Ellen M. P. van Loon, MD1,2, Majanka H. Heijenbrok-Kal, PhD1,2, Wouter S. van Loon, BSc3, Martin J. van den Bent, MD, PhD4, Arnaud J. P. E. Vincent, MD, PhD5, Inge de Koning, PhD1 and Gerard M. Ribbers, MD, PhD1,2
From the 1Rijndam Rehabilitation Center, 2Department of Rehabilitation Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, 3Department of Psychology, Leiden University, Leiden, 4Department of Neurology/ Neuro-oncology, Erasmus MC Cancer Institute and 5Department of Neurosurgery, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands
OBJECTIVE: To systematically review the literature for studies on cognitive functioning in patients with low-grade glioma to evaluate assessment methods and prevalence of cognitive dysfunction.
DATA SOURCES: A search was made in PubMed, Embase, and PsycINFO for articles published between January 2002 and June 2012 using cognition, memory, attention, executive functioning, and low-grade glioma as search terms.
STUDY SELECTION: Two reviewers independently performed the study selection and data extraction. Inclusion criteria were: studies including at least 10 adult patients, with suspected or confirmed low-grade glioma and cognitive functioning as outcome measure.
DATA EXTRACTION: A standard data extraction form was used, with items regarding study quality, patient characteristics, type of measurement instruments, cognitive domain, definition of cognitive dysfunction, and reported prevalence.
DATA SYNTHESIS: Of the 312 articles screened on title/abstract, 69 were screened on full-text and, finally, 17 were included. A total of 46 different measurement instruments were found for the assessment of cognitive functioning; 5 of these were used 5 or more times. There was variability in the definition of cognitive dysfunction. The reported prevalence of cognitive dysfunction ranged from 19% to 83%.
CONCLUSION: Many patients with low-grade glioma experience cognitive dysfunction. However, there is no consensus on how to assess cognitive functioning in these patients.
Key words: brain tumour; cognitive functioning; glioma; neuropsychological testing.
J Rehabil Med 2015; 47: 481–488
Correspondence address: Ellen van Coevorden-van Loon, Rijndam Rehabilitation Center, Rotterdam Neurorehabilitation Research (RoNeRes), PO Box 23181, NL-3001 KD Rotterdam, The Netherlands. E-mail: EvCoevorden@rijndam.nl
Accepted Apr 14, 2015; Epub ahead of print May 18, 2015
INTRODUCTION
Low-grade gliomas (LGGs) are primary brain tumours arising from glial cells, the supporting cells of the central nervous system (CNS). Low-grade gliomas can include astrocytomas, oligodendrogliomas, ependymomas or mixed gliomas (e.g. oligoastrocytomas). According to the World Health Organization (WHO) LGGs can be classified as grade I or grade II, based on the presence of histopathological features (e.g. atypical cells, mitoses, endothelial proliferation, and necrosis) (1).
Only 15–20% of all gliomas are considered to be low-grade ones (2, 3). The mean incidence in Europe of LGG is approximately 1/100,000 persons/year (2, 3). The peak incidence is in young adults, aged approximately 30–40 years (4, 5). The survival rate of patients with LGG is increasing due to improved neurosurgical techniques, advanced radiotherapy and chemotherapy, with median survival times ranging from 5 to 15 years (6, 7). There is still controversy with respect to the best timing of treatment, in particular surgery and radiotherapy. As such, therapy choices may vary across clinicians or hospitals, while patients with LGGs may face long-term consequences in which cognitive, emotional, linguistic and sensorimotor dysfunction may interfere with daily activities and social participation (8–10).
Besides surgery, chemotherapy, radiotherapy and psychosocial support, some patients might benefit from cognitive rehabilitation. There is a growing awareness that these patients might also benefit from multidisciplinary rehabilitation programmes. Multidisciplinary rehabilitation programmes may improve functional outcome, mood, vocation and quality of life in patients with a brain tumour (9, 11–14). However, subacute rehabilitation in patients with LGG is not common practice and has not been well studied. There is still a large gap between current research in the area of the functional consequences of LGG and its actual treatment in multidisciplinary rehabilitation programmes (15).
Cognitive functioning is pivotal for social participation and quality of life (16). In patients with LGG, cognitive functioning may vary over time and is influenced by a combination of tumour characteristics (location, type, and size of tumour), treatment modalities (surgery, radiation therapy, chemotherapy), comorbidity (epilepsy, use of anticonvulsants) and contextual factors such as educational level and coping style (17, 18). A decline in functioning will occur due to the progressive nature of the condition. Such a decline may support clinical decision-making (for example, to decide on planning surgical intervention and/or radiotherapy) or it may cause unresponsiveness to rehabilitation.
Cognitive deficits in patients with a brain tumour can affect attention, memory and executive functioning (19, 20) with a reported prevalence of 29–90% (9, 20–22). Besides variability in the type of tumour, the use of various neuropsychological instruments, different cut-off scores and normative data may explain this wide range (23). Knowledge about cognitive deficits is important as they provide insight into prognosis and follow-up of the disease, and can be used to evaluate treatment (side-) effects and to target cognitive rehabilitation (24).
Therefore, this systematic review focuses on how to measure cognitive functioning in patients with LGG. These patients are relatively young and, with a life expectancy of 5–15 years, and might benefit from multidisciplinary (cognitive) rehabilitation programmes. We focus on the 3 pillars of cognitive functioning: memory, attention and executive functioning. The primary aim of this study is to systematically review the literature on how memory, attention and executive functioning are assessed in patients with LGG. The secondary aim is to compare the reported prevalence of cognitive dysfunction in patient populations with LGG.
METHODS
Data sources
A search was performed in PubMed, PsychINFO and Embase for articles published between January 2002 and June 2012. In addition, the reference lists of all identified publications were checked. The search strategy was developed and tested for PubMed and adapted for PsychINFO and Embase. The following search terms were used: low-grade glioma, cognition, memory, attention, and executive functioning (Table I).
|
Table I. Search strategy used for the present review |
|
PubMed |
|
((Glioma[mh] OR glioma*[tiab] OR astrocytoma*[tiab] OR ependymoma*[tiab] OR oligodendroma*[tiab]) AND (low grade*[tiab] OR grade I*[tiab] OR grade II*[tiab] OR grade 1*[tiab] OR grade 2*[tiab] OR grade1*[tiab] OR grade2*[tiab])) AND (cognitive[tiab] OR cognition[tiab] OR cogniti*[tiab] OR memory[tiab] OR memor*[tiab] OR attention[tiab] OR executive[tiab]) |
|
Embase |
|
((Glioma/exp OR astrocytoma/exp OR glioma*:ab,ti OR astrocytoma*:ab,ti OR ependymoma*:ab,ti OR oligodendroma*:ab,ti) AND (‘low grade’:ab,ti OR ‘grade I’:ab,ti OR ‘grade II’:ab,ti OR ‘grade 1’:ab,ti OR ‘grade 2’:ab,ti OR ‘grade1’:ab,ti OR ‘grade2’:ab,ti)) AND (cognitive:ab,ti OR cognition:ab,ti OR cogniti*:ab,ti OR memory:ab,ti OR memor*:ab,ti OR attention:ab,ti OR executive:ab,ti) |
|
psycINFO |
|
(OR) glioma* astrocytoma* ependymoma* oligodendroma* (AND) (low grade) (AND) (OR) cognitive cognition cogniti* memory memor* attention executive |
Study selection
Studies were included if all of the following criteria were met: (i) the study population included patients with suspected or confirmed LGG; (ii) the results of these patients were distinguishable from any other patient group; (iii) the study population consisted of ≥ 10 patients; (iv) patients were at least 16 years of age at the time of diagnosis; and (v) cognitive functioning was 1 of the outcome measures.
Articles were excluded if: (i) the study design was a review or case study; (ii) no full text was available; (iii) written in a language other than English, Dutch, German or French; (iv) reported only duplicate data; or (v) assessed language capabilities only (i.e. aphasia examinations). Tests of verbal fluency were not considered to be instruments used only for assessing language capabilities, as these can also serve as suitable measures of executive functioning (25).
Two authors (EvL, WvL) independently performed selection of the studies. The first selection was based on title and abstract, and relevant articles were retrieved in full text. Full-text papers were also retrieved if abstracts were missing or if they provided insufficient information to enable selection. The final selection was based on scrutinizing the full-text articles. In case of disagreement between the reviewers, consensus was sought. If the disagreement was not resolved, a third reviewer (GR) made the final decision. The reference lists of the selected articles were reviewed by the first author to identify additional articles.
Data extraction
Two authors independently extracted data from the selected articles using a standard data extraction form. Data included items of study quality (study design, selection of study population, definition of disease, description of treatment, follow-up time, numbers lost to follow-up), population characteristics (number of patients, diagnosis, mean age, male/female ratio, mean time post-diagnosis), and items of cognitive assessment. Items of cognitive assessment included: (i) the cognitive domains tested: attention, memory, executive functioning and/or other cognitive domains (e.g. visio-construction) were primarily based on Lezak (25); (ii) the definition of cognitive dysfunction; (iii) cognitive tests used; and (iv) the reported prevalence of cognitive dysfunction in the study population. Brief cognitive screening instruments, such as the Mini-Mental State Examination (MMSE), were grouped under “other” cognitive domains since these instruments provide only a brief and basic assessment of multiple cognitive domains. In cases of missing data the corresponding authors were contacted by email.
Data synthesis
The focus of this systematic review was to study the instruments used for the cognitive assessment of patients with LGG, and the reported prevalence of cognitive dysfunction. Therefore, the qualitative results of this review are presented in tables using descriptive statistics to characterize the study populations. The measurement instruments used for the assessment of cognitive functioning were studied in detail and briefly described based on the existing literature, particularly Neuropsychological Assessment by Lezak (25), which is a standard in this field (26, 27). Because neuropsychological tests are often described using different names, to avoid confusion the name of the instrument as described by Lezak (25) was used, if available.
RESULTS
The initial literature search yielded 515 articles. After exclusion of duplicate articles, 312 articles were included in the first screening phase (title/abstract), resulting in 69 articles eligible for the second screening phase. After review of the full text, 22 potentially eligible articles remained. After a third round of critical full-text analysis, 4 articles (28–31) were excluded because the data overlapped with previous reports, and 1 article (32) was excluded because it reported only on tests assessing language capabilities. Finally, 17 articles were included in the present review (Fig. 1. shows the selection procedure).
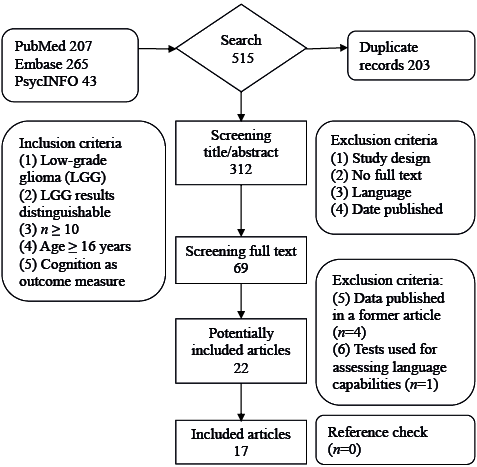
Fig. 1. Study selection procedure.
Study population
Of the 17 articles, those of Moritz-Gasser et al. (33) and Shankar & Rajshekhar (34) both reported results of a retrospective and a prospective study population in 1 publication; therefore, we decided to split both articles into 2 separate studies, resulting in a total of 19 studies.
The articles of Klein et al. (35) and the follow-up study of Douw et al. (7) have overlapping (sub)populations, and the study population of Laack et al. (36) consists of a subpopulation of the study of Brown (37). Finally, all 19 study populations were included in our analyses, because all underwent different cognitive tests and/or outcome measurements.
In this review, the total study population consisted of 775 patients with LGG. In 513 patients a complete description of the type of LGG was provided: the most common diagnosis was astrocytoma (61.0%), followed by oligodendroglioma (24.6%) and oligoastrocytoma (13.3%). The mean age of the total population (average of reported means) was 42.3 (range 38.1–46.7) years and the reported time post-diagnosis ranged from 1 month to 32 years (mean 63 months).
Of the 17 studies, 13 (representing 566 patients) featured cognition as a primary outcome measure. Most studies evaluated (only) the effect of a specific form of treatment: radiotherapy in 7 studies (n = 260), awake surgery in 4 (n = 49), and a combination of chemotherapy and awake surgery in 1 (n = 10). Seven studies (n = 180) were performed before starting any treatment.
Eight studies (n = 318) had a pre-post study design and 11 (n = 457) had a cross-sectional study design. The follow-up period ranged from 3 to 40 months.
Table II presents an overview of the studies and the baseline characteristics of the patient populations.
|
Table II. Overview of the baseline characteristics of the study populations |
||||||||||||||
|
Article reference |
A n (%) |
O n (%) |
OA n (%) |
E n (%) |
Ot n (%) |
U n (%) |
n |
Age1 (years) Mean (SD) |
Tx |
Time post-diagnosis1 (months) |
Time follow-up² (months) |
|||
|
Mean (SD) |
Range |
Mean (SD) |
Range |
|||||||||||
|
Blonski et al., 2012 (38) |
2 (20) |
6 (60) |
2 (20) |
. |
. |
. |
10 |
44.2 (10.4) |
CH+S |
58.1 (17) |
31–90 |
. |
. |
|
|
Bosma et al., 2008 (6) |
10 (59) |
4 (24) |
2 (12) |
. |
. |
1 (6) |
17 |
42.7 (11.2) |
RT±CH |
96 (?) |
12–228 |
. |
. |
|
|
Brown et al., 2003 (37) |
34 (18) |
? |
? |
. |
. |
. |
187 |
? (?) |
RT |
? (?) |
? |
60 (?) |
? |
|
|
Correa et al., 2007 (4) |
9 (23) |
19 (48) |
10 (25) |
. |
. |
2 (5) |
40 |
41.5 (9.4) |
±RT±CH |
? (?) |
3–142 |
. |
. |
|
|
Douw et al., 2009 (7) |
47 (72) |
12 (18) |
6 (9) |
. |
. |
. |
65 |
44.2 (11.9) |
±RT |
149 (46.8) |
72–336 |
. |
. |
|
|
Dutta et al., 2009 (51) |
? |
? |
? |
? |
? |
. |
33 |
? (?) |
Pre-RT |
? (?) |
? |
. |
. |
|
|
Ek et al., 2010 (39) |
5 (36) |
1 (7) |
. |
. |
. |
8 (57) |
14 |
38.4 (9.5) |
RT |
6.1c (?) |
? |
. |
. |
|
|
Klein et al., 2002 (35) |
139 (71) |
43 (22) |
13 (7) |
. |
. |
. |
195 |
40.8 (11.6) |
±RT |
67.2 (44.4) |
– |
. |
. |
|
|
Laack et al., 2005 (36) |
2 (10) |
9 (45) |
9 (45) |
. |
. |
. |
20 |
? (?) |
RT |
? (?) |
? |
38a (?) |
22–40 |
|
|
Miotto et al., 2011 (5) |
? |
? |
? |
? |
? |
. |
19 |
46.0 (11.6) |
Pre-S |
? (?) |
? |
. |
. |
|
|
Moritz-Gasser et al., 2012ab (33) |
? |
? |
? |
? |
? |
. |
11 |
38.1 (8.1) |
AS |
? (?) |
? |
. |
. |
|
|
Moritz-Gasser et al., 2012ab (33) |
? |
? |
? |
? |
? |
. |
12 |
38.3 (10.7) |
AS |
? (?) |
? |
9a (?) |
6–12 |
|
|
Pahlson et al., 2003 (41) |
12 (60) |
3 (15) |
3 (15) |
2 (10) |
. |
. |
20 |
46.7 (10.3) |
AS |
126 (113) |
7–384 |
. |
. |
|
|
Ruge et al., 2011 (42) |
30 (91) |
2 (6) |
1 (3) |
. |
. |
. |
33 |
44.4 (11.2) |
Pre-Tx |
0 (0) |
0 |
. |
. |
|
|
Santini et al., 2012 (40) |
2 (14) |
8 (57) |
4 (29) |
. |
. |
. |
14 |
? (?) |
AS |
5d (?) |
1–9d |
|
? (?) |
3–6 |
|
Sarubbo et al., 2011 (2) |
6 (50) |
5 (42) |
. |
. |
1a (8) |
. |
12 |
41.9 (?) |
AS |
? (?) |
? |
36 (?) |
? |
|
|
Shankar & Rajshekar, 2003ab (34) |
14 (100) |
. |
. |
. |
. |
. |
14 |
? (?) |
RT |
? (?) |
? |
34 (26) |
? |
|
|
Shankar & Rajshekar, 2003ab (34) |
16 (100) |
. |
. |
. |
. |
. |
16 |
? (?) |
RT |
0 (0) |
0 |
20 (6) |
? |
|
|
Yavas et al., 2011 (52) |
19 (44) |
14 (33) |
5 (12) |
. |
5b (12) |
. |
43 |
36* (?) |
RT |
? (?) |
? |
36 (?) |
? |
|
|
1At the first reported time of measurement, 2period between the first and last reported times of measurement; abarticle included 2 separate studies. aGanglioglioma; b1 ganglioglioma and 4 not otherwise classified LGG, cdata included 2 high-grade glioma patients, ddata included 8 high-grade glioma patients. *Median. A: astrocytoma; O: oligodendroma; OA: oligoastrocytoma; E: ependyoma; Ot: other type of LGG; U: histological unconfirmed tumour patients; Tx: type of treatment; CH: chemotherapy; S: surgical resection; RT: radiotherapy; AS: awake surgery; SD: standard deviation. |
||||||||||||||
Measurement instruments
We identified 46 different instruments used for the assessment of cognitive functioning (Table III). Five of these were used ≥ 5 times, i.e. the MMSE, Stroop Test, Trail Making Test, and verbal fluency tests. Tests of verbal fluency were reported under various names and were grouped into tests of phonemic and semantic verbal fluency. Four tests (Facial Recognition Test, Working Memory Task, William’s Delayed Recall Test and the Memory Comparison Test) are not included in Table III because the exact nature of these tests could not be established. Most of the tests were domain-specific. The MMSE and the Functional Assessment Measure provide a global (screening) measure of cognitive function.
|
Table III. Overview of the identified measurement instruments |
||
|
Measurement instrument |
Short task descriptiona |
Articlesb |
|
Auditory Consonant Trigrams |
Hold consonant trigrams (e.g. C-W-L) in mind for recall, while performing a distraction task. |
(4) |
|
Bells Test |
Circle 35 bells scattered amongst 315 objects pseudo-randomly distributed on a page, as quickly as possible. |
(38) |
|
Benton Visual Retention Test |
Recall a 3-figure design by drawing. |
(36) |
|
Block Design |
Use blocks to construct replicas of model designs. |
(39, 41) |
|
Brief Test of Attention |
Count letters or numbers or both from lists read aloud. |
(4) |
|
Brief Visuospatial Memory Test |
Reproduce a 2 × 3 visual array of geometric figures after a 10-s exposure. Recall after 25 min. |
(4, 5) |
|
Brixton Spatial Anticipation Test |
Predict the location of a coloured circle among white circles, determined by 1 of 9 rules based on its position on preceding cards. |
(38) |
|
Concept Shifting Test |
Cross out numbers consecutively, letters alphabetically, alternating numbers and letters, and empty circles in a clockwise fashion (53). |
(6, 7, 35) |
|
Cube Analysis |
Count the total number of blocks in 3-dimensional piles, presented in 2-dimensional drawings. |
(38) |
|
Digit Span |
Repeat sequences of random numbers of increasing length as presented (Digits Forward), or in reversed order (Digits Backward). |
(4, 5, 38, 40) |
|
Digit Symbol-Coding |
Symbols are paired with a randomly assigned number from 1 to 9. Fill in the blanks with nonsense symbols according to a key. |
(38) |
|
Dutch Adult Reading Test |
Dutch version of the National Adult Reading Test (NART). Read aloud 50 phonetically irregular words (54). |
(35) |
|
Table III. Contd. |
||
|
Measurement instrument |
Short task descriptiona |
Articlesb |
|
Functional Assessment Measure |
Test consists of 12 items, including 3 motor items and 9 cognitive/psychosocial items (55). |
(51) |
|
Grooved Pegboard |
Insert pegs that have a ridge along 1 side in a board with a 5×5 set of slotted holes by rotation of the pegs. |
(4) |
|
Hopkins Verbal Learning Test |
20–25 min delayed recall trial of a 12-word list, followed by a recognition trial with distractions. |
(4, 5) |
|
Incomplete Letters |
Identify a letter in 20 cards featuring an incomplete rendering. |
(5, 38) |
|
Information (subtest) |
Answer questions of increasing difficulty designed to test general knowledge about your country, subtest of Wechsler Adult Intelligence Scale. |
(41) |
|
Judgment of Line Orientation |
Match pairs of angled line segments with the same segments presented amongst numbered radii forming a semi-circle. |
(4, 35, 41) |
|
Letter Digit Substitution Test |
Letters are paired with randomly assigned digits from 1 to 9. Match the letters with digits according to a key (56). |
(6, 7, 35) |
|
Letter-Number Sequencing |
Alternating numbers and letters are read aloud. Subjects need to repeat the numbers first, followed by the letters, both in ascending order. |
(33, 38) |
|
Line Bisection Test |
Subjects have to “cut” line segments of various sizes in half by placing a pencil mark. |
(35) |
|
Matrix Reasoning |
Incomplete matrices need to be completed, choosing the item that best completes the pattern(s) from a multiple-choice array. |
(5) |
|
Mini-Mental State Examination |
Screening test of cognitive functions: orientation for time and place, memory, attention, and language (55). |
(2, 4, 36–38, 52) |
|
Müncher Verbaler Gedachtnistest |
German version of the California Verbal Learning Test. 15-word lists are used to assess immediate and delayed recall with distraction (57). |
(42) |
|
Picture Arrangement |
Subjects have to arrange sets of cartoon pictures in such an order that they represent the most sensible story. |
(41) |
|
Position Discrimination |
Two identical squares with a black dot are presented: 1 in the centre, 1 off-centred. Pick the square that contains the centred dot. |
(5) |
|
Post Graduate Institute Scale of Memory |
Memory assessment battery standardized for the Indian population (58). |
(34) |
|
(Rey) Auditory Verbal Learning Test |
15-word lists are used to assess immediate and delayed recall, and recognition with distraction. |
(36, 39–41) |
|
(Rey-Osterrieth) Complex Figure Test |
Test consists of a complex figure that the subjects need to copy, recall immediately, after a delay, or a combination of the 3. |
(38–40) |
|
Selective Reminding |
Subjects hear a list of words for immediate recall, repeatedly followed by a list of omitted words until all words are recalled. |
(38) |
|
Similarities |
Subjects are presented with pairs of concepts and have to explain what each pair has in common. |
(38) |
|
Stroop Test |
Subjects are presented with colour words printed in a different colour ink and have to name the colour of the ink rather than reading the words. |
(4, 6, 7, 35, 36, 38) |
|
Symbol Digit Modalities Test |
Digits paired with a randomly assigned symbol are presented. Fill in the blanks with numbers according to a key. |
(5, 39, 41) |
|
Testbatterie zur Aufmerksamkeitsprufung |
This test examines 3 basic components of attention including alertness, selective and divided attention (59). |
(42) |
|
Trail Making Test |
Subjects draw lines to connect consecutively numbered circles (part A) and to connect alternating numbers and letters (part B). |
(4, 33, 36, 38, 40) |
|
Verbal Fluency, phonemic |
Subjects need to say as many words as they can that begin with a given letter of the alphabet, during a certain amount of time. |
(4, 5, 33, 36, 38–41) |
|
Verbal Fluency, semantic |
Subjects need to say as many words as they can that belong to a given semantic category (e.g. “animals”), during a certain amount of time. |
(4–7, 33, 35, 38) |
|
Visual Memory Span |
Subjects are asked to reproduce spatial patterns on an array of blocks, either forwards or backwards. |
(38) |
|
Visual Verbal Learning Test |
15 common monosyllabic nouns are used to assess free recall, repeatedly followed by a list of mistakes, delayed recall, and recognition. |
(6, 7, 35) |
|
Vocabulary |
Subjects are presented with 30 words, 1 at a time and in order of difficulty, of which they have to explain the meaning. |
(5) |
|
Wechsler Adult Intelligence Scale (WAIS) |
Test consists of a test battery for measuring intelligence. |
(36) |
|
Wisconsin Card Sorting Test |
Work out the correct principle by which to sort the cards from the examiner’s “right” or “wrong” responses to the placement of the cards. |
(5) |
|
aPrimarily based on Lezak (25), if additional references are used these are mentioned; barticles that used this specific measurement instrument. |
||
Cognitive assessment
Memory was assessed in 13 studies, attention in 12, and executive functioning in 11.
In 11 studies all 3 domains were measured, of which 6 studies also included other domains, such as information processing and language. One study focused only on the memory domain. In 4 studies only a global cognitive screening test was used, the MMSE in 3 studies and the Functional Assessment Measure in 1 study. Table IV presents an overview of the study outcomes.
|
Table IV. Overview of the prevalence and definition of cognitive dysfunction, measurement time and cognitive domains assessed |
||||||||
|
Article reference |
Prevalence of cognitive dysfunctiona n/N (%) |
Measurement time |
Definition of cognitive dysfunctionb |
Cognitive domainsc |
||||
|
Pre Tx |
Post Tx |
A* |
M* |
E* |
O* |
|||
|
Blonski et al., 2012 (38) |
7/10 (70%) |
× |
Z-score ≥ 2 SD below the norm score or inferior to the 5th percentile |
1 |
1 |
1 |
1 |
|
|
Bosma et al., 2008 (6) |
. |
× |
Mann-Whitney U test, p < 0.05 |
1 |
1 |
1 |
0 |
|
|
Brown, 2003 (37) |
36/187 (19%) |
× |
MMSE score ≤ 26 |
0 |
0 |
0 |
1 |
|
|
Correa et al., 2007 (4) |
. |
× |
Z-score ≥ 1.5 SD below the norm score |
1 |
1 |
1 |
1 |
|
|
Douw et al., 2009 (7) |
RT+ 17/32 (53%) RT– 9/33 (27%) |
× |
Z-score ≥ 2 SD below the norm score in at least 5 of the 18 tests |
1 |
1 |
1 |
0 |
|
|
Dutta et al., 2009 (51) |
. |
× |
Not reported |
0 |
0 |
0 |
1 |
|
|
Ek et al., 2010 (39) |
6/16 (38%) |
× |
Deficit score = Z-score ≥ 1 SD below the norm score; Global Deficit score ≥ 0.5 (mean of 7 tests) |
1 |
1 |
1 |
0 |
|
|
Klein et al., 2002 (35) |
66/195 (34%) |
× |
Z-score ≥ 2 SD below the norm score in at least 4 of 20 tests |
1 |
1 |
1 |
1 |
|
|
Laack et al., 2005 (36) |
. |
× |
Clinically graded +1 (above average) to –4 (severely impaired) |
1 |
1 |
1 |
1 |
|
|
Miotto et al., 2011 (5) |
. |
× |
Not reported |
1 |
1 |
1 |
1 |
|
|
Moritz-Gasser et al., 2012ab (33) |
5/11 (45%) |
× |
Z-score ≥ 2 SD below the norm score |
1 |
1 |
1 |
0 |
|
|
Pahlson et al., 2003 (41) |
20/24 (83%) |
× |
Z-score ≥ 1 SD below the norm score |
1 |
1 |
1 |
1 |
|
|
Ruge et al., 2011 (42) |
. |
× |
Mann-Whitney U test, p < 0.05 |
1 |
1 |
0 |
0 |
|
|
Santini et al., 2012 (40) |
13/22 (59%) |
× |
Z-score ≥ 2 SD below the norm score |
1 |
1 |
1 |
0 |
|
|
Sarubbo et al., 2011 (2) |
. |
× |
× |
Not reported |
0 |
0 |
0 |
1 |
|
Shankar & Rajshekar 2003ab (34) |
. |
× |
Not reported |
0 |
1 |
0 |
0 |
|
|
Yavas et al., 2011 (52) |
. |
× |
× |
Not reported |
0 |
0 |
0 |
1 |
|
aPrevalence of cognitive dysfunction in study population, bdefinition of cognitive dysfunction used in this study population, ccognitive domains tested in this study, primarily based on Lezak (25). *A: attention; M: memory; E: executive functioning; O: other domains; 1: tested; 0: not tested; Tx: treatment. abarticle included 2 separate studies. |
||||||||
The definition of cognitive dysfunction varied considerably between articles. Seven studies defined cognitive dysfunction as a number of standard deviations (SD) below the mean of a normative sample, represented in terms of Z-scores (4, 7, 33, 35, 38–40). The cut-off points ranged from 1–2 SD below the mean of a normative sample, with 2 SD being the most common cut-off point. Five studies reported other definitions of cognitive dysfunction. Brown (37) defined cognitive dysfunction using a test-specific cut-off score ≤ 26 on the MMSE. Laack et al. (36) assessed cognitive performance on a clinical scale with a Board-certified neuropsychologist. Pahlson et al. (41) used a system in which Z-scores were classified into cognitive dysfunction classes ranging from mild to severe dysfunction. Ruge et al. (42) and Bosma et al. (6) used statistical significance testing to investigate whether study population results differed from healthy control scores (p ≤ 0.05). Five articles did not report any definition of cognitive dysfunction.
The prevalence of cognitive dysfunction could be deducted from 8 of the 17 articles (47%) and ranged from 19% to 83%.
DISCUSSION
The primary aim of this study was to systematically review the literature for assessment methods used for cognitive functioning in patients with LGG. Our search focused on memory, attention and executive functioning, i.e. the 3 main pillars of cognition (25).
In the 17 reviewed articles, 775 patients with LGG were assessed using 46 different instruments. Only 5 instruments were used 5 times or more, i.e. the MMSE, Stroop Test, Trail Making Test and tests of phonemic and semantic verbal fluency. In these latter studies, the main reason for cognitive testing was to evaluate the effects of treatment.
The fact that 46 different instruments were found indicates that no single standard test battery is used for the assessment of cognitive function in patients with LGG. Wilde et al. (43) recommended that cognitive tests should meet the following 7 criteria: widespread use, adequate psychometric properties, availability of norms, applicability across a range of injury severity and functional levels, accessible through the public domain, ease of administration, and brevity. An additional feature of the test should be its sensitivity to even small changes in cognitive functioning in patients with LGG.
To create more uniformity in studies on patients with LGG, Correa et al. (44) suggested using battery testing for attention, executive functioning, motor function, verbal memory and premorbid IQ estimation and quality of life. This battery would include at least the Digit Span, Trail Making Test A/B, Brief Test of Attention, Hopkins Verbal Learning Test, the Grooved Pegboard test and the Barona Index. However, with the exception of 1 study by Correa et al. (4), none of the articles in the present review included all of these tests. Meyers and Wefel et al. (45, 46) constructed a test battery for brain tumour patients, including patients with LGG, including the Digit Span, Digit Symbol, Block Design and Similarities of the WAIS III, Trail Making Test A/B, Hopkins Verbal Learning Test, Grip Strength, Grooved Pegboard, and a multilingual aphasia examination consisting of the Boston Naming Test, Token Test and the Controlled Oral Word Association Test. However, none of the articles in this current review included all of these tests. This battery is currently widely used in prospective randomized multicentre phase III trials, for both high- and low-grade gliomas.
As an alternative to formal testing, the use of patient-reported outcome measures (PROMs) with self-report questionnaires on cognitive complaints is suggested. Self-reported outcomes of the patients and their caregivers may provide useful additional information. The European Organization for Research and Treatment of Cancer brain cancer quality-of-life module (EORTC QLQ-BN20) (47) and the Cognitive Functional Scale, developed for use in the Medical Outcomes Study (MOS-CFS) (48), are examples of such PROMs. Aaronson et al. (49) used the MOS-CFS and concluded that 25% of the patients with LGG had frequent problems with memory and concentration. Complaints of being confused, having problems maintaining attention, having difficulty with solving problems, or having slowed reactions, were reported in 10–18% of patients with LGG.
Our second aim was to compare the reported prevalence of cognitive dysfunction in patients with LGG. The prevalence of cognitive dysfunction in the included articles ranged from 19% to 83%. This wide range may depend on the characteristics of the glioma (type, location, and size), the type of treatment, the time of measurement, the neuropsychological tests used, and the definition of cognitive dysfunction, all of which varied substantially between studies. This heterogeneity makes it difficult to draw general conclusions about the prevalence of cognitive dysfunction in patients with LGG.
Every effort should be made to reliably assess cognitive function in patients with LGG as this may support clinical decision-making; for example, to decide on planning surgical intervention or for targeting rehabilitation programmes.
Unfortunately, most studies included in this review had no (or only a very short) follow-up period.
Finally, it should be noted that this review focused on memory, attention and executive functioning. Cognitive domains, such as language, visuo-construction and perception, fell outside the scope of this research. This could be a limitation of this review, resulting in an underestimation of the cognitive disorders of patients with LGG as well as the variety of instruments used to measure these disorders. In a retrospective study, Lageman et al. (50) concluded that in clinical trials, visuo-construction, processing speed and verbal memory may be the most important domains to assess when evaluating cognitive deficits in patients with a primary brain tumour.
In conclusion, patients with LGG are often young and may have a life expectancy of 5–15 years, in which they may experience cognitive dysfunction and complaints that might benefit from multidisciplinary rehabilitation programmes. The present systematic review has shown that a wide range of neuropsychological tests are used, together with various criteria, to define cognitive dysfunction. Future studies should focus on the reliable assessment of cognitive function in patients with LGG in order to support clinical decision-making, and improve the targeting of rehabilitation programmes.
REFERENCES
