobiology, Care Sciences and Society, Division of Physiotherapy, Karolinska Institutet, 2Department of Physiotherapy, Karolinska University Hospital, Solna and 3Faculty of Medicine and Health, Surgery, Örebro University, Örebro, Sweden
OBJECTIVE: Breathing exercises after cardiac surgery are often performed in a sitting position. It is unknown whether oxygenation would be better in the standing position. The aim of this study was to evaluate oxygenation and subjective breathing ability during sitting vs standing performance of deep breathing exercises on the second day after cardiac surgery.
METHODS: Patients undergoing coronary artery bypass grafting (n = 189) were randomized to sitting (controls) or standing. Both groups performed 3 × 10 deep breaths with a positive expiratory pressure device. Peripheral oxygen saturation was measured before, directly after, and 15 min after the intervention. Subjective breathing ability, blood pressure, heart rate, and pain were assessed.
RESULTS: Oxygenation improved significantly in the standing group compared with controls directly after the breathing exercises (p < 0.001) and after 15 min rest (p = 0.027). The standing group reported better deep breathing ability compared with controls (p = 0.004). A slightly increased heart rate was found in the standing group (p = 0.047).
CONCLUSION: After cardiac surgery, breathing exercises with positive expiratory pressure, performed in a standing position, significantly improved oxygenation and subjective breathing ability compared with sitting performance. Performance of breathing exercises in the standing position is feasible and could be a valuable treatment for patients with postoperative hypoxaemia.
Key words: breathing exercises; cardiac surgery; oxygenation.
J Rehabil Med 2015; 47: 00–00
Correspondence address: Elisabeth Westerdahl, Physiotherapist, Research Centre, Region Örebro County, PO Box 1324, SE-701 13 Örebro, Sweden. E-mail: elisabeth.westerdahl@regionorebrolan.se
Accepted May 12, 2015; Epub ahead of print Jul 1, 2015
INTRODUCTION
Following cardiac surgery, impaired lung function and oxygenation is common in the immediate postoperative period (1–6). Atelectasis in the basal parts of the lungs on the first and second postoperative days has been described in 95–100% of all patients who have undergone coronary artery bypass grafting (CABG) (1, 7). Postoperative physiotherapy is provided during the inpatient phase (2, 8–11). Early mobilization and deep breathing exercises with or without mechanical devices are used to increase lung volume and oxygenation, and to prevent postoperative pulmonary complications (1, 2, 8, 9, 12). It is well known that functional residual capacity (FRC) is affected by surgery and anaesthesia (13), and a reduction in FRC of approximately 20–25% has been found during the third and fourth postoperative days after cardiac surgery compared with preoperative values (4, 12). Jenkins et al. (4) showed that a slumped sitting position has a detrimental effect on FRC compared with upright sitting in cardiac surgery patients.
Research in recent years has shown that deep breathing exercises, with or without devices that provide positive expiratory pressure (PEP), is beneficial to prevent lung function impairments (1, 2). Since an upright position has a positive effect on lung volumes by increasing FRC (4), we hypothesized that the effects of deep breathing exercises could be enhanced in a standing position. To our knowledge there has been no evaluation of standing performance of breathing exercises after cardiac surgery.
The primary aim of this study was to evaluate oxygenation after one session of deep breathing exercises with PEP performed in a sitting position compared with a standing position on the second postoperative day after CABG. Secondary aims were to study subjective breathing ability and adverse effects. Our hypothesis was that standing performance of breathing exercises would increase oxygenation and improve subjective breathing ability compared with sitting performance.
METHODS
Sample
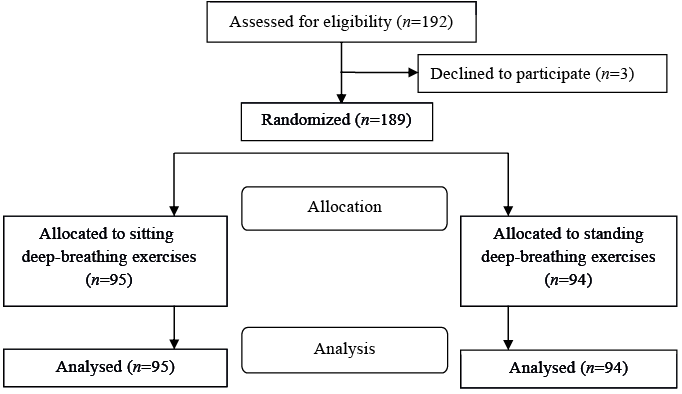
A total of 192 patients who had undergone isolated CABG at Karolinska University Hospital, Solna, Sweden, between April 2010 and October 2011, were consecutively invited to participate in the study on the second postoperative day. Three patients declined without stating a reason (1 female, 2 males) (Fig. 1). The remaining 189 patients had undergone elective, sub-acute, or emergency CABG. Inclusion criteria were that the patient should understand spoken and written Swedish, and should have no thoracic drainage, no symptomatic hypotensive blood pressure (BP), and no other impairments or symptoms that would prevent mobilization to a standing position. The predetermined exclusion criterion was cardiac arrhythmia during the intervention that prevented standing.
The study was approved in April 2010 by the Regional Ethical Review Board in Stockholm, Sweden, (2010/430), and was designed and reported in accordance with the Consolidated Standards for Reporting of Trials (CONSORT) checklist.
Surgery and postoperative care
The patients received general anaesthesia, and CABG was performed through a median sternotomy. The mediastinum and/or one or both pleura were drained. After extubation all patients received oxygen (1–10 l/min) to maintain peripheral oxygen saturation (SpO2) above 90%. All patients spent the first postoperative night in the intensive care unit (ICU), and most arrived at the ward at midday on the first postoperative day. All patients received pain relief according to standard procedures; 1 g paracetamol orally 4 times a day, and a patient-controlled analgesia pump with morphine, or conventional nurse-administered oral morphine analgesia.
Study procedure
The patients were invited to participate in the study by the responsible physiotherapist at midday on the second postoperative day. The patients received oral and written information about the study and signed a written informed consent before baseline measurements were taken. Each patient was randomized to either a sitting control group (n = 95) or a standing intervention group (n = 94) after baseline measurements by picking a numbered letter. An independent statistician had created a computer-generated randomization list, and sealed opaque randomization letters had been prepared and numbered by an independent secretary in another university faculty. Patients not already seated in a chair were assisted to do so before the baseline measurements and randomization were performed. All patients had been without supplemental oxygen and had not performed any breathing exercises or mobilization for 10–15 min before the initial measurements.
Intervention
The breathing exercises consisted of 3 sets of 10 deep breaths performed with PEP. The physiotherapist gave the same instructions on how to perform the breathing exercises to both groups. A bottle filled with 10 cm water and a 30–45-cm long plastic tube (1 cm internal diameter) was used to give an expiratory pressure of 10 cmH2O. The bottle was held by the patient with one or both hands. The instructions were: “Breathe in as much air as you can and then slowly blow out through the plastic tube, being careful not to empty your lungs. Try to save some air at every exhalation.” The patients were allowed to let go of the plastic tube if they preferred to inhale through the mouth instead of the nose.
Patients in the control group were positioned in an armchair with full support at the back, and the standing group performed the breathing exercises in a standing position with the chair directly behind them. The physiotherapist counted out loud, when needed, during the breathing sets. If the breathing exercises were not performed satisfactorily, the same breathing instructions were given again. Between each set of breathing exercises, patients were given a short break in the same position as that in which the breathing intervention took place; the patients in the standing group were not allowed to sit down. During the intervention, the physiotherapist noted any complications. The breathing exercises took, on average, 5 min to perform and the patients were allowed to cough if needed.
Outcome measurements
The primary outcome measurement was SpO2, and the secondary outcome measurements were subjective breathing ability, BP, heart rate (HR), pain at rest, and pain while taking a maximal deep breath.
All measurements were performed immediately before and after the intervention, with the patient sitting in an armchair with full support at the back, regardless of randomization allocation. A third and final set of measurements was taken after 15 min sitting rest. Neither patients nor the physiotherapist were blinded during the gathering of data.
SpO2, BP and HR were measured simultaneously with a portable Spot Vital Signs LXi (Welch Allyn Inc., Skaneateles Falls, NY, USA). The probe was attached to a finger on the right hand, immediately (approximately 10–15 s) after the breathing exercises for both groups. BP was measured on the left arm according to routine procedure in the ward. All collected measurements were registered on a paper record, and entered into a computer file.
Sternotomy pain was quantified, at rest and while performing a single maximal deep breath, on a numeric rating scale ranging from 0 (no pain) to 10 (worst pain imaginable) (14). Directly after the baseline measurement and after the second measurement the patients were also asked to score their subjective ability to take a maximal deep breath by answering the arbitrary study-specific question “How deep a breath can you take now?” on a numerical rating scale ranging from 0 (not able to breathe at all) to 10 (able to take maximal deep breaths). Surgical and demographic data for the 189 patients were extracted from medical records.
Statistical analysis
All data were analysed with version 20.0 of IBM SPSS Statistics for Windows (Armonk, NY, USA). Results are presented as mean ± standard deviation (SD) and/or median [min–max].
Within-group differences before and after the breathing intervention were compared with the Wilcoxon rank-sum test, and between-group differences were analysed with the Mann–Whitney U test as the data were non-normally distributed. All analyses were performed according to intention-to-treat. To obtain a power of 80%, a sample size of 84 patients in each study group was needed to detect a clinically important between-group difference of 2% in SpO2 (90% vs 92%), assuming a SD of 4.6%. Another 24 patients were included to allow for possible drop-outs, giving a total sample of 192 patients. For all statistical tests, a p-value less than 0.05 was considered significant.
RESULTS
Demographic and surgical data for the 189 patients are shown in Table I. No significant differences were found between the two groups in terms of demographic or surgical data. All patients fulfilled the protocol as planned and were included in all statistical analyses. One patient was considered an extreme outlier (Fig. 2), but was included in the analysis according to the intention-to-treat design.
|
Table I. Demographic and surgical data (n = 189) |
|||
|
Variables |
Control group (n = 95) |
Standing group (n = 94) |
p-value |
|
Males/females, n |
83/12 |
74/20 |
0.114 |
|
Age, years, mean (SD) |
67 (9) |
65 (9) |
0.189 |
|
BMI, kg/m2, mean (SD) |
28 (5) |
28 (4) |
0.248 |
|
SpO2 preoperatively, %, mean (SD) |
97 (1) |
96 (2) |
0.122 |
|
ECC, min, mean (SD) |
61 (21) |
69 (24) |
0.610 |
|
AoO, min, mean (SD) Surgery time, min, mean (SD) Mechanical ventilation, min, mean (SD) |
42 (14) 157 (40) 339 (136) |
45 (17) 163 (41) 348 (172) |
0.416 0.491 0.867 |
|
Distal anastomoses, n, mean (SD) |
3 (1) |
3 (1) |
0.733 |
|
AoO: aortic occlusion; BMI: body mass index; ECC: extra corporeal circulation; n: numbers; SpO2: peripheral oxygen saturation; SD: standard deviation. |
|||
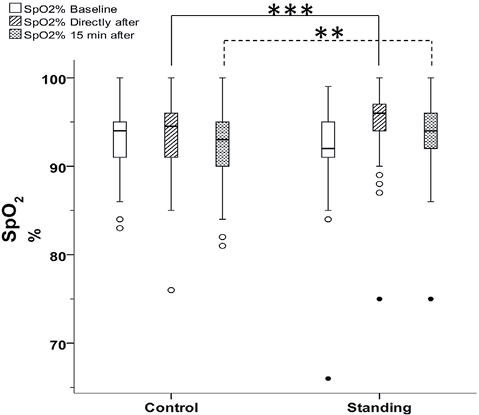
Fig. 2. Box-plots illustrating peripheral oxygen saturation (SpO2) in the control and standing group at baseline, directly after deep breathing exercises with positive expiratory pressure and after 15 min sitting rest. Circles: outliers; filled circles: extreme outliers. There was a significant difference between the groups (Mann–Whitney U test) directly after deep breathing exercises (***p < 0.001) and after 15 min sitting rest (**p = 0.027) (n = 189).
Peripheral oxygen saturation
There was no significant difference in SpO2 between the control group and the standing group at baseline (Table II). In the total sample (n = 189), SpO2 increased significantly directly after the breathing exercises (p < 0.001), and a significant change was still present after 15 min sitting rest (p = 0.004) compared with baseline values (Table II).
Directly after the breathing exercises, the standing group had a higher SpO2 than the control group (p < 0.001). After 15 min sitting rest there was still a significant difference between the groups (p = 0.027), as shown in Fig. 2.
|
Table II. Peripheral oxygen saturation at baseline, directly after deep breathing exercises with positive expiratory pressure, and after 15 min sitting rest (n = 189) |
|||
|
Control group (n = 95) Mean (SD) Median [min–max] |
Standing group (n = 94) Mean (SD) Median [min–max] |
p-valuea |
|
|
Baseline |
93.0 (3.4) 94 [83–100] |
92.3 (4.2) 92 [66–99] |
0.219 |
|
Directly after intervention |
93.5 (3.8) 94 [76–100] |
95.2 (3.5) 96 [75–100] |
< 0.001* |
|
15 min after intervention |
92.5 (3.6) 93 [81–100] |
93.7 (3.5) 94 [75–100] |
0.027* |
|
ap-values refer to the difference between control group and standing group. *Mann–Whitney U test p < 0.05 for between-group comparison. |
|||
Subjective breathing ability
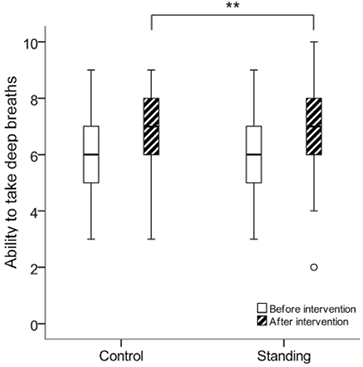
The subjective scoring of the ability to take a single maximal deep breath did not differ at baseline between the control group, median [min–max] (6 [3–9]) and the standing group (6 [3–9]) (p = 0.717). A significantly improved ability to take deep breaths was reported in both groups after the breathing exercises (p < 0.001). Between-group analysis revealed a significant difference between the control group (7 [3–9]) and the standing group (7 [2–10]) (p = 0.004), with the standing group being able to take deeper breaths (Fig. 3).
Blood pressure and heart rate
There were no significant differences in BP between the control group and the standing group at baseline (mean 119/71 (SD 19/8) vs 119/72 (SD 16/8) mmHg; p = 0.904/p = 0.665), directly after the breathing exercises (mean 121/70 (SD 20/8) vs 122/72 (SD 19/8) mmHg); p = 0.701/p = 0.252), or after 15 min sitting rest (mean 118/70 (SD 21/8) vs 116/72 (SD 15/9) mmHg; p = 0.692/p = 0.870). No significant difference in HR was present between the control and standing group at baseline (mean 83 (SD 13) vs 86 (SD 13) beats/min; p = 0.050), but directly after the breathing exercises a significant difference was seen (mean 84 (SD 13) vs 88 (SD 14) beats/min; p = 0.047). After 15 min sitting rest there was no significant difference between the groups (mean 82 (SD 13) vs 86 (SD 14) beats/min; p = 0.121).
Pain at rest and while performing deep breathing
There were no significant differences between the control and standing groups regarding postoperative pain at rest at; baseline median [min-max] (0 [0–4]) vs (0 [0–5]; p = 0.619), directly after intervention (0 [0–7]) vs (0 [0–4]; p = 0.639), or after 15 min sitting rest (0 [0–4]) vs (0 [0–3]; p = 0.578).
There were no significant differences between the groups regarding pain while breathing deeply at baseline (3 [0–6]) vs (3 [0–7]; p = 0.914), directly after the intervention (2 [0–7]) vs (2 [0–7]; p = 0.394), and after 15 min sitting rest (2 [0–5]) vs (2 [0–7]; p = 0.116).
DISCUSSION
To our knowledge this is the first study to describe the effects on oxygenation after cardiac surgery of standing performance of deep breathing exercises compared with sitting performance. Both groups significantly increased SpO2 directly after the breathing exercises compared with baseline values. This finding is in line with previous studies showing that deep breathing exercises increase oxygenation in the immediate period after cardiac surgery (1, 9). Standing performance of the breathing exercises with PEP significantly increased SpO2 compared with sitting performance. Similar improvements in oxygenation related to change in body positioning have been described by Mynster et al. (15), who showed a significant increase of 1–2% in SpO2 when patients changed position from supine to sitting and then to standing on the first and fourth days after laparotomy. An important finding in our study was that SpO2 remained increased after 15 min sitting rest. However, further studies are necessary to evaluate how SpO2 levels change over a longer time.
It is possible that a standing position increases SpO2 by itself, and consequently the unique effect of the intervention is not totally clear, since the mobilization might be as effective as the breathing exercises. However, the current study shows that performing breathing exercises in a standing position is more effective than in a sitting position. An improvement in SpO2 of 1–3% in absolute values obtained by standing while conducting the breathing exercises may be clinically important, especially if the effect of the breathing exercises persists after treatment. The total mean time without supplemental oxygen during the study was 35–40 min. Below the 90% saturation point of the haemoglobin dissociation curve there is a steep drop in arterial oxygen tension, and those few percent may be important given the risk of postoperative myocardial ischaemia (16); this was a reason for the relatively short follow-up time of 15 min after the intervention. One patient in the intervention group had low SpO2 values, but did not present any other signs of discomfort or illness, and was not excluded from analysis according to intention-to-treat.
A shortcoming in the present study was that no arterial blood gases were assessed, which would have provided values on arterial oxygen and carbon dioxide tension (1, 2, 7, 9). However, for practical reasons it was not possible to draw repeated arterial blood samples within this short study period. According to standard procedures at the clinic, arterial catheters were removed from the patients before they left the ICU.
The patients in both groups reported that they could take deeper breaths after the breathing exercises. However, the standing group reported significantly better improvement than the control group, which can be explained by more efficient breathing in the standing position. Changing position from lying to sitting and then to standing has been shown to increase lung volume (4, 17). This indicates that the standing position may have an additional effect on lung volume in breathing exercises, which could further improve oxygenation, as shown in this study. Further evaluation, in studies comparing breathing exercises against mobilization alone, is needed to determine which component is most important. Westerdahl et al. showed that atelectatic areas were significantly reduced after deep breathing exercises performed in a sitting position on the second (1) and fourth days (2) after CABG. It is possible that standing performance of deep breathing with PEP has a more beneficial effect on atelectasis and would optimize the breathing intervention, but unfortunately atelectasis was not examined in the present study.
We chose to evaluate a frequency of 3 sets of 10 deep breaths with PEP, as this is most often prescribed in Swedish hospitals after cardiac surgery (18). Whether an increased number of breaths during each session, or repetitive sessions, would lead to an even greater improvement in SpO2 and subjective breathing ability remains to be evaluated.
It is possible that patients’ expectations, traditions, and nursing habits lead to patients on the ward being allowed to lie in bed and rest for too long, even though preoperative information stressing the importance of early mobilization and breathing exercises is routinely given (8). Physiotherapist-led supervision and instruction in an optimal breathing technique may be of great value in the early postoperative period.
No adverse effects on BP or HR in either group were noticed, even if there was a significantly higher HR in the standing group directly after the breathing exercises of 4 beats/min. We consider this a trivial difference and interpret that the performance of standing breathing exercises is feasible with regards to the central circulation early after cardiac surgery.
The patients’ estimated pain from the sternotomy wound was just below, or of a similar magnitude, to that found in earlier studies (9, 19–22). As shown in the present study, we did not find any significant differences between the groups in pain experienced at rest or when performing deep breathing. These findings are similar to the results of Milgrom et al. (22). We considered it important to evaluate pain while the patients were performing a maximal deep breath, considering that evaluating pain during rest gives insufficient information.
All data in this study were gathered by the same physiotherapist, who was not blinded to the patients’ group allocation. The potential risk of bias was discussed in the research group during the planning of the study, but as the information and instructions were provided in a uniform way, the risk of influencing the patients and their responses was considered minimal.
We have shown that treatment in a standing position is safe and feasible as early as the second day after surgery, and it is possible that the results would have an even larger impact during the following postoperative days. The results may not be fully applicable to patients who are still undergoing thoracic drainage or who have other limitations or restrictions in the immediate postoperative period. Unless there are contraindications to standing, we can recommend that patients who have undergone surgery perform deep breathing exercises in a standing position. The combination of standing and deep breathing with PEP may be useful in the treatment of patients with impaired oxygenation after cardiac surgery.
In conclusion, the performance of deep breathing exercises with PEP in a standing position improved SpO2 more than a sitting performance on the second postoperative day after CABG. The effect lasted for at least 15 min after the treatment session. The standing group reported a significantly better ability to take deep breaths compared with the sitting control group.
ACKNOWLEDGEMENTS
Financial support was provided by grants from the Swedish Research Council (Reg. No. 2009-1385) and the Departments of Thoracic Surgery and Physiotherapy at Karolinska University Hospital, Solna, Sweden.
REFERENCES
