Mark de Niet, MSc1, Susanne T. de Bot, MD2, Bart P. C. van de Warrenburg, MD, PhD2, Vivian Weerdesteyn, PhD1,3 and Alexander C. Geurts, MD, PhD1,3
From the 1Department of Rehabilitation, and 2Department of Neurology, Donders Institute for Brain, Cognition, and Behaviour, Radboud University Medical Centre and 3St Maartenskliniek, Nijmegen, The Netherlands
OBJECTIVE: Although calf muscle spasticity is often treated with botulinum toxin type-A, the effects on balance and gait are ambiguous. Hereditary spastic paraplegia is characterized by progressive spasticity and relatively mild muscle weakness of the lower limbs. It is therefore a good model to evaluate the functional effects of botulinum toxin type-A.
DESIGN: Explorative pre-post intervention study.
SUBJECTS: Fifteen subjects with pure hereditary spastic paraplegia.
METHODS: Patients with symptomatic calf muscle spasticity and preserved calf muscle strength received botulinum toxin type-A injections in each triceps surae (Dysport®, 500–750 MU) followed by daily stretching exercises (18 weeks). Before intervention (T0), and 4 (T1) and 18 (T2) weeks thereafter, gait, balance, motor selectivity, calf muscle tone and strength were tested.
RESULTS: Mean comfortable gait velocity increased from T0 (0.90 m/s (standard deviation (SD) 0.18)) to T1 (0.98 m/s (SD 0.20)), which effect persisted at T2, whereas balance and other functional measures remained unchanged. Calf muscle tone declined from T0 (median 2; range 1–2) to T1 (median 0; range 0–1), which effect partially persisted at T2 (median 1; range 0–2). Calf muscle strength did not change.
CONCLUSION: Botulinum toxin type-A treatment and subsequent muscle stretching of the calves improved comfortable gait velocity and reduced muscle tone in patients with hereditary spastic paraplegia, while preserving muscle strength. Balance remained unaffected.
Key words: hereditary spastic paraplegia; botulinum toxin type-A; muscle spasticity; gait; balance.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Alexander C. Geurts, Department of Rehabilitation, Radboud University Medical Centre, PO Box 9101, Nijmegen, The Netherlands. E-mail: sander.geurts@radboudumc.nl
Accepted Aug 27, 2014; Epub ahead of print Oct 16, 2014
Introduction
Leg muscle spasticity is associated with deteriorated gait and balance performance in patients with upper motor neurone syndrome (UMNS) (1–3). In clinical practice, muscle spasticity can be treated with spasmolytic drugs (4, 5), of which local injections with botulinum toxin type-A (BTX-A) are increasingly favoured over oral medication. BTX-A selectively impairs the neural activation of targeted muscles, particularly their involuntary activity due to spasticity (6). It has been demonstrated that BTX-A can successfully reduce spasticity, with the degree of improvement depending on the dosage (4, 5). However, the functional effects of this treatment are ambiguous (7). An important reason for this may be that most patients with UMNS not only experience “positive” signs, such as spasticity, but also “negative” signs, such as muscle weakness and muscle fatigue (8). The presence and severity of these negative signs may substantially influence the individual functional effects of BTX-A treatment. In order to improve clinical decision-making, it is thus important to understand the functional effects of BTX-A injections on both spasticity and motor control.
Patients with hereditary spastic paraplegia (HSP) constitute a good clinical model to investigate the effects of BTX-A treatment on motor control. HSP has a prevalence ranging between 2 and 10 per 100,000 persons and is characterized by slowly progressive leg muscle spasticity (9). The disease can be divided into “pure” and “complicated” phenotypes. In contrast to the complicated phenotype, patients with the pure form of HSP have relatively little somatosensory impairment and often only mild loss of muscle strength. These patients are mainly disabled by lower limb spasticity and associated muscle stiffness. Thus, in these patients, functional effects of BTX-A treatment can be assessed relatively unconfounded by paresis and somatosensory impairment.
To our knowledge, only a few uncontrolled studies have investigated the effects of BTX-A injections on motor control and functional performance in patients with HSP (10–12). These studies showed that BTX-A injections in the calves and/or hip adductors may lead to reduced levels of spasticity and some increase in gait velocity. However, functional improvements were found only in a limited number of participants, which may have been due to genetic heterogeneity (pure and complicated phenotypes), inclusion of different degrees of severity of motor impairments, and varying BTX-A treatment protocols (dosages and target muscles). In addition, these studies did not incorporate stretching exercises, which may have attenuated potential treatment effects. Indeed, a recent consensus on the application of BTX-A treatment recommended stretching the injected muscles after the treatment (4, 13). Lastly, previous studies did not investigate the effects of BTX-A on balance or other mobility-related skills in patients with HSP.
Hence, the aim of this explorative study was to investigate the functional effects of BTX-A injections in the calves, followed by 18 weeks of daily calf muscle stretching, in patients with pure HSP, symptomatic calf muscle spasticity and preserved calf muscle strength. It was hypothesized that BTX-A treatment and subsequent stretching would improve both gait velocity and balance performance due to a reduction in disabling calf muscle tone, with no change in muscle strength.
Methods
Participants
All patients with symptomatic HSP who visited the outpatient departments of Neurology and Rehabilitation Medicine of our university hospital during a period of one year were eligible. In addition, active recruitment took place through the national patient organization “Spierziekten Nederland” and the hospital database. Inclusion criteria were: (i) having a form of autosomal dominant “pure” HSP (either genetically proven, i.e. subtypes SPG-4, SPG-3A and SPG-8, or based on family history); (ii) having clinical symptoms related to calf muscle spasticity (e.g. muscle cramps, stiffness, pain, clonus) either persistently or intermittently; (iii) being a community ambulator (Functional Ambulation Categories score 5 (range 0–5)); (iv) having bilateral premature calf muscle activity during the loading and/or midstance phase of gait, as determined by surface electromyography (EMG); (v) having balance- and/or gait-related activity limitations in daily life based on the patient’s experiences; and (vi) aged between 18 and 75 years. Exclusion criteria were: unilateral or bilateral: (i) calf muscle tone < 1 or > 2 on the Modified Ashworth Scale (MAS, range 0–5) with the knee either flexed or extended; (ii) muscle strength of the calf or tibialis anterior muscle < 4 on the Medical Research Council scale (MRC, range 0–5); (iii) passive ankle range of motion (PROM) < 10° dorsiflexion with the knee extended.
A total of 77 eligible patients with pure HSP were identified and contacted by the primary investigator (MdN). Of these, 46 patients were excluded based on a preliminary interview in which willingness to participate and inclusion criteria 2, 3 and 5 were checked. Thirty-one patients were invited to attend the outpatient department for additional, physical screening (ACG) and EMG recording during gait (MdN). Based upon these examinations, 16 patients with pure HSP were eventually included (Table I for characteristics). Reasons for exclusion of 15 patients were related to a MAS score of the calf < 1 (n = 5) or > 2 (n = 8), or a MRC score of the calf < 4 (n = 2). In addition, 10 healthy subjects with the same age and sex distribution participated as controls (only for assessments of gait velocity and dynamic posturography). All subjects gave their written informed consent prior to participation. The study was approved by the regional medical ethics committee and conducted according to the principles of the Declaration of Helsinki (14).
|
Table I. Characteristics of patients and controls |
||
|
Patients (n = 15) |
Controls (n = 10) |
|
|
Age, years, mean (SD) [range] |
47.7 (12.3) [20–66] |
46.1 (11.8) [22–63] |
|
Gender, male/female |
12/3 |
7/3 |
|
Gene SPG-4 SPG-3A SPG-8 AD |
8 1 1 5 |
|
|
MRC 5 4 |
9 6 |
|
|
Sensibility, mean (SD) [range] |
3.9 (2.0) [0–7]* |
|
|
*Assessed with Rydel-Seiffer tuning fork. MRC: Medical Research Council scale; AD: autosomal dominant inheritance; SD: standard deviation. |
||
Intervention
A solution of 500 MU Dysport® in 5 ml saline 0.9% was used. The total dosage was equal for both legs and was dependent on the level of spasticity. Patients with bilateral calf muscle tone MAS 1 received 500 MU in each leg, whereas patients with unilateral or bilateral calf muscle tone MAS 2 received 750 MU in each leg. The BTX-A was distributed evenly over the 3 heads of the triceps surae, i.e. soleus (5 sites) and medial (2 sites) and lateral (2 sites) heads of the gastrocnemius. Intramuscular electrical stimulation was used for optimal localization of the injection sites.
During the 18-week intervention period, participants were instructed to perform 10 min stretching exercises of the calf muscles (with the knees both flexed and extended) twice daily. These exercises were demonstrated to all patients at the day of the injections until they were able to perform the exercises correctly themselves. During the intervention period, patients were asked every 2 weeks by the primary investigator (MdN) whether they did their daily exercises. Partners were also stimulated to motivate the patients. No formal exercise diary was kept.
Outcome measures
The primary outcome measure was comfortable gait velocity, tested barefooted with the 10-m walking test (10MWT) (15). The 10MWT (15) has been shown to be a valid and reliable instrument in neurological patients. As the objective was also to record gait kinetics and kinematics (not reported in this study), we used a 6-camera Vicon motion analysis system (Vicon MX, Oxford Metrics, Oxford, UK) to measure gait speed. Reflective markers were placed on the skin according to the PlugInGait full-body model (BodyBuilder, Vicon Motion Systems, Lake Forest, CA, USA). From the marker position recordings, we calculated the walking speed over the middle 6 m (the first and last 2 m were discarded to allow for acceleration and deceleration).
Secondary outcomes were aimed at muscle tone and strength, leg motor selectivity, maximum gait velocity, timed-up-and-go performance, functional balance, dynamic posturography, and balance confidence. Muscle tone of the calf and tibialis anterior (TA) was assessed using the Modified Ashworth Scale (MAS, range 0–5, (16)): 0 = no increase in muscle tone; 1 = slight increase in tone, manifested by a catch and release or by minimal resistance at the end of range of motion (ROM); 2 = increase in tone, manifested by a catch followed by resistance through < 50% of ROM; 3 = marked increase in tone through > 50% of ROM; 4 = severe increase in tone through whole ROM (passive movement difficult); 5 = body segment rigid in flexion or extension (contracture). The calf muscle was tested with the knee both flexed (soleus) and extended (gastrocnemius).
Muscle strength of the calf and TA was assessed with the Medical Research Council scale (MRC, range 0–5): 0 = no movement observed; 1 = only a trace or flicker of movement is seen or felt in the muscle or fasciculations are observed; 2 = muscle can move only if the resistance of gravity is removed; 3 = muscle strength is such that the joint can be moved against gravity only with the examiner’s resistance completely removed; 4 = muscle strength is reduced but movement is possible against resistance (i.e. being able to stand on the heels when testing the TA and to stand on the toes when testing the calf, bilaterally); 5 = muscle contracts normally against full resistance (i.e. being able to stand on the heels and toes, unilaterally) (17); for scores 4 and 5 balance support was allowed.
In addition to clinical muscle testing, maximal voluntary isometric calf muscle strength was measured using the Quantitative Muscle Assessment (QMA) fixed myometry testing system (Aeverll Medical LLC, Gainesville, GA, USA) (18). With this aim, patients were lying on a bench in a fixed position with the knees extended and ankle joints in a neutral position. In this position, patients had to plantarflex 1 ankle joint as firmly as possible against a rope attached to the forefoot and connected to a force transducer. This test was repeated 3 times for the left and the right ankle separately, allowing sufficient resting time in between the tests. The QMA score was the greatest strength (kg) of 3 attempts for each side.
Leg motor selectivity was tested using the Fugl Meyer Assessment (FMA) (19). The lower extremity part of the FMA was scored for each leg and expressed as a percentage of the maximum score (range 0–34).
Maximum gait velocity was tested barefooted using the 10MWT comparable to the test of comfortable gait velocity. Participants were instructed to walk as fast as possible without increasing their fall risk. In addition, the Timed-Up-and-Go (TUG) test (20) was performed with shoes, but without walking aids. Functional balance was assessed barefooted with the BBS (range 0–56) (21).
Dynamic posturography was applied to test the postural responses to platform perturbations (for detailed descriptions of the characteristics of these perturbations see de Niet et al. (22)). Patients stood barefoot on a moveable platform (size 1.2 × 1.8 m; BAAT Medical Products BV, Hengelo, The Netherlands) with their eyes open, knees extended, and their feet at shoulder width. To prevent falls, they wore a safety harness suspended from the ceiling. Platform rotations resulted in either dorsiflexion (toes-up perturbation (TUP)) or plantarflexion (toes-down perturbation (TDP)) at the ankle joints. The platform rotated at a maximum angular velocity of 51 °/s from a horizontal position to 3, 5, 7 or 9° inclination. In addition, platform translations (accelerations of 0.25, 0.5, 0.75 and 1 m/s2) were applied in either the backward (forward perturbation (FP)) or forward (backward perturbation (BP)) direction. More specifically, TUPs were imposed to test the disinhibition of destabilizing calf muscle responses to stretch (as a key symptom of spasticity), whereas FPs were applied to test the potential effect of calf muscle weakness on stabilizing calf muscle responses. Each type of perturbation was repeated 4 times at each intensity level, so that a total number of 64 perturbations were imposed. A trial was classified as successful when the subject could sustain the perturbation without taking a step, grabbing for support, or bending the knees; otherwise it was classified as a failure. Trials at the highest intensity that were not performed by patients due to fatigue or fear of falling were also scored as failures. The overall balance performance was determined as the percentage of trials successfully executed for each type of perturbation (dynamic balance score).
In addition, to obtain a subjective measure of balance and mobility, the Activities-specific Balance Confidence Scale – short version (ABC-questionnaire) (23, 24) was used.
Procedure
Within 3 weeks after inclusion, each patient with HSP was treated with bilateral BTX-A injections in the calf muscles by the same clinician (ACG). They were evaluated on all outcome measures by the same investigator (MdN) 1 week before treatment (T0), 4 weeks after treatment (T1) and 18 weeks after treatment (T2). T1 was set at 4 weeks after treatment, because at this time the physiological effects of BTX-A on spasticity were expected to have reached a maximum (25). Thereafter, the effects of BTX-A were expected to diminish progressively until about 4 months after the injections. Hence, T2 was set at 18 weeks post-treatment to test the possible presence of a residual effect of the treatment not directly related to the physiological effects of BTX-A.
Patients’ experiences
In addition to the formal outcome measures, patients were interviewed (semi-structured) at T2 for the treatment outcome in terms of functional gain (specifically focused on gait and balance) and general effects (e.g. perceived muscle stiffness). Finally, patients were asked whether they would consider continuation of treatment of the calf muscles with BTX-A (yes/no/undecided).
Statistics
The bilateral scores for muscle tone (MAS), strength (MRC, QMA) and motor selectivity (FMA) were averaged into a single score for both sides of the body, resulting in an individual score for each assessment. Continuous and interval measures (gait velocities, dynamic balance score, BBS, TUG, QMA, FMA and ABC questionnaire) were tested for time effects (T0, T1 and T2) using one-way ANOVA for repeated measures. The other outcomes (MAS and MRC) were tested for time effects by means of the non-parametric Friedman’s test. To determine at what time interval significant Time effects occurred, we used post-hoc paired-samples t-tests and Wilcoxon signed-rank tests with Bonferroni adjustments for continuous/interval and ordinal variables, respectively. Finally, the patients were divided in subgroups based on both the MAS and MRC scores of the calf at T0, and compared with regard to the change in gait velocity between T0 and T1 using a Mann-Whitney U test. To compare gait velocity and dynamic balance performance of the patients with the performance by healthy controls, independent t-tests were used. The alpha level for all statistics was set at 0.05. Patients’ evaluations were qualitatively analysed.
Results
Participants
Every patient, except for 2, participated in all assessments. One patient was lost to follow-up after T1, because the measurements were too demanding. Hence, 15 patients were analysed, for whom the patient characteristics are given in Table I. One of these patients did not complete the dynamic balance assessment at T2 due to technical problems. Maximum gait velocity was set equal to comfortable velocity in 2 other patients, because they came close to falling during the examination of maximum gait velocity. According to the inclusion criteria, at T0 the MAS scores of the calf were 1 or 2 in all patients (MAS 2; gastrocnemius n = 8, soleus n = 6), whereas in most patients (n = 9) MRC scores of the calf were 5, indicating normal muscle strength.
Primary outcome
Table II summarizes the group results for all patients at all measurements. The comfortable gait velocity showed a main effect of time (F(2,28) = 11.93, p < 0.001), which consisted of a 9% increase from baseline at T1 (p = 0.006) and a 12% increase from baseline at T2 (p = 0.002). The change in comfortable gait velocity did not differ between patients with an initial MAS 1 score and those with an initial MAS 2 score of the calves (gastrocnemius, p = 0.209; soleus, p = 0.112) (Fig. 1). In addition, patients with MRC 5 of the calves at baseline did not show a different change in comfortable gait velocity compared with those with an initial MRC 4 grade (p = 0.088) (Fig. 2).
|
Table II. Primary and secondary outcomes for patients with hereditary spastic paraplegia at T0, T1 and T2 (n = 15) |
|||||||
|
Test |
T0 |
T1 |
T2 |
Friedman’s test/ Repeated measures |
p-value |
Controls |
t-test p-value T0 vs control |
|
Muscle tone, median [IQR] |
|||||||
|
MAS – G |
1 [1–2] |
0 [0–0] |
1 [1–1] |
χ2(2, n = 15) = 26.462 |
< 0.001a |
||
|
MAS – Sol |
2 [1–2] |
0 [0–1] |
1 [1–1] |
χ2(2, n = 15) = 26.528 |
< 0.001a |
||
|
MAS – TA |
0 [0] |
0 [0] |
0 [0] |
n.a. |
|||
|
Muscle strength |
|||||||
|
MRC – G/Sol, median [IQR] |
5 [4–5] |
4 [4–5] |
5 [4–5] |
χ2(2, n = 15) = 8.4 |
0.015b |
||
|
MRC – TA, median [IQR] |
5 [5] |
5 [5] |
5 [5] |
n.a. |
|||
|
QMA – G/Sol (kg), mean (SD) [95% CI] |
52.6 (18.1) [43.5–61.8] |
50.5 (15.9) [42.6–58.5] |
53.6 (15.8) [45.7–61.6] |
F(2,28) = 1.762 |
0.190 |
||
|
Assessments |
|||||||
|
BBS, median [IQR] |
54 [36–56] |
55 [37–56] |
54 [39–56] |
F(2,28) = 1.27 |
0.295 |
||
|
FMA (%), mean (SD) [95% CI] |
82.3 (6.7) [78.9–85.7] |
81.3 (5.3) [78.2–83.5] |
81.5 (5.4) [78.7–84.2] |
F(2,28) = 1.29 |
0.292 |
||
|
Comfortable gait velocity (m/s), mean (SD) [95% CI] |
0.90 (0.18) [0.80–0.97] |
0.98 (0.22) [0.87–1.09] |
1.01 (0.19) [0.91–1.11] |
F(2,28) = 11.93 |
< 0.001c |
1.32 (0.14) [1.23–1.4] |
< 0.001 |
|
Maximum gait velocity (m/s), mean (SD) [95% CI] |
1.33 (0.34) [1.16–1.50] |
1.33 (0.33) [1.17–1.50] |
1.33 (0.37) [1.14–1.51] |
F(2,28) = 0.010 |
0.990 |
1.95 (0.31) [1,76–2.14] |
< 0.001 |
|
TUG (s), mean (SD) [95% CI] |
10.4 (2.8) [8.9–11.8] |
10.5 (2.3) [9.3–11.7] |
10.9 (2.5) [9.7–12.2] |
F(2,26) = 2.32 |
0.118 |
|
|
|
ABC questionnaire (%), mean (SD) [95% CI] |
30.1 (10.7) [24.7–35.5] |
29.8 (11.2) [24.2–35.5] |
28.8 (12.) [22.7–34.9] |
F(2,28) = 0.749 |
0.482 |
|
|
|
Balance perturbations, median [IQR] |
|
||||||
|
TUP (%) |
46.0 (24.3) [33.7–58.3] |
49.6 (25.1) [36.9–62.2] |
50.0 (23.4) [38.2–61.8] |
F(2,26) = 1.667 |
0.208 |
90.6 (13.3) [82.4–98.8] |
< 0.001 |
|
TDP (%) |
87.5 (17.3) [78.7–96.3] |
87.5 (20.7) [77.0–98.0] |
86.2 (22.9) [74.6–97.7] |
F(2,26) = 0.063 |
0.939 |
100 (0.0) |
0.042 |
|
FP (%) |
39.3 (20.0) [29.2–49.4] |
36.6 (18.8) [27.1–46.1] |
41.1 (23.5) [29.2–53] |
F(2,26) = 1.723 |
0.198 |
73.8 (14.1) [65–82.5] |
< 0.001 |
|
BP (%) |
39.7 (10.6) [34.4–45.1] |
42.0 (11.9) [36–48] |
40.6 (11.2) [35–46.3] |
F(2,26) = 0.588 |
0.562 |
61.3 (15.5) [51.6–70.9] |
< 0.001 |
|
a(T0 > T2 > T1), b(T0/2 > T1,T0 = T2), c(T1/2 > T0,T1 = T2). Bold values indicate statistical significance. MAS: Modified Ashworth Scale; MRC: Medical Research Council scale; G: gastrocnemius muscle; Sol: soleus muscle; QMA: Quantitative Muscle Assessment; BBS: Berg Balance Scale; FMA: Fugl Meyer Assessment – leg score; ABC-questionnaire: Activities-specific Balance Confidence Scale – short version; TUP: toes-up perturbation; TDP: toes-down perturbation; FP: forward perturbation; BP: backward perturbation; SD: standard deviation; CI: confidence interval. |
|||||||
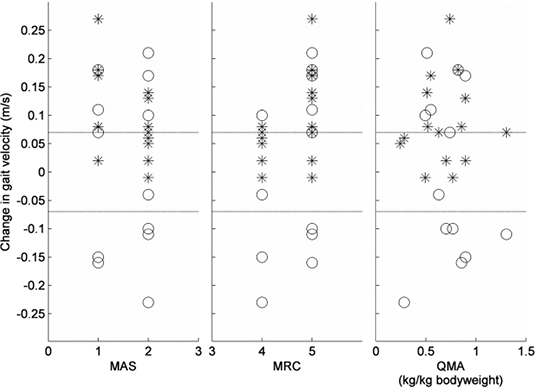
Fig. 1. Change in gait velocity between baseline (T0) and T1 represented for different scores on the Modified Ashworth Scale (MAS), Medical Research Council scale (MRC) and Quantitative Muscle Assessment (QMA) at baseline. * = Change in comfortable gait velocity, O = Change in maximum gait velocity. Dotted lines represent ± 0.07m/s (defined as clinically relevant change in gait velocity).
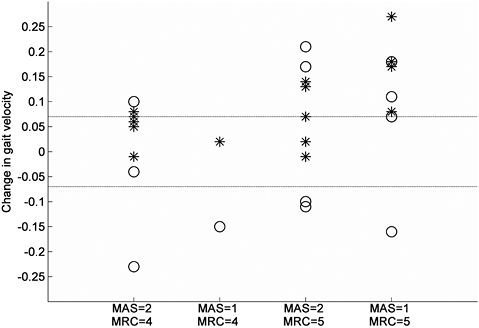
Fig. 2. Change in gait velocity between T0 and T1 for various subgroups based on Modified Ashworth Scale (MAS) and Medical Research Council scale (MRC) scores of the calf at T0. * = Change in comfortable gait velocity, O = Change in maximum gait velocity. Dotted lines represent ± 0.07m/s (defined as clinically relevant change in gait velocity).
Secondary outcomes
Calf muscle tone diminished across time (gastrocnemius: χ2 (2, n = 15) = 26.462; soleus: χ2 (2, n = 15)=26.528, both p < 0.001). This reduction partially persisted at T2 (gastrocnemius: p = 0.004; soleus: p = 0.002). Calf muscle strength assessed with the MRC also showed a time effect (χ2 (2, n = 15) = 8.4, p < 0.001). At T1, calf muscle strength was reduced compared with T0 (p = 0.046), but values returned to baseline level at T2 (p = 0.317). In contrast, muscle strength measured by QMA did not show any time effects (F(2,28) = 1.762, p = 0.190). Likewise, other clinical measures (FMA, TUG, BBS, and ABC questionnaire) remained stable over time. In addition, maximum gait velocity did not show any time effect (F(2,24) = 0.010, p = 0.990) (Table II).
Differences from healthy controls
At baseline, patients walked more slowly than healthy controls at both maximum (mean 1.33 (standard deviation; SD 0.34) vs 1.95 (SD 0.31), p < 0.001) and comfortable (mean 0.90 (SD 0.18) vs 1.32 (SD 0.28), p < 0.001) velocity. Furthermore, dynamic balance performance was worse in the patients than in the controls for all perturbation directions. None of these values showed significant changes across time (Table II).
Patients’ experiences
Table III summarizes the experiences mentioned after the treatment at T2. Nine patients were very satisfied with the effects and requested continuation of treatment of the calf muscles, whereas 3 patients were undecided whether they wanted to undergo this treatment again. Three patients were disappointed in the effects and did not consider continuation of treatment. Disappointment was related mainly to an experienced loss of calf muscle strength or loss of functional capacities. The experienced detrimental effects had all disappeared at T2.
|
Table III. Patients’ experiences after treatment (T2, n = 15) |
|
|
|
n |
|
Perceived effects |
|
|
Gait |
|
|
Increase in efficacy (i.e. lower energy costs) |
5 |
|
Less stumbling |
2 |
|
Balance |
|
|
Increase in balance |
2 |
|
Other |
|
|
Reduction in muscle cramps/spasms |
9 |
|
Reduction in muscle stiffness (also expressed as more relaxed feeling in muscles) |
4 |
|
Improved physical fitness |
3 |
|
No effects perceived |
3 |
|
Better rest at night |
2 |
|
Cessation of disability progression |
1 |
|
(Transient) side-effects |
|
|
Decreased balance confidence |
2 |
|
Reduction in muscle strength |
2 |
|
More stumbling |
2 |
Discussion
The aim of this study was to evaluate the effects of BTX-A treatment and subsequent stretching of the calf muscles on gait and balance performance in patients with pure HSP who had symptomatic calf muscle spasticity and relatively preserved muscle strength. Our hypothesis that this treatment would improve gait velocity was partly corroborated. Only comfortable gait velocity improved by 9–12% after treatment. This effect was probably mediated by a significant reduction in calf muscle tone, which may have promoted the rotation of the tibiae in the ankle joints during the midstance phase of gait, allowing a smoother roll-off at both sides of the body. Indeed, a median improvement of 1 point on the MAS is generally considered to be clinically relevant (26). Interestingly, the beneficial effects of BTX-A treatment appeared to be independent of the initial level of calf muscle tone or strength, although a tendency was observed that patients with MRC 5 and MAS 1 scores of the calf benefitted most consistently (Fig. 1). Indeed, all 4 patients with such scores reached a clinically relevant change of ≥ 0.07 m/s in their comfortable gait velocity. This specific finding, as well as the overall pattern of results, indicates that the effects of BTX-A treatment on muscle tone probably outweigh the possible detrimental (side) effects on muscle strength. The MRC scores of the calf suggested that, overall, a small temporary reduction in muscle force may have occurred 4 weeks after treatment. Yet, the stable QMA values indicated that this temporary loss of calf muscle strength was relatively minor. Interestingly, the beneficial effects on both comfortable gait velocity and muscle tone persisted until 18 weeks after treatment, despite the fact that the biological effects of BTX-A are commonly thought to have ceased after this time interval. It may be that the stretching component of the treatment protocol was responsible for the observed long-term effects.
The observed reduction in muscle tone after BTX-A treatment has been described extensively in previous studies (27, 28), but the effect of BTX-A injections on muscle strength has received considerably less attention (10). Some studies have shown that patients may lose muscle strength, but the results of these studies were ambiguous (10) and not always focused on the treated muscles (11). In the present study, we assessed muscle strength with 2 separate tests; the most commonly used clinical assessment (MRC scoring) and a QMA. The clinical assessment showed that the median MRC score of the calf decreased from 5 to 4 at 4 weeks after treatment due to a temporary effect that was observed in 4 patients. However, the QMA test did not show a significant decrement, which suggests that any reduction in calf muscle force after treatment was not merely temporary, but also clinically insignificant. This notion is supported by the fact that the maximum gait velocity remained unchanged after treatment. Indeed, there were just as many patients showing an improvement as there were patients showing a decline in maximum gait velocity (Fig. 1).
Besides a significant group effect, the comfortable gait velocity showed a clinically relevant change (≥ 0.07 m/s) in 10 of the 15 subjects (67%). This result indicates that patients with HSP may indeed experience a functional benefit from BTX-A injections in spastic calf muscles. At the same time, it indicates that such a benefit is not reached in every patient. Predicting which type of patient will experience functional benefits from BTX-A treatment has shown to be difficult (11). Also in this study, no predictors of success could be identified, although patients with initial MRC 5 and MAS 1 scores of the calves tended to exhibit the greatest improvement in comfortable gait velocity. Overall, it was reassuring that none of the patients with HSP showed a decline in comfortable gait velocity after BTX-A treatment of the calves, indicating the safety of this procedure with regard to ambulation.
Contrary to our hypothesis, balance performance and other functional measures remained unchanged after treatment. It may be that some of the outcome measures applied were not sensitive enough to detect functional improvements (29). On the other hand, one could question the clinical relevance of such small improvements. Another possibility is that, because of the chronic and progressive character of HSP, patients would need more time and repeated BTX-A treatment to be able to improve their balance-related skills and confidence. Future studies with repeated sessions of BTX-A injections in spastic muscles might shed light on this possibility. Probably the most important consideration for the lack of benefit with regard to balance and functional performance is that calf muscle spasticity may not be the main determinant of balance-related disabilities. Evidence for this notion has recently been found by a study in which patients with pure HSP were subjected to dynamic platform perturbations similar to those applied in the present study. It was found that a delay of approximately 35 ms in the postural responses of the distal leg muscles (gastrocnemius, tibialis anterior) was the most important factor for explaining impaired balance responses to platform translations (30), while calf muscle spasticity was explanatory only for impaired responses to toes-up perturbations (22). This latter type or perturbations is, however, relatively rare in daily life.
Patients’ experiences
Most patients were completely (n = 9) or partially (n = 3) satisfied with the effects of treatment. Besides improvements in functional performance, several patients experienced less burden from physical problems such as spasms and muscle stiffness, better sleep, and/or a feeling of improved physical fitness. These subjective improvements are in line with previous studies in patients with HSP (10, 11), indicating that focal spasmolysis may have beneficial effects unrelated to functional improvements.
Study limitations
An obvious limitation of the present study is the relatively small sample size, which is inherent in the inclusion of a homogeneous group of patients with pure HSP, as well as the lack of a control condition (e.g. placebo treatment). The use of rather stringent inclusion and exclusion criteria also limits the generalizability of our findings. Future research should preferably be multi-centred to increase the number of participants and allow a randomized controlled design. In addition, the functional effects of botulinum toxin should be assessed in relation to those of orthotic interventions.
Conclusion
To our knowledge, this is the first study to investigate the functional effects of BTX-A injections in symptomatic spastic calf muscles in a homogeneous group of patients with pure HSP and with preserved calf muscle strength. We learned that this combined treatment gives a clinically significant and long-term improvement in comfortable gait velocity in most patients, probably mediated by a reduction in calf muscle tone, without a risk of inducing disabling calf muscle weakness or reducing maximum gait velocity. In addition, it became evident that improvement in balance-related skills and confidence cannot be expected within the same time-frame. Future studies might provide better insight into the underlying causes of the absence of functional effects on balance.
Acknowledgement
Alexander Geurts was supported by an unrestricted grant from Ipsen Farmaceutica BV in the Netherlands.
References
