Hiroyuki Kato, MD, PhD1 and Masahiro Izumiyama, MD, PhD2
From the 1Department of Neurology, International University of Health and Welfare Hospital, Nasushiobara and 2Department of Neurology, Sendai Nakae Hospital, Sendai, Japan
OBJECTIVE: Most rehabilitative interventions following stroke emphasize the improvement of motor deficits but rarely address sensory function and sensorimotor control. We report here a case of cerebral infarction localized to the postcentral gyrus that presented with severe impairment of motor control due to profound proprioceptive sensory loss. We attempted to demonstrate the mechanism for the motor impairment using functional magnetic resonance imaging (fMRI).
CASE REPORT: A 70-year-old woman developed abrupt loss of motor control of the right hand, concomitant with the loss of proprioception of the hand. An fMRI was conducted 12 days after stroke onset. Movement of the unaffected hand activated the normal sensorimotor network in the brain, including the contralateral primary sensorimotor cortex, supplementary motor areas, and ipsilateral cerebellum. However, movement of the affected hand activated only the contralateral primary motor cortex and activation of the cerebral sensorimotor network was severely depressed. Diffusion tensor tractography revealed that the corticospinal tracts were intact. Intensive rehabilitation and the use of visual support enabled the patient to live an independent life.
CONCLUSION: Loss of motor control may occur even with a normal corticospinal tract when proprioception is severely impaired by dysfunction of the sensorimotor network in the brain.
Key words: proprioception; motor control; functional MRI; diffusion tensor imaging; stroke; rehabilitation.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Hiroyuki Kato, Department of Neurology, International University of Health and Welfare Hospital, 537-3 Iguchi, Nasushiobara 329-2763, Japan. E-mail: katoh@iuhw.ac.jp
Accepted Aug 14, 2014; Epub ahead of print Sep 30, 2014
INTRODUCTION
The nervous system is equipped with various receptors that record information about movement and forces. This function is called proprioception. The role of sensory information in the control of voluntary movement is one of the most essential questions in sensorimotor control. A profound loss of proprioception leads to the failure of motor control resulting from the failure of proper integration of cortical sensory and motor function (1, 2). The most evident way to answer this question is to study the effect of damage to the sensory system on the execution of movement. In patients with stroke, sensory deficits are typically combined with motor deficits. However, there can be pure sensory strokes with lesions confined to the primary sensory cortex (S1). Such patients may exhibit severe impairment of voluntary movement (3) or even involuntary movement called pseudochoreoathetosis (4).
Functional magnetic resonance imaging (fMRI) provides a non-invasive imaging method for evaluating sensorimotor function following stroke (5). Furthermore, diffusion tensor tractography (DTT) is a technique that allows visualization of the main fibre bundles of the brain, such as the corticospinal tract (CST), by virtue of its ability to image water diffusion characteristics (6).
In this study, we report a patient who exhibited abrupt loss of proprioception and motor control without overt impairment of motor function following a stroke that was confined to the postcentral gyrus. We performed fMRI and DTT in this patient and discussed these findings and the anatomical basis of this unique condition.
CASE REPORT
A 70-year-old, right-handed woman noted that her right hand moved involuntarily when she was working in the field. She was transferred to an emergency hospital. She had been treated for hypertension for 3 years and had a history of surgical treatment for breast cancer. Her consciousness was normal. Her blood pressure was 144/77, and she had no cardiac arrhythmias. She had no clear palsy, but the clinicians noticed that she had involuntary movements of the right hand, which tended to occur when she tried to move the hand. Associated movement of the right hand was observed when she moved her left hand. A brain MRI was performed, and she was diagnosed with a cerebral infarct. She was admitted to the hospital to receive treatment for stroke.
Five days later, she was referred to our hospital for further investigation and rehabilitation. On admission, she spoke normally and was able to walk independently. She had a full range of eye movement and no facial weakness. When she stretched out both hands to the front, she exhibited athetoid movements in the right hand. She had lost fine manual skills in the right hand. She had difficulty flexing the right fingers one by one or making a V-form with the index and middle fingers. When her eyes were closed, she could not perform these tasks at all. The grip strengths of the hand were 12 kg for both sides. When she gripped with her left hand, there was an associated movement in the right hand. Her tendon reflexes were normal on both sides, and her plantar reflexes were negative. The finger-to-nose test was unsteady on the right. The sensory examination revealed complete absence of joint position sense in the right hand. Light-touch and pain sensation was decreased in the right hand compared with the left hand. Both graphaesthesia and stereognosis were severely impaired in the right hand. She was unable to use chopsticks and used a spoon while eating, and the right hand movement was clumsy and spatially disorientated. She could write (with her eyes open) but had difficulty in sustaining a pen in the hand. She could throw a ball but did not notice if the ball slipped off her hand.
After 2 months of intensive rehabilitation, she was discharged from the hospital when her hand movement had partially improved with the support of visual feedback. One year later, her proprioceptive loss was still profound, but her right hand movement was well-controlled under visual support. She was able to use chopsticks again by then and was living an independent life.
Brain magnetic resonance imaging
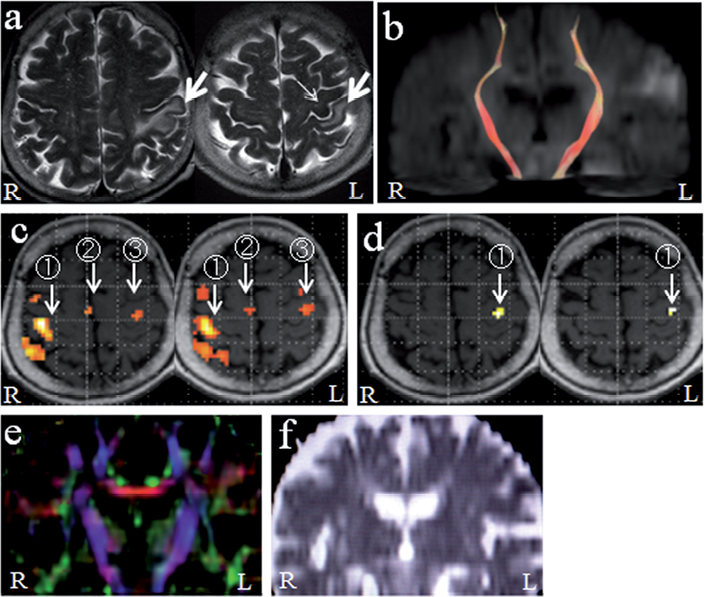
A brain MRI (1.5T Siemens Magnetom Symphony) revealed an infarct localized to the left postcentral gyrus (Fig. 1a). The precentral gyrus, including the precentral knob (primary motor cortex (M1) of the hand) and other motor areas (premotor and supplementary motor areas (SMAs)) were intact.
Fig. 1. (a) T2-weighted images (TR 5,000 ms, TE 113 ms) of the patient 12 days after stroke onset. Cerebral infarction was confined to the left postcentral gyrus (large arrows). The precentral knob (primary motor cortex of the hand) was intact (small arrow). (b) Diffusion tensor tractography of the corticospinal tract. The corticospinal tracts of both sides were intact. (c) fMRI during unaffected (left) hand movement. Normal activation was observed in contralateral (right) primary sensorimotor cortex (1), supplementary motor areas (2), and ipsilateral (left) primary motor cortex (3). (d) fMRI during affected (right) hand movement. Activation was seen only in the contralateral (left) primary motor cortex to a limited degree (1). (e) Fractional anisotropy mapping. Corticospinal tracts are shown in blue. No asymmetry is seen between bilateral corticospinal tracts. (f) Apparent diffusion coefficient mapping. No asymmetry is seen between bilateral corticospinal tracts.
Functional magnetic resonance imaging
The fMRI was performed 12 days after the onset of stroke as described previously (5). In brief, blood oxygen level-dependent images were obtained using a gradient-echo, single shot echo planar imaging pulse sequence. The acquisition parameters were as follows: repetition time = 3,000 ms, time of echo = 50 ms, flip angle = 90°, slice thickness = 3 mm, 30 slices through the whole brain, field of view = 192 × 192 mm with a 128 × 128 matrix. During the fMRI scan, the patient performed a self-paced hand movement task (opening-closing of the hand). The cycle of rest and task (30 s each) was repeated 5 times (a total of 5 min) during each hand movement. Data analysis was performed using Statistical Parametric Mapping 2 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/), which was implemented in MATLAB (The MathWorks Inc., Nattick, MA, USA). We used 100 volumes of 30 slices for analysis. After realignment and smoothing, the general linear model was used for the detection of activated voxels (movement > rest). The voxels were considered as significantly activated if p < 0.05 using the family-wise error rate analysis. The activation images were overlaid on the corresponding T1-weighted anatomic images.
The patient performed the hand movement task satisfactorily with both hands, at rates of 20 times/30 s with the left (non-affected) hand and 22–24 times/30 s with the right (affected) hand. When she moved the left hand, there were slight associated movements in the right hand.
The unaffected hand movement activated the normal sensorimotor network of the brain, including the contralateral primary sensorimotor cortex (SM1), parietal cortex, premotor cortex and SMAs, and ipsilateral anterior lobe of the cerebellum (Fig. 1c). There was also a slight activation in the ipsilateral M1 (Fig. 1c). The affected hand movement activated only the contralateral M1, to a lesser extent than the unaffected hand movement (Fig. 1d), and there was no activation in the sensory cortex and other motor areas.
The fMRI was performed again 42 days later. The findings were essentially the same, except for the appearance of activation in the SMA during the affected hand movement.
Diffusion tensor tractography
Diffusion tensor imaging data were acquired on the same day using a single shot echo planar imaging pulse sequence. The imaging parameters were as follows: repetition time = 5,200 ms, time of echo = 108 ms, slice thickness = 5 mm, 25 slices, field of view = 220 × 220 mm with a 256 × 256 matrix, b value = 1,000 mm2s–1, motion-probing gradient in 6 orientations. DTT was obtained using the Diffusion Tensor Visualizer (http://www.ut-radiology.umin.jp/people/masutani/dTV/dTV_frame-e.htm) and VOLUME-ONE (http://www.volume-one.org/) softwares. Fibre tracking of the CST was performed as reported previously (6), by selecting the posterior limb of the internal capsule as the seed and the M1 as the target, and the threshold fractional anisotropy (FA) was equal to 0.18. We produced FA and apparent diffusion coefficient (ADC) maps, and also measured regional FA and ADC values in the CSTs at the internal capsule.
The CSTs of both hemispheres were visualized similarly, which showed that the CST of the left side was intact (Fig. 1b). We observed no asymmetry in the FA and ADC maps (Fig. 1e, 1f). The FA and ADC values showed no asymmetry between left and right sides (FA, 0.65 ± 0.054 and 0.64 ± 0.036, respectively; ADC, 0.70 ± 0.029 × 10–3 mm2s–1 and 0.71 ± 0.036 × 10–3 mm2s–1, respectively).
DISCUSSION
This case clearly shows that loss of proprioception without overt damage to the motor system can lead to severe impairment of motor control. The brain lesion on MRI was confined to the postcentral gyrus (S1), and there was no anatomical damage beyond the central sulcus to the precentral gyrus (M1) and premotor and SMAs. The lesion represents the rare occurrence of a destruction of the S1 without involvement of the motor areas. DTT confirmed that the CST on the lesion side was intact. Therefore, it was believed that loss of proprioceptive control at the cortical level produced major impairments in execution of the voluntary movements of the hand. We evaluated the FA map because decreased FA in the CST predicts long-term motor outcome after stroke (7), but did not observe such an FA asymmetry. We produced the ADC map because the CST could involve damaged fibres originating from the postcentral gyrus, but could not visualize such a change.
The patient could perform the simple hand movement fMRI task without visual control. The fMRI during the unaffected hand movement displayed the normal activation pattern of the cortical sensorimotor network, including activation of contralateral SM1, the SMAs and the ipsilateral cerebellum, as reported previously (5). In addition, the ipsilateral M1 was slightly activated. This ipsilateral activation is often observed during non-dominant hand movement (8). However, the presence of associated movement in the affected hand is also likely to be responsible for this ipsilateral activation. In contrast, during the affected hand movement, only the contralateral M1 was activated to a smaller extent, and there was no activation in the S1 or other motor areas. Thus, the affected hand movement was associated with only limited activation of the sensorimotor network in the affected brain hemisphere. The impairment of sensory feedback was considered to be responsible for this lowered activation of the sensorimotor network.
In a case with a cortical lesion limited to the left postcentral gyrus extending into supramarginal gyrus, Jeannerod et al. (3) conducted a thorough investigation of prehensile movements of the contralateral hand. The performance of the right hand in motor tasks was severely impaired, and the severity of the deficit depended critically on the availability of visual feedback, with complete failure when the hand was not visible. Thus, data from the patients with sensory loss due to brain lesions clearly emphasize the central role of somatosensory feedback for the manipulative function of the hand.
On the other hand, lesions anywhere along the proprioceptive sensory pathways can cause abnormal involuntary movements called pseudochoreoathetosis (4). Sharp et al. (4) postulated that the loss of proprioception causes alterations in the cortical sensory inputs to the striatum and, finally, variable mixtures of involuntary movements. However, why only a small proportion of patients with proprioceptive sensory loss develop involuntary movements is unknown. Our patients experienced transient involuntary movements of the affected hand during the acute stage of stroke. We only observed a remnant of this involuntary movement 5 days after onset, but the pseudochoreoathetosis may have occurred because of the abrupt loss of proprioception of the hand. The involuntary movement appeared to reflect the effect of deafferentation on motor control, resulting in the inability to follow-up an action once it has been initiated.
Conventionally, most rehabilitative interventions for stroke patients emphasize the improvement of motor deficits, but rarely address sensory capability and sensorimotor control following stroke. However, it should be noted that techniques focused on sensory stimulation have been studied in stroke patients (9). Our case offers the unique opportunity for studying the substitutive role of other sensory modalities, particularly vision when somatosensory control is lacking. It is critical for stroke patients with sensory problems to incorporate appropriate strategies for dealing with sensory impairment into traditional hand function rehabilitation programmes (10).
In conclusion, we report here a case of cerebral infarction that was confined to the left postcentral gyrus. The patient displayed loss of proprioception and severe impairment of the voluntary movement of the right hand. An fMRI revealed severe functional impairment of the sensorimotor network in the brain during movement of the affected hand. The present case clearly shows that sensory feedback is critical for the execution of voluntary manipulation of the hand.
ACKNOWLEDGEMENTS
The authors would like to thank Mr Katsuhiro Aki at the MRI section of Sendai Nakae Hospital for his help in performing the fMRI studies, and Ms Saika Kanno (occupational therapist) and Ms Yuka Kohno (speech therapist) at the rehabilitation section of Sendai Nakae Hospital for their expert rehabilitative evaluations. This study was supported by Grant-in-Aid for Scientific Research (25350615), Japan Society for the Promotion of Science.
REFERENCES
