Annette A. A. van Kuijk, MD, PhD1,3, Ralf Kosters, PA2, Martin Vugts, MD2 and Alexander C. H. Geurts, MD, PhD2,3
From the 1Libra Rehabilitation & Audiology, Blixembosch, Eindhoven, 2Rehabilitation Centre Tolbrug/Jeroen Bosch Hospital, ‘s-Hertogenbosch and 3Department of Rehabilitation, Radboud University Medical Centre, Nijmegen, The Netherlands
OBJECTIVE: To assess the effectiveness of currently available treatment options for idiopathic toe walking on the 3 main levels of the World Health Organization International Classification of Functioning, Disability, and Health for Children and Youth (ICF-CY).
DESIGN: A systematic search from 1966 to December 2013 in MEDLINE, Current Contents, CINAHL and the Cochrane Library of full-length articles addressing clinical efficacy of treatment in children aged 2–18 years.
METHODS: Studies were evaluated using both the Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence and the Methodological Index for Non-Randomised Studies (MINORS). Outcomes were analysed in accordance with the ICF-CY.
RESULTS: One randomized controlled trial and 18 observational studies were identified. The randomized controlled trial was scored OCEBM level 1, whereas the other studies were scored level 4. The MINORS scores ranged from 2 to 18.
CONCLUSION: There is preliminary evidence for beneficial effects of serial casting and surgery on passive ankle dorsiflexion as well as on walking kinetics and kinematics, although normalization does not seem to occur. Botulinum toxin type A does not improve the results of casting. Only after surgery are sustainable effects lasting > 1 year reported. Effectiveness on functional activities and social participation has yet to be demonstrated.
Key words: idiopathic toe walking; treatment; review; ITW.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Annette A. A. van Kuijk, Libra Rehabilitation & Audiology, Blixembosch, PO Box 1355, NL-5602 BJ Eindhoven, The Netherlands. E-mail: Annet.vanKuijk@radboudumc.nl
Accepted May 26, 2014; Epub ahead of print Sep 15, 2014
Introduction
Toe walking is the inability to generate a heel strike during the initial contact phase of the gait cycle, and the absence of full foot contact during the entire standing phase. It is a pattern sometimes observed in healthy developing children less than 2 years old who are learning to walk independently (1). Persistent toe walking beyond 2 years of age can be the first sign of an upper motor neurone or neuromuscular disease. In children with upper motor neurone or neuromuscular diseases there is a striking imbalance between flexor and extensor muscles of the distal lower limb either due to spasticity or to selective weakness, causing a toe-toe gait pattern. Persistent toe walking has also been reported in children with language or cognitive developmental delay (2–4), as well as in children with autistic spectrum disorders (5, 6).
In nearly 5% of the healthy childhood population, however, no apparent cause of persistent toe walking can be identified (7). These children seem to prefer to ambulate persistently in a toe-toe gait pattern, but are able, at least in the first years of toe walking, to voluntarily perform heel-toe gait. This is called idiopathic or habitual toe walking (ITW). ITW is considered a diagnosis of exclusion, and the aetiology and pathophysiology of this condition remain unknown (8). As such, the child with ITW should demonstrate an unremarkable medical history with a normal birth and development as well as a normal neurological and orthopaedic status and no prominent neuropsychiatric disorders (8, 9).
In some of the children with ITW, it appears that there may be a relationship between persistent toe walking and a decreased range of motion at the ankle joint (equinus deformity) (10–13). Hall et al. (14) were the first to propose a pre-existent congenital shortening of the calf muscle, but subsequent studies have called this idea into question by documenting absence of (muscle-tendon) contractures in many of these children at birth (10, 15, 16). In a cross-sectional study of a general population of children, adolescents, and young adults in the Netherlands, children who had been walking on their toes had a 3 times higher chance to develop a decreased ankle dorsiflexion range during growth into their adolescence compared with children without a history of ITW (10). As yet, sound longitudinal data to determine whether ankle equinus is the primary cause (14) or the consequence of ITW are lacking. Other problems attributed to ITW in the literature are secondary pes planovalgus, undifferentiated leg pain, limping, and fatigue (9, 11, 16–18). It is hypothesized that ITW may lead to walking instability with injuries due to tripping and falling (9). Data to support the theory that ITW may lead to poor functional outcome in the long term are, however, lacking.
Because it appears that there may be a relationship between persistent toe walking and ankle equinus, interventions are primarily aimed at maintaining or increasing ankle dorsiflexion range. A number of non-invasive and invasive treatment options have been proposed, including physical therapy (PT), orthopaedic footwear, serial casting, orthoses, Botulinum toxin type A (BTX-A), and soft-tissue surgery (9, 11, 19). All of these interventions claim to correct the equinus position of the ankle with the expectation that gait changes would follow the positional correction. However, at present, consensus about medical management has not been established. Moreover, the effects of the different interventions on the level of activities and participation of the child have not been established. Therefore, the aim of the present study was to perform a systematic review of the literature to assess the effects of the currently available treatment options for patients with ITW on the 3 main levels of the World Health Organization International Classification of Functioning, Disability, and Health for Children and Youth (WHO ICF-CY): (i) body structure and function; (ii) activities; and (iii) participation.
Methods
Data sources
Material for review was selected from a systematic search in the databases of MEDLINE (January 1966–December 2013), Current Contents (January 1966–December 2013), CINAHL (January 1982–December 2013), and the Cochrane Library.
Study selection
The following MeSH headings and keywords were used: either “equinus gait or walking”, or “toe gait or walking”, or “tiptoe gait or walking”, or “forefoot gait or walking”, or “short Achilles tendon”, or “short tendo calcaneus” as well as a combination of 1 of the above-mentioned keywords and either “idiopathic”, or “idiosyncratic”, or “habitual”, or “persistent”, or “dystonic”. Identifying relevant references from the retrieved articles extended the search.
As case series are probably the most frequent type of surgical and orthotic reports in the literature, it was decided not to restrict the selection to a specific study design. As a consequence, after the primary search, studies were included if they used either within-group pre-post treatment comparisons or between-groups comparisons in a (randomized) controlled design. In addition, studies were required to meet the following inclusion criteria: (i) investigating treatment of ITW in children aged 2–18 years; (ii) primarily addressing aspects of clinical efficacy of treatment; and (iii) being written as a full-length article in the English, German, French, or Dutch language. Studies were excluded (iv) in the case of heterogeneous patient samples in which the patients with ITW could not be identified, and (v) if papers were not available in medical libraries in the Netherlands. Ultimately, the remaining studies were selected for detailed methodological evaluation. Two authors independently performed the study selection and methodological evaluation. In the case of disagreement, a third reviewer was consulted.
Data extraction
Assessment of methodological quality. The Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence were used to grade the selected studies (20). The Methodological Index for Non-Randomised Studies (MINORS) was applied to further assess the quality of each observational study (21). The MINORS is a validated list designed to assess the methodological quality of observational or non-randomized studies (either comparative or non-comparative) which comprises 12 items, of which the last 4 items apply only to comparative studies. Items are scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The maximum score is 16 for non-comparative studies and 24 for comparative studies (21).
Quantitative methodological evaluation. Because only 1 randomized controlled trial (RCT) could be identified, no pooling of data could be performed, either in a meta-analysis or in a best-evidence synthesis. The outcomes of the selected studies were analysed following the 3 main levels of the WHO ICF-CY: (i) body structure and function; (ii) activities; and (iii) participation (qualitative synthesis). The ICF-CY is a classification of health and non-health related domains at both individual and population level, commonly used within the field of rehabilitation medicine (www.who.int/classifications/icf/en/).
Results
Study selection
The initial systematic search strategy in PubMed identified 105 relevant citations (Fig. 1). The search in other databases yielded 2 additional articles. On the basis of title and abstract, 82 studies were excluded. Exclusion criteria were: (i) use of a patient sample other than ITW or age of the study sample at the time of intervention beyond 18 years (n = 25); (ii) not primarily addressing aspects of clinical efficacy of treatment (n = 51); and (iii) being written as a full-length article in languages other than English, German, French or Dutch (n = 6). Full texts of the remaining 25 studies were examined. Screening the references of these studies revealed 9 additional articles. From these 34 selected studies 15 were excluded due to: (i) use of a patient sample other than ITW or age of the study sample at the time of intervention beyond 18 years (n = 3); (ii) not primarily addressing aspects of clinical efficacy of treatment (n = 11); and (iv) heterogeneous patient samples in which the patients with ITW could not be identified (n = 1). Ultimately, 19 studies met all inclusion and exclusion criteria and were systematically analysed.
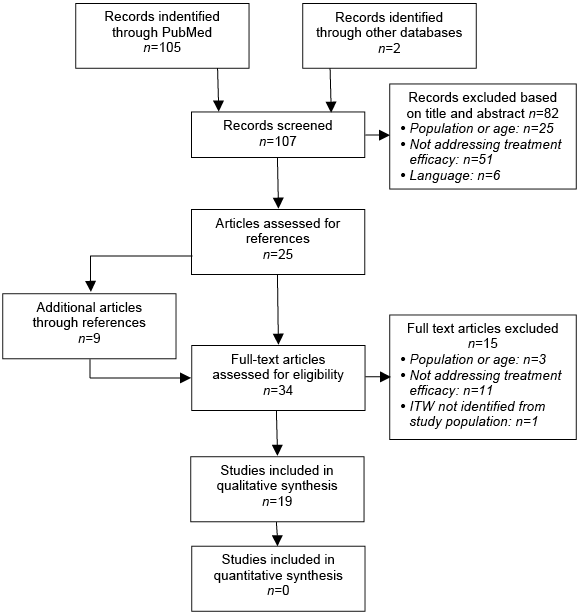
Fig. 1. Study selection process. ITW; idiopathic or habitual toe walking.
Methodological quality
Table I shows the methodological characteristics of the 19 studies included in the review. Most studies used a within-group design with pre-post treatment comparisons and were classified as RCT (n = 1) (22), case series (n = 8) (14, 17, 18, 23–27), qualitative study (n = 1) (28), cross-sectional study (n = 1) (29), historically controlled study (n = 1) (30), or cohort studies (n = 7) (15, 16, 31–33, 34, 35). Only 9 studies used a prospective study design, whereas 10 studies were conducted retrospectively.
Only one study randomly assigned treatments to the participants (22). Two studies incorporated a non-treatment group (16, 32), whereas 2 other studies used a group of children with cerebral palsy for comparison (31, 35). One study incorporated data from an age-matched control group on repetitive single-heel rises on the force plate (29), whereas 7 studies incorporated historical data from a laboratory database of healthy controls (23, 29, 30, 33). In only one of these studies, the historical data were used for quantitative comparisons (30). One study was scored as OCEBM level 1 (22), all other studies were scored as level 4. The MINORS scores of the latter studies ranged from 2 to 18, emphasizing the methodological heterogeneity amongst the observational designs.
|
Table I. Methodological characteristics |
||||||||
|
Authors |
Study design |
Minors score |
Number of subjects |
Age at intervention, years Mean (range) |
Population characteristics |
Intervention |
Follow-up |
Lost to follow-up |
|
Brouwer et al., 2000 (31) |
Cohort; prospective |
18 |
8 |
7.5 (5–10) |
Hospital-based: referred for serial casting for ITW. ITW defined as persistent TW without identifiable cause; Inclusion: normal neurological and musculoskeletal examinations. Exclusion: conditions associated with gastroc-soleus equinus, abnormal muscle tone, muscular dystrophy, or other neurological disorders. |
Cast: Bilateral below-knee serial walking cast for 5.0 ± 0.4 weeks; changes at 1–2 weekly intervals. |
6 weeks after final cast removal |
0 |
|
Brunt et al., 2004 (24) |
Case series; prospective |
10 |
5 |
4.34 (3.25–6.25) |
Hospital-based; referred for BTX-A for ITW. ITW defined as persistent TW after independent gait has been attained. Exclusion: history of prematurity, low birthweight, developmental delays, headaches, back pain, muscle weakness, leg pain, and/or abnormal neurological physical findings. |
BTX-A: Bilateral 12 MU/Kg in each gastrocs and soleus muscle, max 400 MU (Allergan®); 20 days post- BTX-A followed by PT 2 × /week aimed at locomotor training, active dorsiflexion exercises, passive stretching plus home assignments. |
Mean 20 (range 16–28) days post-BTX-A; n = 4: 12 months post-BTX-A |
1 |
|
Clark et al., 2010 (17) |
Case series; prospective |
11 |
5 |
4.2 (2.9–5.4) |
PT practice-based; referred for PT for ITW. ITW defined as persistent TW > age 2 years without neurological impairments. Inclusion: age 30–72 months; parents estimate of toe-walking frequency ≥ 50%; pADF ≥ neutral; < 1.5 SD gross motor subtest PDMS-2; available 16 weeks research activity. |
PT: 2 × 1 h motor control sessions/week for 9 weeks plus home assignments: Play choices adapted to individual therapy goals. |
4 weeks post-treatment |
0 |

|
Table I. Contd. |
||||||||
|
Authors |
Study design |
Minors score |
Number of subjects |
Age at intervention, years Mean (range) |
Population characteristics |
Intervention |
Follow-up |
Lost to follow-up |
|
Eastwood et al., 2000 (32) |
Case-control, retrospective |
11 |
136 |
4 (1.5–10); 3.3 (1.5–10.3); 6.9 (2.5–14.5) |
Hospital-based; referred with bilateral symmetrical TW. ITW defined as persistent TW without underlying neuromuscular or psychological conditions. |
Observation (no PT); Cast: Bilateral serial below-knee walking cast for 6 weeks; intervals cast-changes not mentioned; Surg: Baker’s type aponeurotic TAL followed by below-knee walking cast for 6 weeks. |
Mean 3.2 (range 2–12.8); mean 3.7 (range 2–21.5); mean 7.9 (range 2–22) years after presentation. |
0 |
|
Engström et al., 2010 (33) |
Cohort; prospective |
14 |
15 |
8.9 (5–13) |
Hospital-based; referred for BTX-A for ITW. ITW defined as persistent TW without identifiable cause. Inclusion: normal neurological examination. Exclusion: previous TAL, casting, orthotics, or BTX-A |
BTX-A: Bilateral 6 MU/kg in each gastrocs and soleus muscle, max. 400 MU (Allergan®). Followed by exercise stretching calf muscles 5×/week plus 50 steps walk on heels/day. |
12 months after BTX-A. |
4 |
|
Engström et al., 2013 (22) |
RCT |
47 |
9.4 (5.4–13.6) 9.4 (5.0–14.5) |
Hospital based; referred for ITW. ITW defined as persistent TW > 25% of time for last 3 months without identifiable cause. Inclusion: normal orthopaedic & neurological examination. Exclusion: previous TAL, casting, orthotics, or BTX-A, plantar flexor contracture > 10°. |
Cast: Bilateral below-knee serial walking cast for 4 weeks; followed by home assignment: exercise stretching calf muscles 5 × /week plus 50 steps walk on heels/day instructed by PT. BTX-A + cast: Bilateral 12 MU/kg BTX-A (Allergan®) in gastrocs and soleus muscle. Followed by cast 1–2 weeks after BTX-A for 4 weeks; followed by home assignment: exercise stretching calf muscles 5 × /week plus 50 steps walk on heels /day instructed by PT. |
3 and 12 months after cast removal. |
1 |
|
|
Fox et al., 2006 (15) |
Cohort; prospective |
8 |
44 |
6.1 (2–14.3) |
Hospital-based; referred with ITW; ITW defined as persistent TW without developmental, neurological, or neuromuscular conditions. Inclusion: age > 2 years; independent walking achieved; Exclusion: diagnosis of neuromuscular disorder, neurological condition, or autism, developmental delay, orthopaedic problems. |
Cast: Bilateral below-knee serial walking cast for 5.7 ± 1.1 (range 3–10) weeks, changes at 2 weekly intervals; followed by home assignment: passive AT stretching instructed by PT |
Mean: 14 months. |
0 |
|
Gormley et al., 1997 (26) |
Case series; retrospective |
5 |
6 |
Not mentioned; at follow-up: 6.8 (4–10) |
Hospital-based; referred with ITW refractory to prior conservative treatments (AFO/cast/PT). ITW defined as persistent TW without any neurological deficit. |
BTX-A: Bilateral 7.5–10 MU/kg in each gastrocs and soleus muscle, followed by serial casting until 10° of dorsiflexion achieved. |
Mean 9.2 (range 2–18) months. |
0 |

|
Table I. Contd. |
||||||||
|
Authors |
Study design |
Minors score |
Number of subjects |
Age at intervention, years Mean (range) |
Population characteristics |
Intervention |
Follow-up |
Lost to follow-up |
|
Griffin et al., 1977 (23) |
Case series; prospective |
9 |
6 |
Not mentioned; at evaluation: 7 (5–9) |
Hospital-based; referred with ITW. ITW defined as persistent TW with normal neurological and orthopaedic examine and no behavioural problems. |
Cast: Bilateral serial walking casts; n = 3 below-knee casts for 6 weeks, and n = 1 for 8 weeks; n = 1 long leg casts for 6 weeks, and n = 1 for 8 weeks; intervals cast changes not mentioned, followed by dorsiflexion exercises and heel-toe gait training. |
Not specified. |
0 |
|
Hall et al., 1967 (14) |
Case series; prospective |
4 |
20 |
7.5 (not mentioned) |
Hospital-based; referred with bilateral equines deformity without underlying diagnosis. ITW defined as congenital short tendo-calcaneus without identifiable cause, and able to put their foot flat on request. |
Surg: TAL (calcaneal tendons) followed by below-knee cast for 6 weeks; after 3 weeks with weight-bearing. |
Mean 3 (range 1.5–7) years after surgery. |
0 |
|
Hemo et al., 2006 (34) |
Cohort; retrospective |
7 |
15 |
9.0 (4.2–13.1) |
Hospital based; ITW treated by TAL; ITW defined as persistent TW > age 2 years without neurological or orthopaedic abnormality. Inclusion: completed full motion laboratory assessment pre and postoperatively. 12/15 failed prior non-surgical treatment. |
Surg: bilateral TAL (3 percutaneous, 12 open) followed by below-knee casts for 4–6 weeks, followed by AFO full-time initially progressing to night wearing until discontinued completely at FU 1 year. |
GA mean 1.2 years after surgery; Clinical follow-up: mean 2.9 (range 1.1–6.0) years after surgery. |
0 |
|
Hirsch & Wagner, 2004 (18) |
Case series; retrospective |
4 |
14 |
6.5 (3.0–9.9) |
Hospital-based; referred with ITW. ITW defined as persistent TW without signs of neurological, orthopaedic, or psychiatric diseases. Inclusion: age ≥ 13 years Exclusion: prior surgery for ITW. |
PT: passive stretching exercises calf muscle and TA plus home assignment (14), exercises to increase active ADF (6); Cast: Bilateral below-knee casts during 2–4 weeks followed by intensified PT (5) and night splints (3) (fixed or dynamic). |
Mean 14.5 (range 7–21) years after first evaluation. |
0 |
|
Jacks et al., 2004 (27) |
Case series; retrospective |
5 |
10 |
Not specified; (2–17) |
Hospital-based; referred with ITW refractory to previous conservative treatments (PT, serial cast, brace); ITW defined as persistent TW without any underlying cause. Inclusion: age 2–17 years. |
BTX-A: bilateral 10 MU/kg in each gastrocs and soleus muscle followed by serial walking cast until 10° of dorsiflexion achieved, changes at weekly intervals; followed by articulated AFO during day and night and PT instructed home assignment to progressively stretch PF and strengthen DF. |
12 months post-BTX-A. |
0 |
|
Jahn et al., 2009 (35) |
Cohort; retrospective |
9 |
14 |
8.9 (5.6–12.6) |
Hospital-based; surgically treated for ITW and/or tight gastrocs/soleus; ITW defined as persistent TW without known neurological basis for the gait abnormality. |
Surg: TAL (percutaneous or open) or Vulpius (gastrocs recession, soleus intact). |
Mean 13.2 (range 8–20) months post-surgery. |
0 |

|
Table I. Contd. |
||||||||
|
Authors |
Study design |
Minors score |
Number of subjects |
Age at intervention, years Mean (range) |
Population characteristics |
Intervention |
Follow-up |
Lost to follow-up |
|
Katz & Mubarak, 1984 (25) |
Case series; prospective |
2 |
8 |
7 (3–10) |
Hospital-based; referred with TA contractures; ITW defined as TA contractures without any identifiable neurological cause. Inclusion: full-term pregnancies, normal developmental milestones, ≥ average school performance, no behavioural problems. |
PT: n = 2 only TA stretching exercises. Cast: n = 5 bilateral below-knee serial dorsiflexion cut-out walking casts during 7 (2–16) weeks (6); intervals cast changes not mentioned followed by TA stretching exercises (3), negative-heel shoes and brace (1). |
25 (16–38) months. |
1 |
|
Kogan & Smith, 2001 (28) |
Case series; retrospective, qualitative study |
2 |
15 |
Not mentioned |
Hospital-based; ITW treated by TAL; ITW defined as persistent TW > age 2 years without any identifiable cause, and able to put their foot flat on request. |
Surg: percutaneous TAL followed by below-knee casts for 1 month. |
3 months–6.5 years post-surgery. |
5 |
|
McMulkin et al., 2006 (30) |
Historically controlled study |
13 |
14 |
9.3 (6.7–12.6); 8.5 (5.6–11.3) |
Hospital-based; ITW treated by gastrocs/soleus lengthening. ITW defined as persistent TW without signs of UMN lesion, neurological or developmental problems. Inclusion: complete pre- and postoperative LGA. Exclusion: simultaneous or previous surgery. |
Surg: n = 7 TAL (6 percutaneous, 1 open) n = 7 Vulpius. |
Mean 13 (range 10–17) months post-surgery. |
0 |
|
Stott et al., 2004 (29) |
Cross-sectional study |
12 |
13 |
5.1 (3.6–7.58); Casting: 7.7 (6.5–9.5); surgery: 10.5 (7.5–14) |
Hospital-based; skeletally mature patients treated for ITW during childhood; ITW defined as persistent TW without neurological or orthopaedic abnormality. |
Cast: n = 6 bilateral below-knee serial walking casting for 6 weeks; changes at 2 weekly intervals; followed by home assignment: passive stretching exercises; Cast + Surg: n = 7 casting plus bilateral TAL percutaneous (5) or Baker’s type (2). |
Mean 10.8 (range 5.4–15.6) years from last intervention. |
0 |
|
Stricker & Angulo, 1998 (16) |
Case-control, retrospective |
11 |
80 |
Not mentioned; At initial presentation: 3.4 (2–13) |
Hospital-based; ITW treated by serial casting. ITW defined as persistent TW > age 2 years without identifiable cause. Exclusion: < 2 years follow-up; incomplete charts, previous surgery, underlying neurological cause. |
Observation (none, special shoes, heel cord stretching exercises); Cast/brace: bilateral Below-knee walking casts for 6–12 weeks, intervals cast changes not mentioned (n = 8), or solid plastic AFO for 3–8 months (n = 9) followed by home assignment: stretching exercises; Surg: TAL n = 4 bilateral Baker’s type; n = 11 open, followed by cast and AFO 2–6 months. |
Mean 2.8 (2–8) years after initial presentation. |
0 |
|
ADF: ankle dorsiflexion; pADF; passive ankle dorsiflexion: AFO: ankle foot orthosis; BTX-A: botulinum toxin type A; FU: follow-up; (I)TW: (idiopathic) toe walking; LGA: laboratory gait analysis; PT: physical therapy; RCT: randomized controlled trial; TAL: Achilles tendon lengthening; TA: Achilles tendon; MU: mouse units; UMN: upper motor neurone lesion; MINORS: Methodological Index for Non-Randomised Studies; PDMS-2: Peabody Developmental Motor Scales-revised; SD: standard deviation. |
||||||||
Patient selection
In all studies ITW was considered a diagnosis of exclusion. However, the operationalization of the concept “diagnosis of exclusion” differed substantially between the studies. In 1 study the concept was not specified at all (28), in the other 18 studies at least neurological deficits were ruled out, either by history-taking or by medical examination. Four studies additionally excluded children with developmental delay (15, 17, 24, 25), 5 studies excluded psychological or behavioural problems (15, 18, 23, 25, 32), and 8 studies excluded orthopaedic problems other than equinus deformity of the ankle (14, 15, 18, 22, 23, 29, 31, 34).
The inclusion criteria in 10 studies were dominated by their retrospective nature. As a result, study samples differed with regard to patient characteristics such as age and pre-treatment maximal ankle dorsiflexion angle (Figs 2 and 3). Overall, children in the surgical studies showed more severe structural equinus deformity compared with children in the studies investigating conservative treatments (Fig. 2). Only when the Vulpius procedure (gastrocsoleus aponeurotic lengthening) was used for surgery, the maximal passive ankle dorsiflexion angle seemed to fit within the range reported in studies on serial casting. The age of the children at the time of intervention varied widely, both within and between studies (Fig. 3). One study did not mention the age at intervention (28) and in another study age could not be differentiated between the treatment groups (16). Children receiving surgery or BTX-A tended to be older (mean age > 7 years) compared with children receiving PT or serial casting. Similarly, children less than 4 years old were less frequently included in surgical studies, although in 1 study the youngest child receiving surgery was 2.5 years of age (32).
Fig. 2. Age at intervention (range and mean value (Δ) of the group). Black line: surgery, Black line stripes: botulinum toxin (BTX), Black line dots: BTX plus cast, Dark grey line: cast, Dark grey line stripes: PT/observation. TAL: Achilles tendon lengthening; VP: Vulpius procedure.
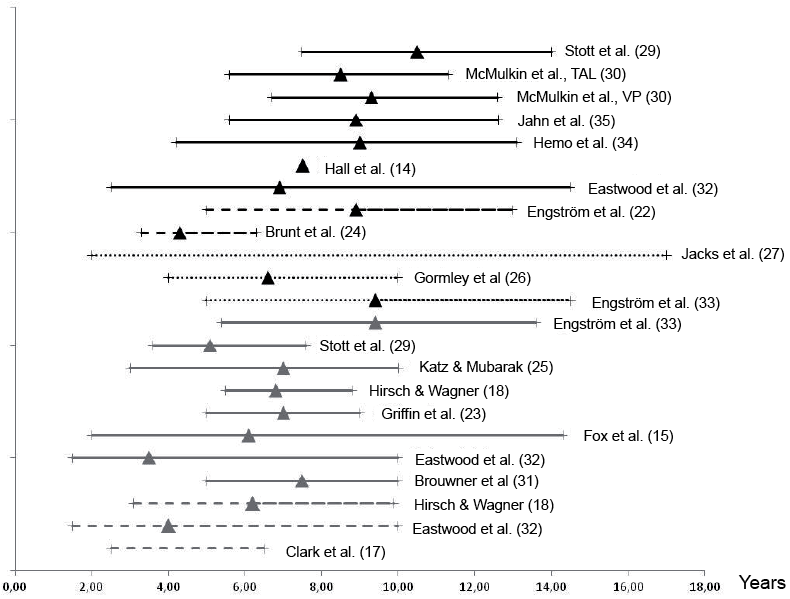
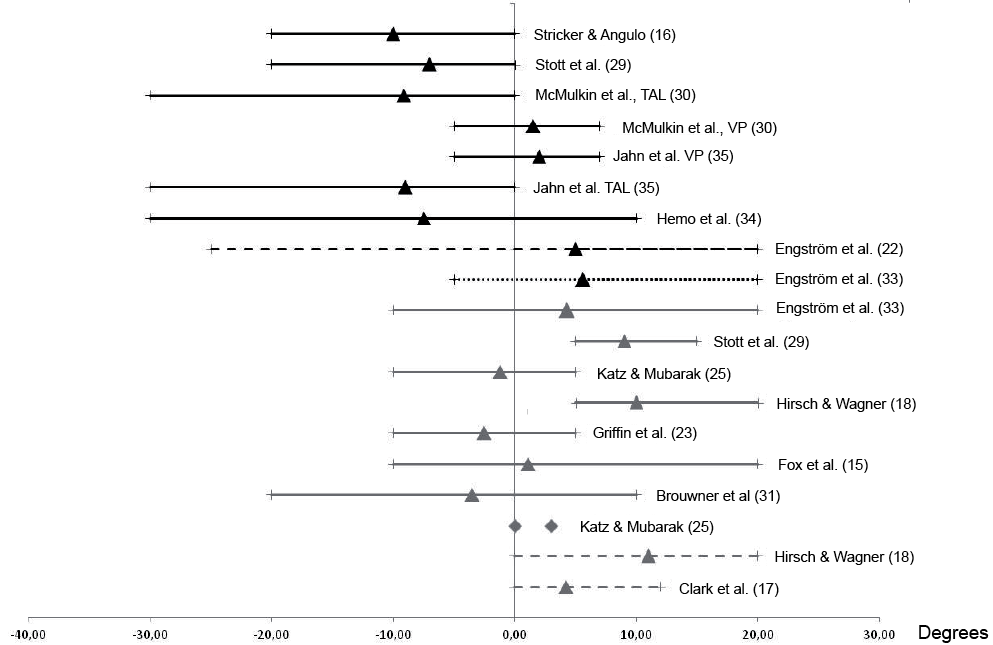
Fig. 3. Maximal ankle dorsiflexion end range of motion (range and mean value (Δ) of group). Black line: surgery, Black line stripes: botulinum toxin (BTX), Black line dots: BTX plus cast, Dark grey line: cast, Dark grey line stripes: TAL: Achilles tendon lengthening; PT/observation; VP: Vulpius procedure.
Treatment
Most studies (n = 13) reported on a single intervention. The effectiveness of PT (motor control intervention) was examined in one study (17), serial casting in 3 (15, 23, 31), BTX-A in 4 (24, 26, 27, 33), and surgery in 5 studies (14, 28, 30, 34, 35). Six studies (1 RCT and 5 non-randomized, uncontrolled studies) compared the effects of different interventions (16, 18, 22, 25, 29, 32). Criteria for the use of a particular type of intervention, however, were not provided. In general, only patients not responding to conservative treatment and with (severe) fixed contractures were included in surgical studies (16, 29, 30, 34). With regard to the same type of intervention, there was no standardized treatment protocol within or between studies, either with respect to treatment algorithm, the use of co-interventions or duration of treatment, or with respect to post-intervention management (Table I).
Outcome
A wide range of outcome measures was used (Table II). The earlier studies assessing conservative treatments and surgery have mainly relied on maximal passive ankle dorsiflexion, qualitative visual observation of gait, surface electromyography, and parental reports. These studies lack comprehensive quantitative assessment of (toe) walking. In the more recently conducted studies, comprehensive gait analysis has been used (22, 29, 30, 33–35).
|
Table II. Main outcome measures |
|||||
|
Authors |
Body Structures and Functions |
Activities Walking capacities |
Participation Caregiver/patient perspective |
||
|
PROM |
Gait assessment |
Others |
|||
|
Brouwer et al., 2000 (31) |
pADF |
OGA (video): Foot-contact pattern Temporospatial: Stride length Gait velocity |
PF strength (isometric) PF extensibility Reflex threshold Velocity of stretch Co-contraction ratio TA/gastrocs |
||
|
Brunt et al., 2004 (24) |
Not mentioned |
OGA: % frequency of foot-contact pattern EMG r.t. foot-contact pattern |
|||
|
Clark et al., 2010 (17) |
pADF |
OGA (GED): % Heel strike |
GMQ- PDMS-2 |
Parents’ report: % heel strike (VAS) |
|
|
Eastwood et al., 2000 (32) |
OGA (wet-foot prints): Foot-contact pattern |
Parents’ report: severity TW % TW |
|||
|
Engström et al., 2010 (33) |
pADF |
LGA: Kinematics Kinetics Temporo-spatial |
PROM hip/knee Alvarez classification of TW |
Parents report: % TW |
|
|
Engström et al., 2013 (22) |
pADF: Knee flexed Knee extended |
LGA: Kinematics Kinetics Temporo-spatial |
PROM hip/knee DF strength (isometric) Alvarez classification of TW Assessment of neuropsychiatric problems (FTF questionnaire) |
Parents report: % TW |
|
|
Fox et al., 2006 (15) |
pADF: Knee flexed Knee extended |
OGA |
Parents’ report: % TW |
||
|
Gormley et al., 1997 (26) |
pADF |
||||
|
Griffin et al., 1977 (23) |
pADF |
OGA: EMG r.t foot-contact pattern |
|||
|
Hall et al., 1967 (14) |
Only descriptive data |
OGA: Foot-contact pattern |
|||
|
Hemo et al., 2006 (34) |
pADF: Knee flexed Knee extended |
LGA: Kinematics Kinetics |
PF strength (number of heel rises) |
||
|
Hirsch & Wagner, 2004 (18) |
pADF |
OGA: Foot-contact pattern |
Parents’ report: Persistent TW Complaints of TW |
||
|
Jacks et al., 2004 (27) |
pADF |
OGA |
Lower-extremity function Assessment Test |
||
|
Jahn et al., 2009 (35) |
pADF: Knee flexed Knee extended |
LGA: Muscle-tendon lengths normalized to neutral posture lengths |
|||
|
Katz & Mubarak, 1984 (25) |
pADF |
OGA: Foot-contact pattern |
|||
|
Kogan & Smith, 2001 (28) |
OGA: Foot-contact pattern |
Parents’ report: Recurrence TW GMF irt normal |
|||
|
McMulkin et al., 2006 (30) |
pADF: Knee flexed Knee extended |
LGA: Kinematics Kinetics Temporospatial Normality Index |
PROM hip/knee |
||


|
Table II. Contd. |
|||||
|
Authors |
Body Structures and Functions |
Activities Walking capacities |
Participation Caregiver/patient perspective |
||
|
PROM |
Gait assessment |
Others |
|||
|
Stott et al., 2004 (29) |
pADF: Knee flexed Knee extended |
LGA: Kinematics Kinetics Observational gait scale |
aADF: Knee flexed Knee extended Ext. tibial torsion Hindfoot valgus Force plate heel rise test: n. max heel rises Total ROM PF power |
Patients’ report: TW treatment satisfaction walking/sports limitations |
|
|
Stricker & Angulo, 1998 (16) |
pADF |
OGA: Foot-contact pattern |
Parents’ report: treatment satisfaction (amount TW) |
||
|
aADF: active ankle dorsiflexion; OGA: observational gait analysis; pADF; passive ankle dorsiflexion: PF: plantar flexor; FTF questionnaire: Five to 15 questionnaire; PROM: passive range of motion; GED: Gait Event Detector; GMF: Gross Motor Function; ROM: range of motion; GMQ- PDMS-2: Gross Motor Quotient – Peabody Developmental Motor Scales-revised, r.t.: related to; LGA: laboratory gait analysis; TW: toe walking. |
|||||

Many studies contained only post-treatment information with no or merely historical pre-treatment data available (16, 18, 26–30, 34, 35). Follow-up varied widely both between and within studies, ranging from 6 weeks up to 22 years post-treatment (Table I). In studies addressing either PT or serial casting, 3 studies mentioned immediate post-intervention results up to 6 weeks after treatment (23, 25, 31), whereas 2 studies mentioned intermediate results (between 6 weeks and 1 year) (15, 22). In the study by Fox et al. (15), however, patients were discharged from follow-up as soon as parents were satisfied with the results of treatment. As a consequence, the time-frame of the follow-up evaluations varied between patients and may have influenced the outcomes. Studies addressing surgery did not mention immediate or intermediate post-operative results on any of the ICF levels. All these studies evaluated their results at least one year after surgery. Five studies specifically looked at the long-term results of surgery, with follow-up periods varying from 2 to 22 years (16, 18, 25, 29, 32).
Ten out of 19 studies mentioned adverse effects and complications, varying from no complications at all (27, 29, 31), to pressure ulcers in the case of casting (15, 16), to moderate pain after BTX-A injection (22, 23), PT (17) or cast (22), and to wound dehiscence (34), Achilles tendinitis (28, 34), or excessive ankle dorsiflexion (35) after Achilles tendon lengthening.
Passive ankle range of motion. Immediately after the termination of serial casting or PT the maximal passive ankle dorsiflexion range increased (15, 17, 23, 31). In these conservatively treated groups (serial casting and/or PT), only 3 studies statistically quantified this immediate improvement in ankle range of motion (15, 17, 31). In 2 of these studies, the gains in ankle dorsiflexion persisted during the 6-week follow-up period (15, 31). One RCT studied the intermediate effects of casting at 3 and 12 months in 2 treatment groups (22). One group receiving 4 weeks of below knee casting only, whereas the other group received BTX-A of the gastro-soleus complex prior to casting. In both groups the ankle dorsiflexion range was increased at, respectively, 3 and 12 months post-casting. Both at 3 and 12 months post-treatment, no added value of BTX-A on improving passive ankle dorsiflexion range could be found. Another prospective cohort study of 15 subjects treated with BTX-A, statistically quantified the results in terms of (increase in) passive ankle range of motion one year after treatment (33). At follow-up 1 year post-treatment, no significant difference in passive ankle dorsiflexion range could be observed compared with pre-treatment (33). In both studies, all children had normal range of motion at the hip and knee joint before treatment, while no or only minor limitations of the ankle were reported (Fig. 2).
The sustainability of the induced gains with conservative treatment (PT or serial casting), however, seemed to be relatively short. On average, 2 years after treatment, maximal ankle dorsiflexion tended to have decreased 6° from the values measured at the termination of the primary treatment (25). At even longer follow-up periods of 2–21.3 years, both Hirsh & Wagner (18) and Stricker & Angulo (16) did not find any systematic improvement in passive ankle dorsiflexion range after conservative treatment. Both studies, however, used historical pre-treatment data and, as such, the validity of this result may be questioned. After Achilles tendon lengthening, the increase in passive ankle dorsiflexion range seemed to persist in the long-term, with reports up to 3 years post-surgery (16, 30, 34, 35). Two studies statistically quantified this increase at 1 and 3 years’ post-surgery, respectively (30, 34).
In addition, Mc Mulkin et al. (30) performed a systematic clinical assessment of all lower extremity joints both pre- and at 1-year post-surgery. Prior to surgery, restricted passive ankle dorsiflexion, as well as decreased popliteal angle, increased external hip rotation, and increased tibial torsion (external rotation) were observed. Although these additional deviations improved significantly at 1-year post-surgery, only the popliteal angle increased to normal values, whereas the other deviations persisted (albeit small). At even longer terms of 5.4–15.6 years after surgery, Stott et al. (29) reported that the actual passive and active degrees of ankle dorsiflexion (with an extended knee) were still below the reported normal range of 10–25° for young adults. This study was, however, limited by its cross-sectional design, preventing definitive statements about the persistence or recurrence of restricted ankle dorsiflexion during physical examination.
Gait analysis. Two small case studies qualitatively studied electromyographic patterns in conjunction with foot contact patterns during toe walking (23, 24). One of these studies used reference data of normal children performing a similar gait pattern (23). During toe-toe walking, out-of-phase triceps surae activity prior to foot contact was observed, both in controls (23) and in children with ITW (23, 24). In the latter group, however, a greater proportion of the swing phase (20–30% vs 5% in controls) was characterized by premature triceps surae activity (23). Moreover, children with ITW showed early offset of (low) amplitude tibialis anterior activity in the swing phase, resulting in absent late-swing and loading-response activity of this muscle (23, 24). While Griffin et al. (23) used serial casting, Brunt et al. (24) selected BTX-A to treat the out-of-phase triceps surae activity. In both studies, electromyographic patterns changed immediately after treatment, i.e. the activation of both muscles closely resembled the normal situation. In the BTX-A treated group these results were still present at 1 year after treatment (24), while reports on the sustainability of the effects of serial casting are lacking.
Five studies directed at the effects of, respectively, casting (22), BTX-A (33), the combination of casting and BTX-A (22), and surgery (30, 34, 35) performed quantitative gait analysis. Children in the cast and BTX-A studies had (near) normal range of motion at all joints before treatment (22, 23), whereas the children in the surgical studies had (severe) equinus contractures (30, 34, 35). Kinematically, all children showed a significantly increased ankle plantar flexion angle throughout the gait cycle before the interventions (22, 30, 33–35). Overall, the muscle-tendon length determined by physical examination did not correlate well with the peak stance-phase muscle- tendon length determined during gait analysis (29, 35). Most children showed an absent first rocker (i.e. plantar flexion after initial contact) (22, 30, 34). Children in the cast and BTX-A study did not show any significant deviations in the pelvis or hip joint kinematics either pre- or post-treatment (33), whereas children in the surgical studies showed increased external hip rotation (30). Children receiving Achilles tendon lengthening had a greater mean pelvic tilt (30) compared with children receiving only a Vulpius procedure (30, 35). Moreover, in the surgical studies both an increased external foot progression angle during the stance phase (30) and a decreased peak knee flexion angle during the swing phase (30, 34) were observed. Some of the children also showed knee hyperextension during the stance phase (22, 34).
One year after each intervention, ankle dorsiflexion during the stance phase had increased significantly (22, 30, 33–35). Both the mean ankle dorsiflexion angle at initial contact and the peak ankle dorsiflexion angle during the stance phase had increased (22, 30, 33, 34). Most children developed a first rocker (28, 29), and the timing of maximal ankle dorsiflexion and the transition into plantar flexion occurred later in the stance phase (22, 34). During the swing phase, a significantly improved peak ankle dorsiflexion angles was also reported (22, 30, 33, 34). An increased peak knee flexion angle was also seen (22, 30, 34), while most children no longer showed knee hyperextension (22, 34). Children receiving Achilles tendon lengthening had a significant decrease in mean pelvic tilt, whereas after a Vulpius procedure no significant changes were seen, probably because pre-operative values were closer to normal (30).
Although the kinematics of gait generally improved, normalization did not occur (22, 30, 34). At 1-year follow-up, children still had a significantly lower peak ankle dorsiflexion angle during the entire gait cycle (22, 30, 34). Children in the surgical studies showed a significantly greater mean pelvic tilt, mean external hip rotation, and greater external foot progression angle during the stance phase (30). At even longer terms of 5.4–15.6 years post-surgery, Stott et al. (29) reported that 11 out of 13 patients still had a restricted peak ankle dorsiflexion angle during the stance phase, more than 2 SD below the reference values normalized for age. All but 1 child showed a normal first rocker, but progression of the tibia during mid-stance was still limited by lack of ankle dorsiflexion in many children. Some of these children also showed increased knee extension during the stance phase, although none showed clear hyperextension. The children who had undergone surgery still had larger thigh-foot angles, indicating increased external tibial torsion in the case of severe equinus contracture before surgery. As already indicated, the validity of the study by Stott et al. (29) was limited by its cross-sectional design.
Kinetically, a double bump pattern of the ankle plantar flexion moment, with a peak occurring before 25% of the gait cycle and inversion of the second rocker could be observed (29). In some children a premature internal ankle plantar flexion moment was present (due to premature calf muscle activity) and an increased knee extension moment in the early stance phase (30, 34). The premature ankle plantar flexion moment decreased (22, 30) and ankle power at push-off (A2 power generation) increased to 67% of normal values, although this effect did not reach statistical significance (30, 34). Within-group analysis showed that patients with the most severe equinus contracture demonstrated the greatest increase in ankle power (> 15 % of pre-operative values) (34).
Parental perception of gait performance and activities. In most studies on either conservative treatments (15, 22, 23, 31, 32), BTX-A (22, 24, 27), or surgery (14, 25, 28, 29), qualitative improvement of gait performance was reported after the intervention. Normalization of gait, however, did not occur in all children, with some children still performing toe or flatfoot contact after treatment (14, 15, 18, 22, 24, 29, 31, 32).
Two retrospective, non-randomized studies compared parents’ perception of toe walking at an average of 3 (range 2–22) years after treatment in 3 different groups: an observational group (no intervention), a serial casting group, and a surgical group (16, 32). Parents reported improved gait in nearly 50% of the children in both the observational and the casting group. Yet, only 6% of the children in the observational group and 10% in the casting group were considered to have a normal gait at all times (32). Only 25% of the parents were satisfied with the results of observation or serial casting, basically due to failure to correct the toe-walking (16). Casting appeared to offer little long-term improvement in walking performance compared with untreated ITW. Because both studies excluded children from follow-up when they had undergone Achilles tendon lengthening for persistent toe walking (after they had been initially treated conservatively), underestimation of failure rates may have occurred (16, 32).
Two studies on BTX-A assessed parents’ perception of toe walking (22, 33). Of the children who were followed for 12 months, only 27–30% were reported to have completely ceased toe walking. The 12-month follow-up seemed to be a good indicator of the outcome 3–5 years later (33).
After surgery most parents reported improved gait performance up to 22 years (14, 28, 30, 34). In the study by Eastwood et al. (32) 72% of the parents reported improved gait after 3 (range 2–22) years, although only 22% of the children had completely ceased toe walking. The failure rate (no complete cessation of toe walking) after surgery in the other studies ranged from 17% to 33% (16, 29, 32, 34). Despite this, a high level of parental satisfaction was reported in the surgery group (16, 28).
With regard to activities, some studies reported disabilities due to ITW in terms of limitations in sporting activities (29), difficulty or minimal assistance needed with running (15), and limitations in activities requiring the use of legs and feet as reported on the Lower Extremity Function Assessment Test (27). Due to the small numbers and the lack of systematic report of reliable outcome measures, however, it is difficult to exclusively relate the reported disabilities to ITW.
Discussion
This systematic review was conducted to gain insight into the available evidence on the efficacy of different treatments for ITW at all levels of the ICF-CY. Several treatment modalities have been used, including motor control interventions, passive stretching, serial casting, BTX-A, and surgery to correct the equinus position. Although these treatments are widely used in clinical practice, the number and quality of the traced publications investigating these treatments is, as yet, limited. Only 1 study randomly assigned different treatments to different groups (22), and only 2 other (non-randomized, retrospective) studies incorporated a non-treatment group (16, 32). The other studies were non-randomized uncontrolled studies, scoring level 4 according to the definitions of the Oxford Centre for Evidence-based Medicine. As more than half of all studies were retrospective in nature or used historical control groups, their results are highly subjective to information bias. In addition, most studies incorporated only small samples of children and, as such, selection bias may have occurred.
As the pathophysiology underlying ITW is unknown, causal treatment is still not possible. Therefore, interventions to address ITW have been directed towards increasing passive ankle dorsiflexion in children aged 4–17 years, with the expectation that gait changes would follow gains in structure. Consensus on the criteria for using a particular type of treatment has not been established, although Achilles tendon lengthening is generally performed in the case of significant equinus contracture when other treatments have failed. As surgery was reserved for the most severe cases, these children tended to be older, were non-responders to previous treatments, and had equinus contractures. Moreover, the increased foot progression angle, anterior pelvic tilt, and external hip rotation observed in many of these children might be secondary to the equinus contractures. As muscle length and bone formation are affected by functional demands, adaptive external rotation at the hip and in the tibia might occur while attempting to place the limb in a plantigrade position despite an ankle contracture (30, 36, 37).
Nonetheless, at the ICF-CY level of body structures and function, improvements have consistently been reported in terms of increased passive ankle dorsiflexion angle immediately after treatment. Only in the BTX-A group no improvement in passive ankle dorsiflexion was observed, probably because these children had no structural limitations in ankle dorsiflexion before treatment. The sustainability of the improvements in the conservative treatment (PT/casting) groups was, however, limited, whereas surgical management seemed to be more effective in restoring passive ankle dorsiflexion angle even in the long-term (> 1 year). However, as surgery was restricted to children with the most severe equinus contractures, the opportunity to increase ankle dorsiflexion end range of motion in these children was substantial. As such, selection bias may be responsible for the favourable outcome of surgery compared with the other treatment alternatives. Improvements in kinematics and kinetics during instrumented gait analysis were observed after both casting, BTX-A, and surgery, however, normalization of the gait pattern did not occur.
It can be questioned whether a gait analysis represents the child’s most common walking performance. It is reported that a person with ITW may spontaneously adopt both a toe and a heel contact pattern during the same gait analysis. Thus, it may be possible that a child with ITW walks less on the toes during gait analysis by using cognitive compensation to control the walking pattern. As such, parents’ perceptions of toe walking may give a better indication of the child’s walking performance in a normal environment. As indicated by the parental reports of toe walking, the carry-over of the heel-toe gait after casting seems to be limited. In the long-term, parental reports did not differ between children treated with serial casting and those that did not receive treatment for ITW (30). Even after surgery, 17–33% of the parents reported incomplete cessation of toe walking. Moreover, the final outcomes of ITW treatment in terms of activities and participation are ill defined. Not only the efficacy of the different treatment modalities on the ICF-CY levels of activities and participation remains unclear, but also the consequences of untreated ITW on these levels of functioning.
Only minimal complications were reported in the studies included in this review. Hence, all reported treatments for ITW seem to be fairly safe. Although most intervention studies suffered from bias with regard to the report of long-term complications, particularly the surgical studies on ITW used follow-up periods of many years, which makes it unlikely that serious long-term complications might have been missed.
Study limitations
There are several limitations to this review that need to be considered when interpreting the results. Pooling of results in a meta-analysis was not possible because of the poor methodological quality of many of the studies. Comparisons between studies were difficult for several reasons. First, systematic differences in the base population both within and between studies were present with regard to age, pre-treatment ankle dorsiflexion angle, and previous treatments for ITW. Secondly, a variety of treatment modalities, algorithms, and post-treatment management strategies were reported. None of the studies controlled for co-interventions, which prohibits to validly attribute the observed effects to a single intervention. Thirdly, a wide range of outcome measures and follow-up periods were applied, making it difficult to compare the effectiveness of different treatments. Hence, as the quality of the included systematic reviews and studies was generally poor, the results must be interpreted with some caution. As articles reviewed were limited to the Dutch, English, French and German languages and to manuscripts available in medical libraries in the Netherlands, some studies may have been missed.
Conclusion
This review emphasizes the importance of adequate patient and goal selection when treating ITW. Moreover, to identify relevant treatment-induced changes, it is essential to obtain more knowledge of the natural course of ITW during growth and development into adulthood. The consequences of ITW with regard to functional activities and social participation of the affected children, as well as the effects of treatment on their functioning have yet to be demonstrated. There is preliminary evidence of the beneficial effects of serial casting and surgery on passive ankle dorsiflexion, and of serial casting, BTX-A and surgery on the kinetics and kinematics of gait. However, the sustainability of beneficial effects after conservative treatment (PT/casting) seems to be short. Larger controlled studies are needed to compare the effectiveness of different treatment modalities for ITW. In addition, outcome measures at all levels of the ICF-CY should be used with sufficiently long-term follow-up.
References
