Hanna Klingshirn, Bc of Health OT, MPH1, Eva Grill, PhD, MPH1,2, Andreas Bender, MD3,4,
Ralf Strobl, PhD, Dipl-Stat1,2, Rene Mittrach, MAS1, Kathrin Braitmayer, MPH1 and
Martin Müller, PhD, MPH1,2
From the 1Institute of Medical Information Processing, Biometrics and Epidemiology, 2German Center for Vertigo
and Balance Disorders, 3Department of Neurology, Ludwig-Maximilians-Universität München, Munich and
4Therapiezentrum Burgau, Burgau, Germany
OBJECTIVE: To characterize existing rehabilitation interventions for patients with disorders of consciousness in long-term care and to evaluate the quality of evidence of these interventions.
DATA SOURCES: Databases MEDLINE, Embase, CINAHL, the Cochrane Library, CareLit and SoLit from January 2003 until July 2013.
STUDY SELECTION: Studies were selected that focused on rehabilitation interventions for patients in a coma, vegetative state or minimally conscious state who were living in a long-term care setting. Interventions related to rehabilitation nursing, physical therapy, occupational therapy, and speech therapy were described. A total of 53 publications was included.
DATA EXTRACTION: Two authors independently extracted the data and assessed the quality of reporting using the National Service Framework (NSF) for Long Term Neurological Conditions (LTNC).
DATA SYNTHESIS: Out of all extracted rehabilitation interventions 12 categories were generated and described. Out of 53 publications 28 (52.8%) contained expert-based evidence and 25 (47.2%) presented research-based evidence.
CONCLUSION: There are a multitude of different rehabilitation interventions for individuals with disorders of consciousness, which are established in clinical practice and supported by expert opinion. However, evidence regarding these interventions is weak and recommendations are strictly limited. The findings of this review may represent a basis for further research.
Key words: rehabilitation; long-term care; consciousness disorders; persistent vegetative state; minimally conscious state.
J Rehabil Med 2015; 47: 577–585
Correspondence address: Martin Müller, Institute of Medical Information Processing, Biometrics and Epidemiology, Ludwig-Maximilians-Universität München, DE-81377 München, Germany. E-mail: martin.mueller@med.uni-muenchen.de
Accepted Apr 14, 2015; Epub ahead of print Jun 16, 2015
INTRODUCTION
Brain injuries of traumatic or non-traumatic origin may have catastrophic consequences for the individual. With improvements in acute medical and post-acute rehabilitation care, the survival rate of these patients, and the number of individuals surviving with severe disorders of consciousness (DOC), increases (1, 2). DOCs are characterized as coma, i.e. complete unresponsiveness with closed eyes, vegetative state (VS), i.e. complete unresponsiveness with open eyes, or minimally conscious state (MCS), i.e. limited conscious interaction with the environment (2). Despite the presumably increasing number of affected individuals, valid epidemiological data on the prevalence and incidence of DOC are limited and show great variation (3). For long-term care facilities and nursing homes in Austria, a prevalence of 3.4 per 100,000 for VS and 1.5 per 100,000 for MCS has been shown (4). Healthcare institutions in France showed a VS/MCS prevalence of 2.8 per 100,000 inhabitants (5).
For the last 20 years, VS has been considered permanent when lasting for longer than 3 months after non-traumatic brain injury or for longer than 12 months after traumatic brain injury (6). However, recent studies have shown that making a prognosis is difficult and there is potential for recovery years after the initial injury (7–11), even despite the presence of negative prognostic markers (12–14).
As such, it seems reasonable to maintain a proper level of rehabilitation care even after discharge from initial inpatient rehabilitation. While it has been shown in the post-acute setting, that rehabilitation may increase the likelihood of functional improvement of patients in VS or MCS (15, 16), the effectiveness of interventions in long-term care is less clear.
International guidelines for the rehabilitation of brain injury recommend a complex and interdisciplinary approach, but hardly consider the specific nature of long-term care (17, 18). In Germany, the Federal Association for Rehabilitation provides guidelines for neurological rehabilitation in long-term care. According to these guidelines, rehabilitation should comprise interventions from physical therapy, occupational therapy, and speech and language therapy to amend rehabilitative nursing care. This guideline suggests the implementation of various treatment concepts that focus on the individuals’ functioning, but which also include measures to support the family, adapt the environment and enable patients to participate in social life (19).
Although it seems legitimate to reason that rehabilitation interventions might increase the chance of functional improvements in DOC, there is no systematic evaluation of the quality of evidence of rehabilitation interventions for individuals with DOC in the specific context of long-term care. The objective of this systematic review is to evaluate the quality of evidence of rehabilitation interventions for patients with DOC in long-term care.
The specific aims of this review are:
• to characterize the rehabilitation interventions applied in long-term care; and
• to evaluate the quality of evidence of these rehabilitation interventions.
METHODS
Data source
A systematic literature review was performed using the databases MEDLINE, Embase, CINAHL and the Cochrane Library from January 2003 until July 2013. This period was chosen to provide an overview of the last 10 years. To present the healthcare situation in Germany, the databases CareLit and SoLit, which mainly contain German language publications, were included during the same period.
Study selection
To identify all relevant studies, a specific, rather than a sensitive, search strategy comprising 2 components was conducted. The first component identified all studies involving patients with DOC, whereas the second component aimed to identify all rehabilitation interventions for people with DOC (Appendix 1). The following inclusion criteria were used:
• patients with DOC presenting as coma, VS or MCS;
• 12 years of age or older (i.e. from adolescence on);
• living in an inpatient or outpatient long-term care setting;
• presence of rehabilitation interventions.
The recommendations of the German Federal Association for Rehabilitation for the long-term care rehabilitation of people with DOC were followed (19), and rehabilitation intervention was defined as non-pharmaceutical and non-surgical interventions provided by therapists including nurses (e.g. rehabilitation nursing, physical therapy, occupational therapy, speech therapy or related). Rehabilitation interventions were also considered, e.g. multidisciplinary patient care, special rehabilitation treatment concepts, the use of adaptive technology and environmental adaption or education and support for family caregivers.
Since a pilot search showed that evidence in the context of long-term rehabilitation care is limited, no restrictions regarding study design were made. The search was restricted to English and German language. If studies were published more than once, the latest publication was included. To ensure the quality of the search strategy, all search strategies were pilot-tested on their ability to find abstracts that were previously identified as relevant.
Data extraction and analysis
Studies were independently screened for inclusion criteria by 2 reviewers (HK and RM) based on title and abstract. The selected publications were subsequently reviewed based on full text. Agreement on the criteria for selecting publications has to be reached by consensus. In case of disagreement during the selection process, a third reviewer (MM) made the final decision. After the final selection, 2 reviewers (HK and RM/ KB) extracted data independently.
The quality of evidence of the reported studies was assessed using the scheme of the UK’s National Service Framework (NSF) for Long Term Neurological Conditions (LTNC) (20). The NSF-scheme was developed for the critical appraisal of literature especially for the setting of neurological long-term care conditions and offers a methodology to assess the evidence of primary research as well as the evidence from expert opinion to support best clinical practice (21, 22).
Within the NSF-scheme “Expert evidence” comprises expert opinions from users, carers and professionals and is not further evaluated in the critical appraisal. Each publication including “research evidence” was rated in 3 categories: “design”, “quality” and “applicability”. The category “design” assesses the used research design (Table I). The assessment of “quality” is based on 5 items and was designed to be applied for both qualitative and quantitative research (Table II). “Applicability” indicates whether the study relates directly or indirectly to long-term neurological conditions (20, 22). Consensus of scoring was achieved through discussion, until there was a complete agreement on scores. In the event of discrepancy a third reviewer (MM) was available to discuss and make a majority decision. We decided to use the NSF approach instead of the more common approach of GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) (23, 24) because the preliminary screening of the included publications showed that their quality in terms of study design and reporting would be below the threshold of GRADE, and the aim of our study was to give an overview of the existing literature rather than providing immediate recommendations for clinical decisions.
|
Table I. Categories of research design for the included studies according to National Service Framework (NSF) typology (n = 53) |
|
|
Research-based evidence |
n (%) |
|
Primary research-based evidence |
|
|
P1 Primary research using quantitative approaches |
14 (26.4) |
|
P2 Primary research using qualitative approaches |
1 (1.9) |
|
P3 Primary research using mixed methods |
1 (1.9) |
|
Secondary research-based evidence |
|
|
S1 Meta-analysis of existing data analysis |
0 (0.0) |
|
S2 Secondary analysis of existing data |
0 (0.0) |
|
Review-based evidence |
|
|
R1 Systematic reviews of existing research |
0 (0.0) |
|
R2 Descriptive or summary reviews of existing research |
9 (17.0) |
|
Total research-based evidence |
25 (47.2) |
|
Expert evidence |
|
|
E1 Opinion/experience of users and/or carers |
0 (0.0) |
|
E2 Opinion/experience of professionals |
28 (52.8) |
|
E1+E2 Both |
0 (0.0) |
|
Total expert evidence |
28 (52.8) |
|
Table II. Quality rating within the National Service Framework (NSF) typology |
|
|
Quality criteria |
Score |
|
1 Are the research question/aim and design clearly stated? |
|
|
2 Is the research design appropriate for the aims and objectives of the research? |
|
|
3 Are the methods clearly described? |
|
|
4 Is the data adequate to support the authors‘ interpretations/conclusion? |
|
|
5 Are the results generalizable? |
|
|
Total |
/10 |
|
Each quality item is scored as follows: Yes = 2, In part = 1, No = 0. High quality = 7–10, Medium quality = 4–6, Poor quality = 0–3. |
|
Data on patient group, setting, rehabilitation interventions and study design were extracted from the selected publications. After extraction, the retrieved rehabilitation interventions were categorized. The categorization was conducted in 3 steps. In the first step each rehabilitation intervention described in the publication was listed in its original wording. In the second step, the names of the interventions were harmonized. The third step was to build major categories summarizing rehabilitation interventions with the same topic or goal. The main goal of this categorization was to arrive at categories without any reference to specific professions, but according to functional and structural aspects.
RESULTS
Study selection
The literature research yielded a total of 2,290 publications for the title and abstract screening. After screening of title and abstract, a total of 151 publications was selected to be assessed for eligibility in full-text. Out of 151 publications 146 were read in full-text and 5 were untraceable. The 2 reviewers (HK and RM) reached consensus on 143 publications, and 3 publications were presented to the third reviewer (MM) for final decision. Finally, 93 publications did not meet the inclusion criteria and a total of 53 publications were selected for inclusion in the analysis. A flow chart of the selection process is shown in Fig. 1.
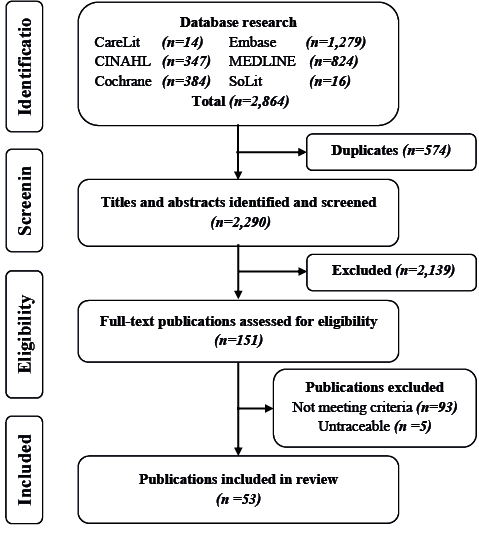
Fig. 1. Search process.
Characteristics of included publications
The patients in the included publications covered the whole spectrum of DOC, particularly VS and MCS. Twenty-three (43.3%) out of 53 publications did not provide a detailed description of the setting. The reported settings include 1 assisted living community (1.9%), 3 specialized long-term care facilities (5.7%), 11 nursing homes or other long-term care facilities (20.8%), and 15 other long-term rehabilitation settings (28.3%). Sixteen (30.2%) out of 53 publications are primary research studies, with 13 (24.5%) quasi-experimental studies, 1 (1.9%) cross-sectional study, 1 (1.9%) mixed method study and 1 (1.9%) qualitative study. Detailed information about patient group, setting, rehabilitation intervention and study design is shown in Tables III and IV.
|
Table III. Characteristics of the included expert evidence |
||||
|
Author, year, country |
Patient group |
Setting |
Rehabilitation interventions |
Study design, evidence |
|
Working party of the Royal College of Physicians, 2003 (37) UK |
Coma, VS, MCS, LIS |
Not precisely described, entire rehabilitation process |
A,B, C, D, E, F, H, L |
Guideline E2 |
|
Andrews, 2005 (27) UK |
VS, MCS |
Not precisely described, entire rehabilitation process |
A, D, F, G, H |
Clinical experience E2 |
|
Ashwal, 2013 (38) USA |
VS, MCS (age < 18 years) |
Not precisely described, entire rehabilitation process |
L |
Brief commentary E2 |
|
Barker, 2005 (43) UK |
VS, MCS |
Not precisely described, entire rehabilitation process |
F, H, K |
Clinical experience E2 |
|
Felgenhauer & Schneider, 2006 (47) Germany |
VS, MCS |
Not precisely described, entire rehabilitation process |
A, G, I, J, L |
Clinical experience E2 |
|
Fisch, 2009 (48) Austria |
VS, MCS |
Not precisely described, entire rehabilitation process |
I |
Clinical experience E2 |
|
Hackenberg-Werner, 2005 (73) Germany |
VS, MCS |
Assisted living community |
G, L |
Clinical experience, case report (7 cases) E2 |
|
Healy, 2010 (39) UK |
Coma, VS, MCS |
From ICU to long-term care |
A, B, C, D, E, F, H, L |
Clinical experience, case report (1 case) E2 |
|
Henze, 2004 (29) Germany |
VS |
Not precisely described, entire rehabilitation process |
A, F, H, I |
Clinical experience E2 |
|
Herr, 2005 (40) Germany |
VS, MCS |
Long-term care facility |
E, G, H, I, L |
Clinical experience E2 |
|
Hockauf et al., 2003 (25) Germany |
VS, MCS |
Special long-term care facility |
A, C, D, F, H, I, K, L |
Clinical experience, case report (1 case) E2 |
|
Hocker & Rabinstein, 2012 (36) USA |
Coma |
Entire rehabilitation process, inclusive long-term care |
A, B, C, D, F, H |
Clinical experience E2 |
|
Hofmann, 2003 (50) Germany |
VS, MCS |
Nursing home, special long-term care facility |
I |
Clinical experience, case report (1 case) E2 |
|
Kölbl-Catic, 2007 (51) Austria |
VS, MCS |
Long-term care facility |
I, L |
Clinical experience E2 |
|
Lavrijsen et al., 2007 (45) Netherlands |
VS |
Long-term care, nursing home |
F, G, H |
Clinical experience, case report (1 case) E2 |
|
Lavrijsen et al., 2005 (41) Netherlands |
VS |
Long-term care, nursing home |
H |
Clinical experience, case report (2 cases) E2 |
|
Pape et al., 2012 (69) USA |
VS, MCS |
Not precisely described, entire rehabilitation process |
I |
Framework E2 |
|
Ludwig, 2009 (31) Germany |
VS, MCS |
Home nursing |
B, C, E, F, G, H, I, J, L |
Clinical experience E2 |
|
Magee, 2005 (74) UK |
VS, MCS |
Not precisely described, entire rehabilitation process |
I |
Clinical experience, case report (1 case) E2 |
|
Menke, 2006 (54) Germany |
VS, MCS |
Residential home |
I |
Clinical experience E2 |
|
Mielke & Ehlers, 2012 (26) Germany |
Chronic DOC |
Home nursing, patient home |
C, G |
Clinical experience, case report (1 case) E2 |
|
Munday, 2005 (32) UK |
VS, MCS |
Rehabilitation centre, acute district hospitals |
B, F, G, H, J, K, L |
Clinical experience E2 |
|
Naude & Hughes, 2005 (68) UK |
VS, MCS |
Entire rehabilitation process, incl. long-term intervention |
I, J |
Clinical experience, case report (1 case) E2 |
|
Piel, 2005 (42) Germany |
VS, MCS |
Long-term care |
B, C, F, G, H, I |
Clinical experience E2 |
|
Staehelin, 2004 (34) Swizerland |
VS |
Not precisely described, entire rehabilitation process |
A, G, H, L |
Guideline E2 |
|
Teigeler, 2007 (35) Germany |
VS, MCS |
Special long-term care facility |
H, I, J, K |
Clinical experience E2 |
|
Thiel, 2005 (55) Germany |
VS, MCS |
Special long-term care facility |
I, K, L |
Clinical experience E2 |
|
Tolle & Reimer, 2003 (56) Canada |
VS |
Long-term care facilities or home nursing |
I |
Conceptual analysis E2 |
|
Rehabilitation interventions: A: adequate nutrition; B: good skin care; C: tracheostomy management and pulmonary care; D: management of bladder and bowel care; E: oral and dental hygiene; F: joint movement and range of motion exercise; G: postural, repositioning and mobility management; H: prevention of secondary complications; I: special treatment concepts; J: adaptive technology and environmental management; K: provision of interaction and communication; L: family support; M: individual preferences and participation. DOC: disorders of consciousness; VS: vegetative state; MCS: minimally conscious state; LIS: locked-in syndrome; ICU: intensive care unit; E2: expert evidence, experience of professionals. |
||||
|
Table IV. Characteristics of included research-based evidence |
||||
|
Author, year, country |
Patient group |
Setting |
Rehabilitation interventions |
Study design, evidence |
|
Anderson & Arciniegas, 2010 (46) USA |
Coma, VS, MCS |
Not precisely described, entire rehabilitation process |
I, J, K, L |
Descriptive review: R2 High Direct |
|
Ashwal, 2004 (77) USA |
VS (age < 15 years) |
Not precisely described, entire rehabilitation process |
A, B, C, F, G, H, J, L |
Descriptive review: R2 Medium Direct |
|
Bernat, 2006 (1) USA |
Chronic DOC, VS, MCS |
Not precisely described, entire rehabilitation process |
A, B, C, D, F, G, I, K |
Descriptive review: R2 High Direct |
|
Elliott & Walker, 2005 (44) UK |
VS, MCS |
Not precisely described, entire rehabilitation process |
F, G, H, I |
Descriptive review: R2 Medium Direct |
|
Heidler, 2008 (49) Germany |
Coma, VS, MCS |
Not precisely described, entire rehabilitation process |
I |
Descriptive Review: R2 Medium Direct |
|
Herkenrath, 2006 (72) Germany |
VS |
Long-term nursing institution |
I |
Qualitative research (12 participants): P2 Medium Direct |
|
Hirschberg & Giacino, 2011 (2) USA |
Coma, VS, MCS |
Not precisely described, entire rehabilitation process |
A, D, F, I, J |
Descriptive review: R2 Medium Direct |
|
Huber et al., 2010 (57) Germany |
VS (caregiving relatives) |
Special rehabilitation centre in addition to inpatient or outpatient long-term care |
I, L |
Mixed methods, survey (n = 11) semi-structured qualitative interviews (n = 3): P3 High Direct |
|
Kratzki & Lücke, 2004 (30) Germany |
VS, MCS (occupational therapists) |
Inpatient or outpatient long-term care |
H, I, J, L |
Cross-sectional study (n = 60): P1 High Direct |
|
Lancioni et al., 2012 (65) Italy |
MCS |
Medical care centre, personal room |
I, J |
N-of-1 trial ABABB1CB1 sequence (n = 1): P1 High Direct |
|
Lancioni et al., 2012 (66) Italy |
MCS, MCS+ |
Special rehabilitation or care centre, participants‘ room |
I, J, K |
N-of-1 trial Study I: ABAB sequence (n = 5); Study II: non-concurrent multiple base design (n = 3): P1 High Direct |
|
Lancioni et al., 2012 (67) Italy |
MCS+ |
Rehabilitation centre, participants‘ rooms |
I, J |
N-of-1 trial Non-concurrent multiple baseline design (n = 3): P1 High Direct |
|
Lancioni et al., 2011 (63) Italy |
VS |
Medical rehabilitation and care centre |
I, J |
N-of-1 trial Study I: ABABCBC sequence (n = 1); Study II: ABABCB sequence (n = 1): P1 High Direct |
|
Lancioni et al., 2011 (70) Italy |
MCS+ |
Special rehabilitation clinic and family home |
J, K |
N-of-1 trial ABAB sequence (n = 2): P1 High Direct |
|
Lancioni et al., 2011 (64) Italy |
MCS |
Care and rehabilitation centre |
I, J |
N-of-1 trial Study I: ABAB sequence (n = 3); Study II: ABAB sequence (n = 1): P1 High Direct |
|
Lancioni et al., 2010 (61) Italy |
VS |
Not precisely described, brain injury 2 years prior study |
I, J |
N-of-1 trial ABAB sequence (n = 1): P1 High Direct |
|
Lancioni et al., 2010 (52) Italy |
VS, MCS |
Not precisely described, entire rehabilitation process |
I, J, K |
Descriptive review: R2 High Direct |
|
Lancioni et al., 2010 (62) Italy |
MCS |
Participants home, brain injury 15 years prior study |
I, J |
N-of-1 trial ABAB sequence (n = 1): P1 High Direct |
|
Lancioni et al., 2009 (58) Italy |
MCS |
Not precisely described, long time after traumatic event |
I, J, K |
N-of-1 trial Study I: ABAB sequence (n = 2); Study II: ABABB1 sequence (n = 2): P1 High Direct |
|
Lancioni et al., 2009 (59) Italy |
VS |
Not precisely described, brain injury 10–5 months prior study |
I, J |
N-of-1 trial ABABCB sequence (n = 3): P1 High Direct |
|
Lancioni et al., 2009 (60) Italy |
VS |
Not precisely described, participants‘ homes |
I, J |
N-of-1 trial ABABCB sequence (n = 3): P1 High Direct |
|
Laureys et al., 2006 (28) Belgium |
VS, MCS |
Not precisely described, entire rehabilitation process |
G, H, I |
Descriptive Review: R2 Medium Direct |
|
Lotze et al., 2011 (53) Germany |
VS, MCS |
Residential home |
I |
N-of-1 trial ABA-BAB sequence (n = 6): P1 High Direct |
|
Noda et al., 2004 (71) Japan |
VS |
Hospital (brain injury 9 months to 4 years prior study) |
I |
Quasi-experimental study: Pre-post-test design (n = 26): P1 Medium Direct |
|
Schiff et al., 2005 (33) USA |
VS |
Not precisely described, entire rehabilitation process |
A, B, C, D, F, H, I, L |
Descriptive Review: R2 Medium Direct |
|
Rehabilitation interventions: A: adequate nutrition; B: good skin care; C: tracheostomy management and pulmonary care; D: management of bladder and bowel care; E: oral and dental hygiene; F: joint movement and range of motion exercise; G: postural, repositioning and mobility management; H: prevention of secondary complications; I: special treatment concepts; J: adaptive technology and environmental management; K: provision of interaction and communication; L: family support. DOC: disorders of consciousness; VS: vegetative state; MCS: minimally conscious state, emerged from Minimally Conscious State (MCS+); P1: primary research using quantitative approaches; P2: primary research using qualitative approaches; P3: primary research using mixed methods; R2: descriptive or summary reviews of existing research. |
||||
Rehabilitation interventions in long-term care
The categorization process resulted in 12 major categories of rehabilitation interventions, with 2 to 8 sub-categories (Table V). Out of the 53 included publications, 41 (77.4%) reported more than 1 of those major categories.
The majority of the rehabilitation interventions were associated with routine care measures (Table V, A–G). Most of them aimed to evoke rehabilitation potentials, e.g. weaning off percutaneous endoscopic gastronomy (PEG)-tube and enabling in oral feeding (25), decannulation to facilitate physiological respiration (26), removal of urinary catheter (27) or postural management to support a stable sitting or standing position (1, 27, 28).
Furthermore, many rehabilitation interventions were identified from the included publications that aim to prevent secondary complications (Table V, H) (25, 27–41, 42). This included malnutrition and complications related to PEG-tube (33, 35), respiratory tract infections or cystitis (25, 27, 33, 36–42), pressure sores (31–33, 35, 36, 42), joint contractures (27, 29, 31–33, 35–37, 42–45) or deep venous thrombosis (33, 36, 42).
Thirty-nine publications reported the use of specialized treatment concepts (Table V, I). The most frequently reported treatment concepts were sensory stimulation techniques (1, 2, 25, 28–31, 33, 35, 40, 44, 46–57), partly in combination with assistive technologies for patient’s self-control (52, 58–68) or including the provision of familiar voices (59, 60, 69).
|
Table V. Categories of rehabilitation interventions described in the included studies |
|
|
Rehabilitation intervention |
Description of rehabilitation intervention |
|
Nutrition |
Enteral or parenteral via endoscopic gastrostomy (PEG) tube (1, 2, 27, 29, 33, 34, 36, 37, 39, 47, 77) Weaning from PEG-tube, swallowing independent and enable oral feeding (25) |
|
Skin care |
Good skin care (1, 37, 39, 77) Prevention of pressure areas and decubitus ulcers (31–33, 36, 42) |
|
Tracheostomy management and pulmonary care |
Supply of tracheostomy equipment and care (1, 26, 31, 77) Liberation from ventilator and tracheostomy (25, 26, 36) Prevention of respiratory infections, e.g. suction to avoid aspiration (1, 26, 33, 36, 37, 39, 42) |
|
Bladder and bowel management |
Management of bladder and bowel incontinence (1, 37, 39, 47) Bowel and bladder regulation, e.g. support bowel action and sphincter control (2, 27, 33) Remove urinary catheter and control incontinence using suitable aids to prevent infections (25, 27, 33, 36) |
|
Oral and dental hygiene |
Care for oral and dental hygiene to avoid secondary complications (37, 39, 40) |
|
Joint movement and range of motion exercise |
Passive joint exercise to prevent joint contractures (1, 2, 27, 29, 31–33, 36, 37, 39, 42–44, 77) Spasticity treatment to prevent muscle contractures (1, 25, 27, 29, 31–33, 36, 42, 43, 45, 77) Deep venous thrombosis prophylaxis (33, 36, 42) |
|
Mobility management |
Frequent repositioning help, e.g. every 4 h in bed (1, 28, 31, 32, 42, 44, 77) Postural management to support a stable sitting or standing position (1, 27, 28) Support transferring, e.g. from bed to wheelchair (31, 32, 40, 42, 45) Mobilization in wheelchair or tilt table (26, 32, 34, 40, 42, 45, 47, 73) |
|
Prevention of secondary complications |
Prevention of secondary complications in general (25, 27–35) Prevention of malnutrition and complications related to PEG-tube (33, 36) Prevention of decubitus ulcers (31–33, 36, 42) Prevention of contractures (27, 29, 31–33, 36, 37, 39, 42–45) Prevention of deep venous thrombosis (33, 36, 42) Prevention of infections, e.g. respiratory infections, cystitis, etc. (25, 27, 33, 36, 37, 39–42, 77) |
|
Special treatment concepts |
Sensory stimulation techniques e.g. multisensory stimulation, sensory regulation or basal stimulation (1, 2, 25, 28–31, 33, 35, 40, 44, 46–57) Sensory stimulation techniques combined with assistive technologies for patient‘s self-control (52, 58–68) Sensory stimulation techniques including familiar voices (59, 60, 69) Music therapeutic interventions (50, 52, 65, 68, 71, 72, 74) Facio-Oral Stimulation techniques (25, 30, 35, 47) Bobath concept (25, 30, 35, 42, 47, 51) Affolter concept (30, 35) Kinaesthetics (25, 30, 35, 40, 47, 51) |
|
Adaptive technology and environmental management |
Environmental management, e.g. minimize overstimulation, natural sleep-wake cycle and regularly scheduled meals (2, 27, 46, 47) Adaptive technologies to access environmental stimulation, communicate or request social contact 52, 58–68, 70) Support family and caregivers in an adequate use of adaptive technologies and medical aids (30–32, 35) |
|
Provision of interaction and communication |
Tolerate delays and afford more time to accomplish verbal or non-verbal response (46) Individualized communication systems (1) Establishing yes-no-codes, e.g. turning the head, nodding or pressing the hand (25, 32, 35, 43, 55) Technological communication aids (52, 58, 66, 70) |
|
Family support |
Inform the family (27, 37–39, 57) Counselling the family (27, 30, 31, 47) Guidance and training of the family (31, 32, 46, 47) Social support of the family (27, 33, 34, 40, 57) Family inclusion (25, 31, 51, 55, 73) |
Also, the use of adaptive technologies and environmental management (Table V, J) was frequently reported in the included studies. This included measures to minimize overstimulation, promotion of natural sleep-wake cycles and the use of medical aids or adaptive technologies. Also, supporting communication and interpersonal interaction (Table V, K) was frequently reported. A number of publications described simple communication strategies, e.g. yes-no-code with pressing the hand (25, 32, 35, 43, 55) or complex adaptive communication technologies (52, 58, 66, 70).
Seventeen included publications reported rehabilitation interventions related to “family support” (Table V, L). This included measures such as information provision (33, 57, 58, 61, 63), counselling (33, 62, 64, 65), guidance and training (37, 62, 65, 66), social support (28, 33, 35, 58, 59) and inclusion in treatment process (31, 34, 39, 65, 66).
Evidence typology and quality assessment
Twenty-eight (52.8%) out of 53 publications contained expert-based evidence. Among those, 14 (50.0%) described the author’s clinical experiences and 9 (32.1%) combined the author’s clinical experiences with case reports (see Table I).
Out of the 53 publications, 25 (47.2%) presented research-based evidence. Of these 25 publications, 16 (64.0%) reported primary research, mostly a quasi-experimental design without control group or randomization and a small sample size. Nine (36.0%) of the 25 publications used review-based approaches, all non-systematic, descriptive or summary reviews of existing research.
A total of 17 out of the 25 studies (68.0%) were rated as “high quality”, 8 (32.0%) as “medium quality”, and none as “low quality” in the quality assessment. Since we only included studies that were conducted in the setting of long-term care, the criterion “applicability” is classified as “direct” for all included studies.
DISCUSSION
This systematic review characterizes rehabilitation interventions for patients with DOC in long-term care and provides further insight into the quality of evidence of interventions in this field. Twelve different major rehabilitation intervention categories were identified, supported by literature of varying quality.
The multitude of categories reflects the complex and multidisciplinary character of optimal care in neurological long-term conditions where interventions often overlap and interact (22).
Even though only a small proportion of publications reporting results of original research were found, the number of reports from current practice shows the high relevance of the topic.
Almost all included publications that were categorized as research-based evidence were carried out by the same group on the topic of adaptive technologies. These may have an important impact on the individuals’ engagement and interaction; however, they were not yet being used in routine care (28, 52, 58, 67, 70). The other publications judged as primary research-based evidence report research on specialized treatment, such as sensory stimulation techniques (53) and music-based stimulation (69, 70) or research on occupational therapy practice (30), all show potential implications for practice, but without providing sufficient evidence for supporting clinical decision-making.
The quality of evidence varies among the 12 major rehabilitation categories identified. For example, evidence regarding the categories “nutrition” and “skin care” are based mainly on clinical experiences, while the evidence on “adaptive technology and environmental management” mainly includes primary research-based evidence. However, as mentioned previously, a higher level of evidence does not necessarily lead to more relevance for clinical practice (52, 58, 67, 70). In the context of long-term conditions, the outcome or treatment goals are usually focused on reduction in the impact of the disease (22). This is also reflected in our review: various rehabilitation interventions aim to support the patient’s individual preferences and provide participation in community life (25, 32, 33, 35, 40, 50, 55, 73).
The included primary research studies are concerned with 3 categories of rehabilitation interventions: “special treatment concepts”, “adaptive technology and environmental management” and “provision of interaction and communication”. Quality evaluation shows that, despite the small sample sizes and studies not exceeding quasi-experimental study designs, the majority of studies were judged as well-constructed according to the NSF typology. The major category “special treatment concepts” involves a multitude of different concepts that were frequently reported in practice, e.g. sensory stimulation techniques (1, 2, 25, 28–31, 33, 35, 40, 44, 46–57), sensory stimulation techniques combined with assistive technologies (52, 58–67), sensory stimulation techniques including familiar voices (59, 60, 69), music therapeutic interventions (50, 52, 65, 68, 71, 72, 74), Facio-Oral Stimulation techniques (25, 30, 35, 47), Bobath concept (25, 30, 35, 42, 47, 51), Affolter concept (30, 35) and kinaesthetics (25, 30, 35, 40, 47, 51). However, even though sensory stimulation techniques for patients in a coma or VS in the acute rehabilitation setting were the most frequent studied approach in long-term care, they showed insufficient evidence in a systematic review to support or rule out the effectiveness of sensory stimulation techniques (75). Furthermore, a series of studies was found that assess the use of adaptive technologies, sometimes combined with measures to provide interaction and communication. These studies may enable people with DOC to develop an active role with increased self-determination (52, 58–67, 70). Nevertheless, the studies have only a small number of participants (1–5 cases), without providing control groups and randomization.
Study strengths and limitations
The choice of the focused settings may require some explanation. We decided to investigate the situation of rehabilitation care provision in the individuals’ permanent living situation after initial inpatient rehabilitation care. This includes individuals living at home, as well as in nursing homes or in specialized long-term care facilities. Settings such as the long-term acute care hospitals, as established in the USA (76) do not meet the focus of our study because those meet the patients’ needs for prolonged acute care rather than providing permanent living situations.
A further point of concern might be the relatively high number of included publications that do not precisely report the study setting. However, most of these publications report guidelines or expert opinions, which are targeted to the general situation of patients with DOC rather than to a specific setting of care, and are therefore unambiguously relevant to our review. In publications that report primary research or systematic reviews, the proportion of unspecified settings is much lower (17%).
A major strength of this review is the use of the UK’s NSF scheme, which makes evidence both from research and from experts’ opinion accessible for evaluation (22). However, even though a large proportion of the retrieved studies reached a good quality rating in this system, one must acknowledge that almost all included studies would fail when applying traditional methods of evidence evaluation, such as GRADE. However, making use of GRADE would have resulted in floor effects, i.e. almost all studies would have been graded at the same low level of evidence, and comparisons between the studies would have been less insightful (21, 23). The NSF’s typology accounts for the specific situation of neurological long-term interventions, includes expert and research evidence, and allows in-depth analysis of the studies included in this review (20–22).
Moreover, this review is based on a broad search strategy using the databases MEDLINE, Embase, CINAHL, the Cochrane Library, CareLit and SoLit. This makes it probable that all relevant studies were included in this review. Since the databases CareLit and SoLit mainly cover German language publications, the situation in Germany may be overrepresented, and this must be taken into consideration when interpreting the results of this study.
Conclusion
This review highlights the multitude of different rehabilitation interventions for individuals with DOC established in clinical practice and supported by expert opinions. Twelve categories are set out in this review in an effort to deal with the variety of rehabilitation interventions that overlap and interact with each other. Overall recommendations are strictly limited. However, the review provides an overview and characterization of all existing rehabilitation interventions for people with DOC in long-term care. The general absence of randomized controlled trials and clinical trials underscores the need for further systematic research to establish evidence-based rehabilitation for this highly vulnerable population. The findings of this review may form the basis for a systematic research programme.
ACKNOWLEDGEMENTS
This study was funded by the German Research Foundation (DFG, grant number MU 3603/1-1).
The authors declare no conflicts of interest.
APPENDIX
|
Appendix I. Search strategy |
||
|
MEDLINE (Ovid) July 2009 |
||
|
# |
Search |
Results |
|
1 |
exp Persistent Vegetative State/ |
2,521 |
|
2 |
unresponsive wakefulness syndrome.mp. |
34 |
|
3 |
vegetative state.mp. |
3,828 |
|
4 |
minimally conscious state.mp. |
347 |
|
5 |
exp Coma, Post-Head Injury/ |
90 |
|
6 |
therapy.mp. |
1,765,258 |
|
7 |
exp Therapeutics/ |
3,342,529 |
|
8 |
exp Patient Care/ |
565,562 |
|
9 |
exp Nursing Care/ |
115,254 |
|
10 |
exp Rehabilitation/ |
147,292 |
|
11 |
1 or 2 or 3 or 4 or 5 |
3,959 |
|
12 |
6 or 7 or 8 or 9 or 10 |
4,248,442 |
|
13 |
11 and 12 |
2,227 |
|
14 |
limit 13 to (year = “2003 -Current“ and |
824 |
|
Search strategy adapted for Embase, CINAHL, Cochrane Library, CareLit and SoLit. |
||
REFERENCES
