Joffrey Drigny, MSc1, Vincent Gremeaux, MD, PhD1–3,4, Olivier Dupuy, PhD1,5–7, Mathieu Gayda, PhD1–3, Louis Bherer, PhD5,6, Martin Juneau, MD1–3 and Anil Nigam, MD1–3
From the 1Cardiovascular Prevention and Rehabilitation Centre (ÉPIC), 2Research Center, Montreal Heart Institute and “Université de Montréal”, 3Department of Medicine, Faculty of Medicine, “Université de Montréal”, Montreal, Canada, 4Plateforme d’investigation technologique, INSERM CIC 1432, CHU Dijon, Dijon, France, 5PERFORM Centre, Department of Psychology, Concordia University, Montréal, Québec and 6Laboratoire LESCA, Centre de Recherche de l’Institut Universitaire de Gériatrie de Montreal, Canada and 7Faculté des Sciences du Sport, Laboratoire MOVE, Université de Poitiers, France
OBJECTIVE: To assess the effect of a 4-month high-intensity interval training programme on cognitive functioning, cerebral oxygenation, central haemodynamic and cardiometabolic parameters and aerobic capacity in obese patients.
METHODS: Cognitive functioning, cerebral oxygenation, central haemodynamic, cardiometabolic and exercise parameters were measured before and after a 4-month high-intensity interval training programme in 6 obese patients (mean age 49 years (standard deviation 8), fat mass percentage 31 ± 7%).
RESULTS: Body composition (body mass, total and trunk fat mass, waist circumference) and fasting insulin were improved after the programme (p < 0.05). V. O2 and power output at ventilatory threshold and peak power output were improved after the programme (p < 0.05). Cognitive functioning, including short-term and verbal memory, attention and processing speed, was significantly improved after training (p < 0.05). Cerebral oxygen extraction was also improved after training (p < 0.05).
CONCLUSION: These preliminary results indicate that a 4-month high-intensity interval training programme in obese patients improved both cognitive functioning and cerebral oxygen extraction, in association with improved exercise capacity and body composition.
Key words: high-intensity interval training; obesity; cognition; cerebral oxygenation.
J Rehabil Med 2014; 46: 1050–1054
Correspondence address: Anil Nigam, Montreal Heart Institute, 5000 Belanger St East, Montreal, QC, Canada. E-mail: anil.nigam@icm-mhi.org
Accepted Sep 10, 2014; Epub ahead of print Oct 7, 2014
INTRODUCTION
Obesity represents a major health problem in our society and increases the risk of mortality and major chronic diseases, such as cardiovascular disease, type 2 diabetes and hypertension. Physical activity is recommended in obese patients as a treatment intervention to improve body composition and global health. Compared with moderate intensity continuous exercise training, high-intensity interval training (HIIT) has a greater effect on body mass loss, adiposity reduction, reduction in waist circumference (WC) and improvement in V. O2max in obese subjects (1).
Obesity is associated with cognitive dysfunction across adulthood and with a higher risk of dementia with ageing (2). Nutritional intervention and body mass loss are known to improve executive/attention functioning and memory in obese patients. Regular physical activity is clearly associated with lower rates of age-related cognitive decline (3) and brain atrophy (4). Furthermore, improvement in exercise capacity after an aerobic training programme in healthy adults has been shown to be associated with improved cognitive performance (3). However, the effects of physical activity on cognitive functioning in obese patients are unknown. In addition, neuro-imaging studies in obese patients have shown that cognitive decline may result from changes in brain structure (5). One mechanism by which physical training may improve cognition is through better cerebral oxygenation, concomitant with an improvement in V. O2max (6).
The aim of this study was to determine the effects of a 4-month HIIT programme on cognitive functioning, cerebral oxygenation, exercise capacity and cardiometabolic parameters in obese patients.
MethodS
Subjects
Six obese patients (mean age 49 years (standard deviation (SD) 8), range 40–56 years) were included in the study. Inclusion criteria were: age > 18 years and fat mass percentage > 25% in men and > 35% in women. Exclusion criteria were: previous cardiac disease (coronary, peripheral heart disease and chronic heart failure), disabling and mental conditions such as dementia or depression in which the person is unable to perform basic and instrumental activities of daily living.
All patients provided written informed consent and the protocol was approved by the ethics committee of Montreal Heart Institute.
Study design
Before and after the training programme, patients underwent a complete medical examination with body composition (segmental bioelectric impedance/Tanita BC 418, Japan), waist circumference and fasting blood analysis (lipid profile, fasting insulin/glycaemia measurement and homeostasis model assessment (HOMA)). Waist circumference was measured with a tape-measure halfway between the lowest rib and the iliac crest. Moreover, patients completed a cognitive functioning evaluation (2nd visit) and maximal exercise testing on an ergocycle (3rd visit) with gas exchange, central haemodynamic and cerebral oxygenation measurement.
The 4-month aerobic exercise training programme comprised 2 sessions of HIIT, 1 session of moderate intensity continuous exercise (1 h at 60% of peak power output) and 2 resistance training sessions per week, as described previously (7). The HIIT sessions comprised 2–3 10-min sets of repeated bouts of 15–30 s at 80% of maximal aerobic power (MAP), interspersed by 15–30 s periods of passive recovery (total duration 34–48 min), with a targeted Borg rating of perceived exertion (RPE) of 15.
Measurements
Evaluation of cognitive functioning. Cognitive functioning was evaluated with a validated paper-and-pencil neuropsychological battery test (8, 9).Testing was carried out by a neuropsychologist. The Geriatric Depression Scale (GDS) and Mini-Mental State Examination (MMSE) were used to exclude patients with depression and mental disease; a score lower than 26/30 on the MMSE or higher than 11 on the GDS resulted in exclusion.
The neuropsychological battery included the following tests:
• Digit Span (Forward and Backward) (short-term and working memory): the neuropsychologist verbally presented a series of numbers at the rate of about 1 per second. Following presentation, subjects were requested to repeat the numbers in the order presented (Digits Forward) or in reverse order (Digits Backward).
• Digit Symbol Substitution Test (attention and processing speed): subjects were instructed to associate symbols with numbers (1–9), in a table of numbers, by referring to a response key. Subjects had 120 s to draw as many symbols as possible.
• Trail making test, part A and B: in part A, the participant was instructed to connect numbers (from 1 to 25) with straight lines as quickly as possible. In part B, which measures flexibility, the participant was instructed to alternate between letters in alphabetical order and numbers in ascending order (1-A-2-B-3-C, etc.) as quickly as possible.
• D-KEFS Color-Word Interference Stroop Test: The Modified Stroop colour test includes 4 conditions and provides a measure of inhibition and mental flexibility. In the reading condition (1), subjects had to read colour words aloud as quickly as possible. In the naming condition (2) subjects had to name the colour of rectangles. In the inhibition condition (3) colour-words were printed in a colour that differed from their meaning (e.g. red printed in green) and the task was to name the ink colour (green) and avoid reading the word. In the flexibility condition (4) subjects had to alternate between naming the colour of the colour-words, and reading the words (when the colour-words appear in a square). Typically, scores in the more difficult conditions (3 and 4) are considered representative of executive control. In all conditions, word lists were printed on a sheet of paper and subjects had to provide their answer verbally as quickly as possible.
• Rey Auditory Verbal Learning Test (RAVLT, long-term verbal memory). In the RAVLT, the participant must learn and remember a list of 15 words immediately after learning them and after a delay.
Maximal cardiopulmonary exercise testing. This test was performed on a ergocycle (Ergoline 800S, Bitz, Germany), with an individualized protocol that included a 2-min warm up at 20 W, followed by a power increase of 10–20 W/min until exhaustion at a free pedalling speed > 60 rpm. Peak power output (PPO) was defined as the power output reached at the last fully completed stage. Gas exchange (Oxycon Pro, Jaegger, Germany) and central haemodynamic variables (bio-impedancemetry; Physioflow Enduro, Manatec Biomedical, Paris, France) were measured for 3 min at rest, during exercise and during 5-min recovery, and were averaged every 15 s. Electrocardigram (ECG) (Marquette, Missouri, USA) and blood pressure measurements were monitored during the test. The maximal average V. O2 value recorded during exercise was considered as the V. O2peak. The ventilatory threshold was determined using a combination of the V-slope, ventilatory equivalents and end-tidal oxygen pressure methods.
Cerebral oxygenation. Cerebral oxygenation was measured using near-infrared spectroscopy (NIRS) system (Oxymon Mk III, Artinis, The Netherlands) during exercise (9). Optodes were placed on the left prefrontal cortical area between Fp1 and Fp3, according to the modified international electroencephalogram (EEG) 10–20 system, with an inter-optode distance of 4.5 cm and signal sampling of 10 Hz. Relative concentration changes (Δµmol) were measured from resting baseline of oxyhaemoglobin (Δ[HbO2]), deoxyhaemoglobin (Δ[HHb]) and total haemoglobin (Δ[HbTot] = [HbO2] + [HHb]). The baseline period was set at the end of the 3-min resting period, defined as 0 µmol (10).
Statistical analysis
Data were expressed as mean and SD. Statistical differences were localized using Wilcoxon’s signed-rank test, with a p-value < 0.05 considered significant, using Statistica software (v.10, Statsoft, Inc., USA). Due to the small sample size in this study, the magnitude of difference was calculated according to Hedge’s (g) formula adapted for a small sample size (11). The magnitude of the difference was compared using Cohen’s d scale and was considered either small (0.2 < g < 0.5), moderate (0.5 < g < 0.8) or large (g > 0.8).
Results
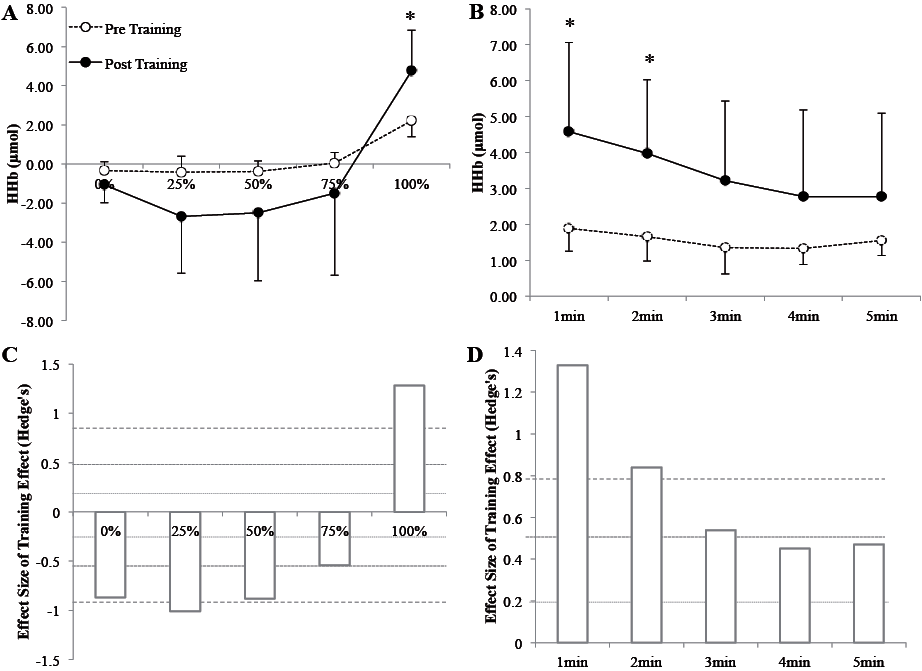
Cardiometabolic parameters are shown in Table I. After training, body mass, body mass index (BMI), waist circumference (WC), total and trunk fat mass were significantly improved (p < 0.05). Blood lipid parameters were not improved after training (p > 0.05). PPO, V. O2 and power at the ventilatory threshold (VT) were significantly improved after training (p < 0.05) (Table I). Concerning cognitive functioning, short-term memory (Forward Digit Span score), attention and processing speed (Digit Symbol Substitution Test score), verbal memory (RAVLT scores: A1-15) and mood (GDS score) were improved after training (p < 0.05) (Table II). Regarding NIRS parameters, Δ[HHb] signal was significantly higher (Fig. 1) after training (p < 0.05) at 100% of PPO and at recovery (min 1 and 2 only).
|
Table I. Body composition, blood analysis, exercise and haemodynamic parameters at baseline and after training in obese patients (n = 6) |
||||
|
Baseline Mean (SD) |
After training Mean (SD) |
p-value |
ES |
|
|
Body composition |
||||
|
Body mass, kg |
86.2 (7.6) |
82.2 (7.7) |
< 0.05 |
–0.44 |
|
BMI, kg/m2 |
29.7 (1.2) |
28.3 (1.3) |
< 0.05 |
–0.93 |
|
Total fat mass, kg |
27.0 (3.9) |
23.2 (3.8) |
< 0.05 |
–0.83 |
|
Trunk fat mass, kg |
15.4 (1.1) |
13.3 (0.8) |
< 0.05 |
–1.71 |
|
Waist circumference, cm |
103.1 (8.1) |
93.6 (9.0) |
< 0.05 |
–0.92 |
|
Blood analysis |
||||
|
Fasting insulin, pmol/l |
48.5 (15.4) |
41.3 (18.1) |
0.07 |
–0.34 |
|
Fasting glycaemia, mmol/l |
5.2 (0.3) |
5.3 (0.4) |
0.71 |
0.25 |
|
HOMA – IR |
0.9 (0.3) |
0.8 (0.3) |
0.17 |
–0.22 |
|
Total cholesterol, mmol/l |
3.9 (0.5) |
3.8 (0.8) |
0.34 |
–0.12 |
|
HDL-cholesterol, mmol/l |
1.3 (0.2) |
1.4 (0.3) |
0.29 |
0.28 |
|
LDL-cholesterol, mmol/l |
2.4 (0.6) |
2.3 (0.6) |
0.60 |
–0.14 |
|
Total cholesterol/HDL-cholesterol |
0.7 (0.2) |
0.6 (0.1) |
0.07 |
–0.69 |
|
Triglycerides, mmol/l |
0.9 (0.2) |
0.8 (0.1) |
0.17 |
–0.57 |
|
Exercise and haemodynamics |
||||
|
Resting SBP, mmHg |
127 (10.5) |
121 (8.2) |
0.13 |
–0.56 |
|
Resting DBP, mmHg |
81.3 (4.5) |
81.3 (1.6) |
< 0.99 |
0.00 |
|
VO2max, ml/min/LBM |
47.6 (2.7) |
53.5 (5.7) |
0.07 |
1.09 |
|
PPO, W |
217 (52) |
253 (53) |
< 0.05 |
0.57 |
|
VO2 at VT, ml/min/LBM |
33.6 (3.0) |
38.3 (3.0) |
< 0.05 |
1.48 |
|
Power at VT, W |
127 (36) |
161 (40) |
< 0.05 |
0.73 |
|
C(a-v)O2, ml O2/100 ml |
16.6 (4.9) |
17.6 (5.9) |
0.50 |
0.16 |
|
Maximal cardiac output, l/min |
17.6 (2.9) |
17.3 (2.3) |
0.68 |
0.06 |
|
Maximal stroke volume, ml |
103 (19) |
104 (14) |
0.13 |
0.53 |
|
Maximal heart rate, beats/min |
181 (9) |
170 (8) |
0.07 |
–0.95 |
|
SD: standard deviation; BMI: body mass index; HOMA – IR: Homeostasis Model Assessment – insulin resistance (fasting insulin (µU/ml) × glucose (mmol/l)/22.5); HDL: high-density lipoprotein; LDL: low-density lipoprotein; SBP: systolic blood pressure; DBP: diastolic blood pressure; LBM: lean body mass; PPO: peak power output; VT: ventilatory threshold; C(a-v)O2: arteriovenous difference; ES: effect size. |
||||
|
Table II. Cognitive functioning at baseline and after training in obese patients (n = 6) |
|||||
|
Baseline Median (IQR) |
After Median (IQR) |
p |
ES |
Mean of tests at 49 years old Mean (SD) |
|
|
Mental disease and depression symptomatology |
|||||
|
GDS (/30) |
2 (0–4) |
0.5 (0–1) |
0.06 |
0.4 |
N/A |
|
MMSE (/30) |
29.5 (29–30) |
29 (28–29) |
> 0.99 |
0.0 |
N/A |
|
Short-term and working memory |
|||||
|
Forward span |
6 (6–7) |
7 (7–8) |
< 0.05 |
1.6 |
6.57 (1.38) |
|
Backward span |
5.5 (4–6) |
6.5 (5–7) |
0.33 |
0.4 |
4.79 (1.42) |
|
Attention and processing speed |
|||||
|
DSST |
76 (68–79) |
82.5 (76–81) |
< 0.05 |
0.4 |
68–72* |
|
Perceptual abilities and processing speed |
|||||
|
Trail A, s |
33.5 (28–48) |
24.5 (23–28) |
0.11 |
–0.8 |
31.78 (9.93) |
|
Stroop 1, s |
28 (26–32) |
27 (24–29) |
< 0.05 |
–0.6 |
28–30* |
|
Stroop 2, s |
21.5 (19–23) |
20.5 (18–25) |
0.60 |
–0.2 |
22* |
|
Cognitive inhibition and flexibility |
|||||
|
Trail B, s |
57.5 (48–75) |
59.5 (47–86) |
0.24 |
0.2 |
63.76 (14.42) |
|
Stroop 3, s |
51 (48–55) |
50.5 (44–51) |
< 0.05 |
–0.6 |
53–57* |
|
Stroop 4, s |
53 (47–57) |
50 (49–51) |
0.75 |
–0.2 |
60–64* |
|
Long-term verbal memory |
|||||
|
Immediate recall |
11 (10–14) |
13.5 (13–15) |
0.22 |
0.50 |
9.6 (3) |
|
Delayed recall |
11.0 (9–13) |
14.5 (13–15) |
< 0.05 |
1.2 |
9.4 (3.3) |
|
Recognition |
15 (14–15) |
15 (15–15) |
0.17 |
0.0 |
12.2 (2.6) |
|
A1–15 |
54 (52–57) |
65 (55–70) |
< 0.05 |
1.1 |
47.4 (8.8) |
|
*These numbers correspond to a scaled score of 10 (50th percentile; see refs in Lagüe-Beauvais et al. (9)). IQR: interquartile range; SD: standard deviation; GDS: Geriatric Depression Scale; MMSE: Mini-Mental State Examination; DSST: Digit Symbol Substitution Test; s: seconds; ES: effect size. |
|||||
Discussion
To our knowledge, this is the first study to assess the effects of HIIT on cognitive performance and cerebral oxygenation in obese patients.
The main finding is that HIIT improved cognitive functioning and cerebral oxygenation. The results concerning body composition and exercise capacity are consistent with some previous studies confirming the effect of HIIT in obese patients (12). In addition, the current study found an improvement in cognitive functioning in obese patients following HIIT, in line with previous results reported in older adults benefiting from aerobic training (3). More specifically, Colcombe & Kramer (3) reported that the executive functions are more sensitive to fitness level than other cognitive functions. Executive functions generally refer to a “high-level” cognitive functioning involved in the control and regulation of cognitive processes, such as planning, inhibiting routine behaviour or switching abilities. In the executive domains, we found a selective positive effect of HIIT on inhibition measured by Stroop 3, whereas we reported no effect on switching abilities measured by Trail B and Stroop 4. Our results are in agreement with the study of Boucard et al. (13), who reported a selective improvement in inhibition but not in flexibility (i.e. switching) in older fit people compared with sedentary control subjects. Our results confirm the effect of HIIT in improving aspects of cognitive functioning, such as processing speed, memory and inhibition, particularly in obese patients, who are at higher risk of developing subclinical cognitive impairment.
Obesity is associated with changes in cerebral volume (5) and can affect cerebral hypoperfusion (14), which could lead to cognitive impairment. In our study, we showed a higher Δ[HHb] signal amplitude during exercise and recovery after training, highlighting the effect of HIIT in cerebral oxygenation adaptation. Seifert et al. (15) demonstrated a decrease in metabolic rate after 3 months of endurance training in overweight males during submaximal exercise, with no changes in cerebral oxygenation. The lack of effect on cerebral oxygenation could be due to the lower training intensity used in this study. In this sense, Fu et al. (16) reported a higher amplitude in cerebral oxygenation in heart failure patients after interval training, whereas moderate intensity continuous exercise had no effect. They also reported a higher amplitude of Δ[HbTot] during maximal exercise and a small increase in Δ[HHb] at 100% of PPO. We also found similar results at 100% of PPO for Δ[HHb] and during the first 2 min of recovery. Our results indicate that cerebral oxygen extraction may be improved after training. Indeed, stability of, or reduction in, [HbO2] associated with increased Δ[HHb] have also been suggested to reflect an accelerated rate of oxygen extraction (10, 16). In addition, our results are in agreement with a recent meta-analysis. Rooks et al. (17), reported that subjects with a high level of fitness presented greater Δ[HHb] concentrations only at the hard or maximal exercise intensities that control unfit subjects. This phenomenon is in accordance with the recent hypothesis that training improves cognition through a higher level of brain mitochondrial proliferation (18). From the clinical standpoint, a reduction in cerebral perfusion during the ageing process can lead to cognitive impairment, and obesity is a major risk factor for such hypoperfusion (14). This pilot study validates the effect of HIIT in improving cerebral oxygenation and reducing the risk of developing mental diseases.
Study limitations
This study has several limitations, including a small sample size and no control group; thus caution is required in interpretation and generalization of the results. It is possible that a type I error could be induced with such a small sample, but the significant results of the study are in agreement with the initial hypotheses and current data from the literature. All results are accompanied by a measure of effect size, calculated according to Hedge’s (g) formula adapted for small sample size. In addition, with no control group, it is not possible to rule-out the confounding effect of practice on cognitive measurements. However, all the neuropsychological tests in the battery used in this study are subject to practice effects, and only select tests emerge as showing improvement post-test. Measuring a specific effect of HIIT on a selective cognitive task avoids a confounding effect of practice. Although these results must be interpreted with caution, there are currently no data available on the effect of HIIT on cerebral oxygenation and cognition in obese patients and further research is needed to expand on the preliminary results of this pilot study. The positive results from our small sample indicate that HIIT may have a potential role as a powerful stimulus to enhance general health in this population. However, further research is necessary to confirm these results.
Conclusion
These preliminary results indicate that, beyond the benefits of HIIT on cardiometabolic health (body composition and fitness), this type of training also improves subjects’ health and brain plasticity. This study indicates the effect of HIIT in health outcomes and a possible role for HIIT in reducing the risk of cardiovascular and mental diseases.
Acknowledgement
This study has been supported by ÉPIC Foundation and Montreal Heart Institute Foundation.
References
