Yi Li, MD, Yun Qu, MD, Mengwei Yuan, MD and Tianhui Du, MD
From the Department of Rehabilitation Medicine, West China Hospital, and Sichuan Provincial Key Laboratory of Rehabilitation Medicine, Sichuan University, Chengdu, Sichuan, China
OBJECTIVE: To perform a meta-analysis of studies investigating the effects of low-frequency repetitive transcranial magnetic stimulation on post-stroke aphasia.
DATA SOURCES: Studies were identified by performing a search of electronic databases (MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Knowledge) for articles published until June 2014.
STUDY SELECTION: Randomized controlled trials (RCTs) reporting treatments with low-frequency repetitive transcranial magnetic stimulation in patients with post-stroke aphasia were included. The outcomes included naming, repetition, comprehension, changes in brain excitability, and adverse events.
DATA EXTRACTION: Two independent reviewers extracted the data. Study quality was evaluated with the PEDro scale.
Data analysis: Of the 879 articles identified, 4 RCTs were included in the final analysis. Data synthesis showed that low-frequency repetitive transcranial magnetic stimulation was beneficial for post-stroke patients in terms of naming (standard mean difference (SMD) 0.51; 95% confidence interval (95% CI) 0.16–0.86) and changes in brain excitability (7.6 ± 33.55; 95% CI –10.7–26.20). However, the changes in repetition (SMD 0.31; 95% CI –0.04–0.65) and comprehension (SMD 0.31; 95% CI –0.14–0.75) after stimulation were not significant. No adverse effects were reported. The included studies were of high methodological quality.
CONCLUSION: These findings indicate that low-frequency repetitive transcranial magnetic stimulation is an effective treatment for recovery of naming. In addition, this treatment favours reorganization of the left-hemispheric language networks.
Key words: transcranial magnetic stimulation; stroke; aphasia; meta-analysis.
J Rehabil Med 2015; 47: 00–00
Correspondence address: Yun Qu, Department of Rehabilitation Medicine, West China Hospital., Sichuan Provincial Key Laboratory of Rehabilitation Medicine, Sichuan University, Chengdu, Sichuan, People’s Republic of China, 610041 Chengdu, China. E-mail: quyben@126.com
Accepted Apr 24, 2015; Epub ahead of print Jul 15, 2015
INTRODUCTION
With an ageing population worldwide, ischaemic stroke has emerged as a leading cause of disability, representing a significant healthcare burden. It has been conservatively estimated that there will be a marked increase in the incidence of stroke to 9 million people annually, particularly in individuals over 65 years of age (1). Post-stroke aphasia, which is a frequent sequela of stroke, occurs in approximately 30% of stroke victims, substantially affecting patients’ quality of life, with significant impairment of both mental and physical components (2). However, compared with spontaneous motor recovery, spontaneous aphasia recovery occurs at a slower rate and over a longer period of time (3). Speech and language therapy (SLT) has been reported to improve various aspects of aphasia, in a study focusing on early intervention as well as training intensity and duration (4). However, the few studies that have evaluated drug therapies have reported limited effects (5). Accordingly, the development of a new treatment option is crucial for patients with post-stroke aphasia.
Repetitive transcranial magnetic stimulation (rTMS), which is a non-invasive and well-tolerated brain stimulation method that acts on the cerebral cortex, may be effective for the treatment of aphasia (6, 7). rTMS induces a magnetic field followed by an electromotive force after penetrating the brain tissue, resulting in changes in cerebral cortex excitability (8). Usually the boundary between inhibitory and excitatory stimulation is set at 4–5 Hz. Lower frequencies can inhibit cortical excitability, whereas higher frequencies have the opposite effect. Under normal physiological conditions, the language centre is located in the dominant hemisphere. However, when aphasic patients perform language tasks, the perilesional areas in the left hemispheric and contralateral homotopic regions show abnormal excitability. This activity is thought to hinder language recovery (9). Therefore, the goals of aphasic recovery are to balance bilateral hemispheric excitability and to reorganize the language network. Other studies have suggested that reducing the excitability of the non-dominant hemisphere with low-frequency repetitive transcranial magnetic stimulation (LF-rTMS) and subsequently relieving the inhibition of the dominant hemisphere could promote a restorative process (10, 11). However, the findings with regard to the effects of LF-rTMS have hitherto been controversial.
Thus, the aim of this study was to investigate the efficacy and safety of LF-rTMS in post-stroke aphasia via a systematic review and a meta-analysis of randomized controlled trials (RCTs).
METHODS
Search strategy and selection criteria
Four online databases (MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL) and the Web of Knowledge) were searched for articles published until June 2014 using the following search terms: (transcranial magnetic stimulation or repetitive transcranial magnetic stimulation or TMS or rTMS) AND (aphasia or language disorders or speech disorders or anomia or aphasi$ or dysphasi$ or anomic).The search was limited to RCTs. The reference lists of the relevant studies were also carefully reviewed to identify additional studies for inclusion.
Studies were included if they met the following criteria: (i) the design was an RCT or a randomized controlled crossover trial and, for the latter, only the first period in the parallel group design was included in the analysis; and (ii) the patients were at least 18 years old; (iii) the experimental group underwent LF-rTMS alone or LF-rTMS plus SLT or any other approach for improving aphasia, and the control group underwent sham LF-rTMS alone or sham LF-rTMS plus SLT or any other approach or no intervention; (iv) the articles were published in peer-reviewed journals in English. The exclusion criteria included: (i) quasi-RCTs; (ii) with several articles from the same study, only the one with the most patients and the latest and most complete data was chosen; (iii) articles presented at international meetings with no specific data provided, even after contacting the author.
Data extraction and quality assessment
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews, 2 independent reviewers (YL and MY) performed the literature search. A third independent reviewer (TD) determined study eligibility when there was a discrepancy. Two investigators (YL and MY) extracted the data pertaining to the patient numbers and characteristics, intervention strategies, mean outcomes and standard deviations of the outcomes as well as the adverse events.
Quality assessments were evaluated with the PEDro scale, which assigns a score from 0 to 10, with a higher score indicating greater quality (12), as follows: 9–10: excellent; 6–8: very good; 4–5: good; < 4: poor (13).
Data synthesis and analysis
Surrogate parameters for language disorders, including naming, repetition and comprehension, were considered our primary outcomes. Secondary outcomes included changes in brain excitability and adverse events.
Data on naming, repetition and comprehension were determined from various aphasia assessment outcomes. Data on excitability changes were derived from the language activation H2O (15) positron emission tomography (PET) study, which consisted of consecutive measurements of relative cerebral blood flow (CBF) in 3-dimensional mode with a verb-generation task. Activation volumes indices (AVI) or laterality indices (LI) were used to evaluate the changes with specific formulas. When using LI, data collection started when the number of true counts was above the baseline level for > 5 kcounts and continued for 45 s. When using AVI, the activation volumes were calculated using all suprathreshold voxels (the Z-score represented the threshold that was transformed from the relative cerebral blood flow changes with a range of greater than 2 for each hemisphere on the PET images) (14). Positive values of LI and AVI deduced from the respective formulas indicated left-hemispheric dominance.
Review Manager software (version 5.3) was used for all statistical analyses. Forest plots were constructed to display the results that were separately summarized with meta-analysis techniques for each end-point. p < 0.05 was considered significant. I2 statistics were used to estimate the statistical heterogeneity among studies (15). When I2 was less than 50%, fixed-effects models were used; otherwise, random-effects models were employed. The summary effect size (SES) was estimated by calculating the standard mean difference (SMD) and 95% confidence interval (CI) derived from the mean and standard deviations of each follow-up value. Effect sizes were classified as small (< 0.2), medium (0.2–0.8) or large (> 0.8). Sensitivity analysis was conducted to verify the stability of the study.
RESULTS
Selected studies and characteristics
A total of 879 articles was identified in the initial search. After excluding duplicates as well as screening the titles and abstracts, 29 studies remained for further assessment. After evaluating the full texts for more details, 12 RCTs satisfied the inclusion criteria. However, after excluding 8 studies (16–23) with duplicate publication (19–23) and no response to the request for data (16–18), 4 RCTs (24–27) with a total of 132 patients were included in the analyses. A flow chart of the structured review is shown in Fig. 1. All the included studies were RCTs. All the interventions except 1 were combined with SLT (27). The mean time post-stroke ranged from 36.7 days to 3.48 years. With the exception of 2 studies (24, 27) that focused on patients with non-fluent aphasia, the remainder included all types of aphasia patients. All the stimulation sites were over the right pars triangularis (PTr). The key features of the included studies are summarized in Table I.

|
Table I. Characteristics of included studies |
||||||||||
|
Study |
n (E/C) |
Handedness (right-handedness) |
Aphasia type |
Mean age, years |
Mean time post-stroke |
Study design |
Site of stimulation |
Treatment intensity |
With SLT (yes/no) |
Outcome measurement |
|
Seniow et al., 2013 (26) |
20/20 |
– |
All types |
60.7 |
36.7 days |
RCT |
PTr |
15 sessions protocol of 30 min 1Hz 90% rMT 1800 pulses |
Yes |
BADE |
|
Heiss et al., 2013 (25) |
15/14 |
39/41 (95%) |
All types |
68.8 |
44.9 days |
RCT |
PTr |
10 sessions protocol of 20 min 1 Hz 90% rMT |
Yes |
AAT, AVI |
|
Tsai et al., 2014 (24) |
33/23 |
56 (100%) |
Non-fluent |
62.6 |
18.1 months |
RCT |
PTr |
10 sessions protocol of 10 min 1 Hz 90% rMT 600 pulses |
Yes |
PNT, CCAT |
|
Barwood et al., 2013 (20) |
6/6 |
12 (100%) |
Non-fluent |
63.9 |
3.48 years |
RCT |
PTr |
10 sessions protocol of 20 min 1 Hz 90% rMT 1,200 pulses |
No |
BADE, BNT |
|
SLT: speech and language therapy; PTr: right pars triangularis; AAT: Aachen Aphasia Test; AVI: Activation Volume Indices; BADE: Boston Diagnostic Aphasia Examination; BNT: Boston Naming Test; CCAT: Concise Chinese Aphasia Test; PNT: Picture Naming Test; E/C: experimental/control groups; RCT: randomized controlled trial; PTr: right pars triangularis. |
||||||||||
The PEDro scores of the included studies ranged from 7 to 9. Concealed allocation was mentioned in 2 articles (24, 26). Assessors and subjects were blinded in all studies, while the administration of LF-rTMS could only be performed with single blinding (Table II).
|
Table II. PEDro assessment quality results of included studies |
||||
|
Seniow et al., 2013 (26) |
Heiss et al., 2013 (25) |
Tsai et al., 2014 (24) |
Barwood et al., 2013 (20) |
|
|
PEDro assessment |
||||
|
Eligibility criteria (not included in total score) |
Yes |
Yes |
Yes |
Yes |
|
Random allocation |
1 |
1 |
1 |
1 |
|
Concealed allocation |
1 |
0 |
1 |
0 |
|
Similar groups at baseline |
1 |
1 |
1 |
1 |
|
Blinding subjects |
1 |
1 |
1 |
1 |
|
Blinding therapists |
0 |
0 |
0 |
0 |
|
Blinding assessors |
1 |
1 |
1 |
1 |
|
Outcome obtained in more than 85% of the subjects |
1 |
0 |
1 |
1 |
|
Intention-to-treat analysis |
1 |
1 |
1 |
1 |
|
Between-group statistical comparison |
1 |
1 |
1 |
1 |
|
Point estimates and measures of variability |
1 |
1 |
1 |
1 |
|
Total |
9 |
7 |
9 |
8 |
|
Quality |
Excellent |
Very good |
Excellent |
Very good |
Primary outcome
Naming. All 4 included articles (n = 132) provided complete data pertaining to naming (24–27). The Picture Naming Test (PNT), the Boston Naming Test (BNT), the Boston Diagnostic Aphasia Examination (BADE) and the Aachen Aphasia Test (AAT) were used to evaluate naming. Because the data were homogeneous (I2 = 0%, Fig. 2) across the studies, the fixed-effects model was chosen. Meta-analysis showed a significant medium SES with an SMD of 0.51, a 95% CI of 0.16–0.86, and p = 0.004.
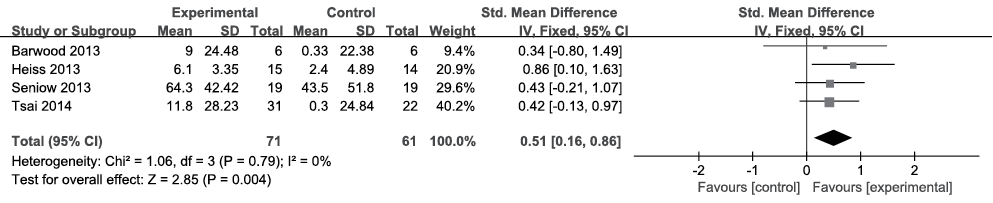
Fig. 2. Summary effect size for naming. SD: standard deviation; 95% CI: 95% confidence interval; Std. mean difference: standard mean difference; IV: in figure caption means inverse variance.
Repetition. All 4 included articles (n = 132) reported data pertaining to repetition (24–27). The Concise Chinese Aphasia Test (CCAT), BADE and AAT were used to evaluate repetition. Because the data were homogeneous (I2 = 0%, Fig. 3) across the studies, the fixed-effects model was chosen. Meta-analysis showed a significant medium SES with an SMD of 0.31, a 95% CI of –0.04–0.65, and p = 0.08.
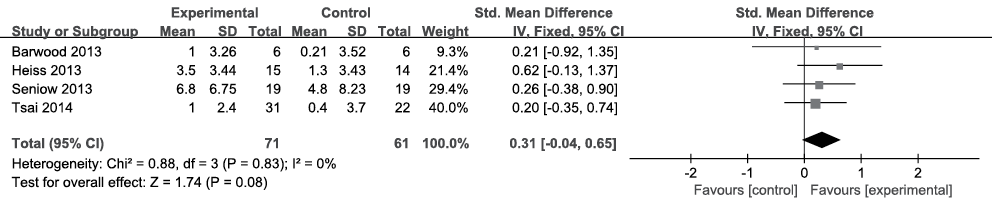
Fig. 3. Summary effect size for repetition. SD: standard deviation; 95% CI: 95% confidence interval; Std. mean difference: standard mean difference; IV: in figure caption means inverse variance.
Comprehension
Of the 4 included articles, 3 (n = 79) reported data pertaining to comprehension (25–27). BADE and AAT were used to evaluate comprehension. Because the data were homogeneous (I2 = 0%, Fig. 4) across the studies, the fixed-effects model was chosen. No treatment effect was found according to meta-analysis with an SMD of 0.31, a 95% CI of –0.14–0.75, and p = 0.18.
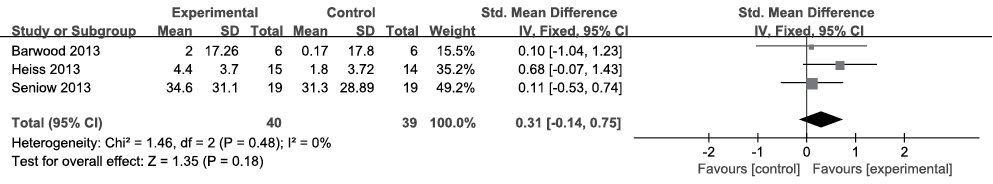
Fig. 4. Summary effect size for comprehension. SD: standard deviation; 95% CI: 95% confidence interval; Std. mean difference: standard mean difference; IV: in figure caption means inverse variance.
Secondary outcomes
Changes in brain excitability. Of the 4 articles, only 1 (25) (n = 29) provided complete data pertaining to changes in brain excitability. AVI was used for assessment. There was a significant difference in AVI between the sham (–16.9 ± 42.81) and rTMS groups (7.6 ± 33.55) after treatment (p = 0.023).
Adverse events. None of the included studies reported any adverse events in response to the treatments.
Sensitivity analysis
The fixed-effects model was replaced with the random-effects model to test the SES. No differences were found after changing the method, demonstrating the high quality of the data.
Publication bias
No publication bias in naming, repetition or comprehension identified by Begg’s funnel plot and Egger’s line regression test (Begg’s test: p = 0.308, p = 0.308, p = 1, respectively, and Egger’s test: p = 0.823, p = 0.721, p = 0.989, respectively). Funnel plots are shown in Fig. 5.
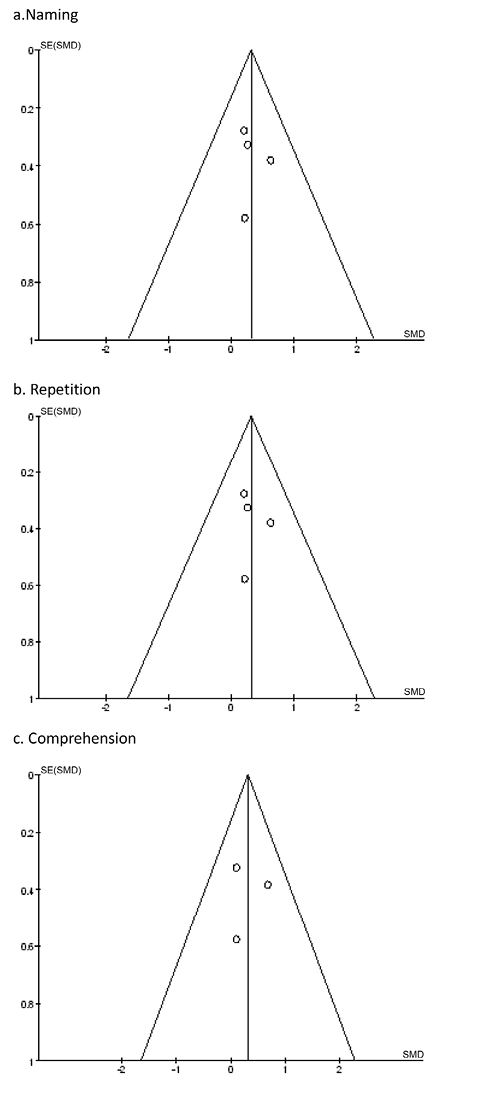
Fig. 5. Funnel plot for the evaluation of publication bias. (a) Naming; (b) repetition; (c) comprehension.
DISCUSSION
This systematic review and meta-analysis summarized the current RCTs, investigating the efficacy and safety of LF-rTMS in post-stroke aphasia. Overall, data synthesis showed that LF-rTMS was beneficial for post-stroke patients with regard to naming (SMD 0.51; 95% CI, 0.16–0.86) and changes in brain excitability (7.6 ± 33.55; 95% CI, –10.7–26.20). However, the changes in repetition (SMD 0.31; 95% CI, –0.04–0.65) and comprehension (SMD 0.31; 95% CI, –0.14–0.75) after stimulation were not significant. No adverse effects were observed due to the intervention.
The relative balance between the 2 cerebral hemispheres is typically preserved by collateral (ipsilateral perilesional area) and transcallosal (contralateral homotopic area) inhibition (28, 29). When a patient has an acute stroke, this balance is disrupted, and the undamaged hemisphere and ipsilateral perilesional regions are released from interacting with each other. This phenomenon has been demonstrated by functional neuroimaging in post-stroke aphasic patients (30). For small lesions outside of the primary language region, a procedure called intrahemispheric compensation is activated, resulting in the activation of the ipsilateral perilesional area (31). For large lesions, procedures are more frequently aimed at reducing transcallosal inhibition rather than targeting intrahemispheric compensation, resulting in the activation of contralateral homotopic areas (28). However, favourable clinical outcomes may be observed following either activation in uninjured brains or reintegration of the left perilesional area by suppressing the overactive right homotopic language area with LF-rTMS (32, 33). Our analysis of changes in brain excitability corroborated the latter explanation. Neuroplastic changes within the cortical and subcortical language network play important roles in aphasia recovery. Several studies have reported that the activation patterns of network activity are concentrated in the non-dominant hemisphere prior to treatment (30, 31). However, this situation has been demonstrated to be completely reversed after treatment, with experimental groups showing shifts in network activity toward the left hemisphere. Nevertheless, only 1 study (23) has reported a moderate relationship between changes in brain excitability and clinical outcomes. Whether these changes in shift are associated with improvements in overall speech and language functions requires further investigation. The promotion of the right homotopic language area has been attributed to aphasia recovery in a growing number of studies, particularly when large parts of the left hemisphere are injured (34). More RCTs are needed to verify which part of the right homotopic language area undertake is involved in recovery.
Our findings are in agreement with the results reported by Barwood et al. (19), who observed improvements in naming after applying LF-rTMS over the PTr in chronic non-fluent aphasic patients. In contrast, Waldowski et al. (21) reported only slight, non-significant differences between experimental and control groups. The reason for the variance may be that the stimulated site in the latter study focused on both the PTr and pars opercularis (POr) rather than on the PTr alone. In general, these 2 regions form the inferior frontal gyrus (IFG) (35). One study (36) reported that over-reaction of the PTr is detrimental to aphasic recovery due to response inhibition. Application of LF-rTMS to this region could reduce the negative impact on the primary language centre in the dominant hemisphere, thus accelerating the recovery process. Moreover, the POr is partially linked to the temporo-parietal cortex and premotor area through the superior longitudinal fasciculus following impairment of the primary language centre, promoting contact among bilateral circuits (37). However, hyperactivity of the PTr could impede this contact until localized inhibitory rTMS is applied, resulting in greater involvement of this region. In addition, the lesion site is a crucial factor. Martin et al. (32) found that, after undergoing rTMS, 1 patient with chronic, non-fluent aphasia exhibited enhanced naming ability, while another did not. The reason for this difference was mainly because the latter patient had a lesion that extended into the IFG, including the left motor cortex, dorsal premotor cortex, the deep white matter adjacent to the left supplementary motor area and the posterior middle frontal gyrus, which is an area that is vital for naming (38). Consequently, structural integrity of the cerebral cortex is a prerequisite for efficacy. Comprehension, which is mainly modulated by the temporal lobe, may not have been affected by LF-rTMS treatment of the conventional stimulation site. Finally, because the target was focused on the unaffected hemisphere, which was located far from the peri-infarct tissue, the risk of seizures was minimized.
There were also some limitations of our analyses. First, we could not eliminate the possibility that some studies, such as those published in a language other than English, were omitted. Secondly, because we were unable to obtain specific data from some of the authors, the results might have been biased. Thirdly, although the included studies were largely of moderate or high quality, the lack of concealed allocation and unmasking of the therapists administering rTMS could have biased the results. Ultimately, due to the limited number of included studies, as well as the small sample sizes, the statistical power was moderate.
In conclusion, LF-rTMS is a relatively safe and effective treatment for post-stroke aphasic patients in terms of naming. In addition, this treatment favours the reorganization of the left-hemispheric language networks. Although rTMS is considered a promising therapy, the specific mechanism underlying its success is unknown. Further investigations should aim to evaluate the different types and phases of aphasia. Greater attention should be paid to exploring other potentially effective stimulation sites and optimal parameters for this type of treatment, not only in the dominant hemisphere but also the right hemisphere, with the aid of imaging and neuronavigational methods for precise localization.
ACKNOWLEDGEMENTS
The authors would like to thank Dengying Kang from the Department of Clinical Epidemiology, West China Hospital, Sichuan University, Chengdu, Sichuan, China, for his valuable statistical insights.
The authors declare no conflicts of interest.
REFERENCES
