Masashi Kumon, MD1, Toshikazu Tani, MD1, Masahiko Ikeuchi, MD1, Kazunobu Kida, MD1, Ryuichi Takemasa, MD1, Noritsuna Nakajima, MD1, Katsuhito Kiyasu, MD1, Nobuaki Tadokoro, MD1 and Shinichirou Taniguchi, MD2
From the 1Department of Orthopaedic Surgery, Kochi Medical School, Kohasu Oko-cho, Nankoku City, Kochi, and 2Department of Orthopaedic Surgery, Kansai Medical University Takii Hospital, Osaka, Japan
OBJECTIVES: To determine whether repetitive tibial nerve stimulation (RTNS) affects neurogenic claudication and F-wave conduction in lumbar spinal stenosis.
DESIGN: An intervention study: before/after trial.
SUBJECTS: Data for 12 central lumbar spinal stenosis patients were compared with 13 age- and sex-matched healthy volunteers.
METHODS: A conditioning RTNS at the ankle, 0.3-ms duration square-wave pulses with an intensity 20% higher than the motor threshold, was applied at a rate of 5/s for 5 min. We assessed the effects of RTNS on the claudication distance at which the lumbar spinal stenosis patients can no longer continue walking due to increasing leg symptoms, and on tibial F-wave measurements.
RESULTS: A comparison between mean pre-RTNS and post-RTNS revealed a significant difference in claudication distance (66 m (standard deviation (SD) 19) vs 133 m (SD 37); p = 0.003), mean F-wave minimal latency (48.3 ms (SD 1.7) vs 44.8 ms (SD 1.0); p = 0.007) and mean F-wave conduction velocity (53.3 m/s (SD 2.0) vs 55.5 m/s (SD 1.9); p = 0.009) in the lumbar spinal stenosis group, but not in the control group.
CONCLUSION: RTNS has beneficial effects on neurogenic claudication and F-wave conduction in central lumbar spinal stenosis patients. This phenomenon may have practical value in providing a new therapeutic modality for lumbar spinal stenosis.
Key words: F-wave; lumbar spinal stenosis; neurogenic claudication; repetitive nerve stimulation.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Masashi Kumon, Orthopaedic Surgery, Kochi Medical School, 783-8505 Nankoku, Japan. E-mail: mk480158@gmail.com
Accepted May 15, 2014; Epub ahead of print Sep 3, 2014
INTRODUCTION
Since the publication of the gate control theory by Melzak & Wall in 1965 (1), the role of nerve stimulation in pain relief has gained credibility and a neuromodulation technique of this type is widely used as an effective method of chronic pain management (2).
In contrast, there has been little research into the beneficial effects of peripheral nerve stimulation on neurogenic claudication in lumbar spinal stenosis (LSS), which was originally reported by Tamaki et al. (3). In LSS patients with neurogenic claudication, they demonstrated that walking distance increased immediately after repetitive stimulation of the peroneal or the tibial nerve for 5 min. This phenomenon may provide not only corroborative information for the diagnosis of LSS, but also a new clinical modality for the management of neurogenic claudication. LSS can be classified radiographically into central narrowing of the spinal canal with thecal sac compression (central stenosis), constriction in the nerve root canals or a combination of both of these factors. Central stenosis typically leads to neurogenic claudication affecting both legs due to compression of the cauda equina (4).
The aim of this study was to attempt to verify the beneficial effects of repetitive tibial nerve stimulation (RTNS) on neurogenic claudication in central LSS patients and to test whether the tibial F-wave measurements, which, unlike EMG and the conventional nerve conduction studies, enable the assessment of motor conduction along the cauda equina (5), account for the effects.
MATERIAL AND METHODS
Subjects
From July 2008 to September 2009, 15 LSS patients consecutively referred to our hospital for decompressive surgery underwent RTNS study. All had neurogenic claudication and radiologically confirmed central LSS affecting both legs. Of these, we analysed 12 patients (8 women) aged 68–84 years (mean age 75 years) in whom RTNS increased claudication distance (LSS group). Claudication distance was defined as the distance at which patients can no longer continue walking due to increasing leg symptoms (6). The remaining 3 patients in whom RTNS had no effect on neurogenic claudication were excluded from the subsequent analyses. In the LSS group, magnetic resonance imaging (MRI) disclosed the most intense compression of the thecal sac at L3–4 in 2 patients and at L4–5 in 10 patients. Based on clinical evaluation, none had polyneuropathies, entrapment neuropathies, vascular insufficiency of the lower limbs, or history of spinal surgeries. For comparison, 13 age- and sex-matched healthy volunteers (6 women) aged 68–79 years (mean 74 years) underwent the same study (control group). All subjects agreed in writing to participate in the study after providing written informed consent approved by the hospital ethics committee.
Walking test
LSS patients walked around a circular course of 30 m on a smooth flat surface in a marked corridor. They were instructed to walk at a comfortable and reasonable speed supervised by one of the authors (MK). Claudication distance (6), at which the patients can no longer continue walking due to increasing leg symptoms, was measured using a distance measuring wheel with digital display of 0.1 m resolution (M474WS-160BS, Shiro Industry Co., Osaka, Japan). The walking test was performed twice; once before and once after RTNS.
F-wave study
The subject lay supine on a table in a quiet room. A Nihonkohden Neuropack (Neuropak MEB2200, Nihonkohden, Tokyo, Japan) was used to measure F-waves, and the signals were displayed and sampled at 10 KHz, with a filter setting of 20 Hz–3 KHz. F-waves were recorded from the abductor hallucis (AH) with a pair of disposable disk electrodes, 12 mm in diameter (Vitrode J-150, Nihonkohden, Tokyo, Japan), placed over the belly and the tendon after application of an abrasive gel to reduce impedance. The stimulating electrodes consisted of a pair of disposable disk electrodes (Vitrode J-150) placed on the tibial nerve at the ankle, with the cathode placed 2 cm distally to the anode (5). The maximal stimulus was determined by delivering 0.1 ms square-wave pulses of increasing intensity to elicit the largest compound muscle action potentials (CMAPs). Supramaximal shocks, adjusted up to a value 20% higher than the maximal stimulus, were delivered at 1 Hz for acquisition of F-waves, which were defined as a deflection of at least 50 μV measured from peak to peak. The late responses with a constant latency and waveform called the A-wave were excluded from the evaluation (5). We tested the leg on the side affected by neuropathic symptoms more than the other in LSS patients and on a side chosen arbitrarily in controls. The subjects underwent the F-wave study twice; once before and once after RTNS.
F-wave measurements consisted of: (i) persistence, or the number of definable F responses per 100 stimuli; (ii) the minimal onset latency; (iii) chronodispersion (7); or the difference between the minimal and maximal F-wave latencies; and (iv) peak-to-peak amplitude averaged for only those trials with detectable responses (response average). In addition, F-wave conduction velocity (FWCV) was calculated as follows: FWCV (m/s) = 2D (mm)/F (ms) – M (ms) – 1.0 (ms), where “D” represents the surface distance measured from the stimulus site to the T12 spinous process by way of the knee and greater trochanter of the femur, and “F” and “M” are latencies of the F-wave and M-response, respectively. Subtracting an estimated minimal delay of 1.0 ms at the motor neurone pool and dividing by 2, (F-M-1)/2 represents the conduction time from the stimulus site to the spinal cord (5). The minimal onset latencies were measured twice by 1 investigator with an interval of more than 3 months. The levels of intra-rater reliability of measuring F-wave latencies were assessed using the intraclass correlation coefficient (ICC).
Repetitive tibial nerve stimulation
Using the same stimulating electrodes as in the F-wave study, a conditioning RTNS, 0.3-ms duration square-wave pulses with an intensity 20% higher than the motor threshold, defined as a stimulus intensity just sufficient to evoke a detectable muscular twitching in the AH, was applied at a rate of 5/s for 5 min. A series of relatively low-voltage stimuli caused neither pain nor discomfort for the subjects.
Sequence of study (Fig. 1)
LSS patients first underwent a walking test. The patient was then seated comfortably on an adjustable armchair for 30 min., which completely relieved the claudication symptoms. Next, baseline F-waves were recorded with the patient lying supine on a table, followed by conditioning RTNS. Immediately after the RTNS, F-wave study and walking assessment were repeated. For controls, the same sequence of studies was followed as used in LSS patients, but walking assessments were eliminated, because they were able to walk continuously for more than 30 min with no leg symptoms.
In 4 of the 12 LSS patients, we also measured the tibial nerve motor conduction time by stimulating the tibial nerve at the knee and recording CMAPs from the AH before and after RTNS.
Statistical analysis
The Shapiro-Wilk test for continuous variables showed that the data were in the form of a non-normal distribution. Therefore, statistical analyses included the Mann-Whitney U test for statistical differences between the LSS group and control group data, and Wilcoxon signed-rank test for evaluating differences between pre- and post-RTNS data. Values are given as mean (standard deviations (SD)) with a significance level of p < 0.05. All statistical analyses were performed with SPSS software, version 16 (SPSS Inc., Chicago, IL, USA).
RESULTS
Comparison of the mean baseline F-wave data obtained prior to RTNS between the LSS and the control groups showed significant differences in persistence (72.3% SD 5.0) vs 90.7% (SD 3.3); p = 0.019) and mean chronodispersion (12.7 ms (SD 6.2) vs 7.5 ms (SD 2.8); p = 0.015), but not in mean minimal latency (48.3 ms (SD 1.7) vs 44.8 ms (SD 1.0); p = 0.21), mean velocity (53.3 m/s (SD 2.0) vs 58.8 m/s (SD 1.3); p = 0.088) and mean amplitude (361 μV (SD 43) vs 346 μV (SD 28); p = 0.83) (Table I).
|
Table I. Baseline F-wave data |
|||
|
LSS group Mean (SD) |
Control group Mean (SD) |
p-valuea |
|
|
Persistence, % |
72.3 (5.0) |
90.7 (3.3) |
0.019 |
|
Minimal onset latency, ms |
48.3 (1.7) |
44.8 (1.0) |
0.21 |
|
Conduction velocity, m/s |
53.3 (2.0) |
58.8 (1.3) |
0.088 |
|
Chronodispersion, ms |
12.7 (6.2) |
7.5 (2.8) |
0.015 |
|
Peak to peak amplitude, μV |
361 (43) |
346 (28) |
0.83 |
|
aMann-Whitney U test. LSS: lumbar spinal stenosis; SD: standard deviation. |
|||
In the LSS group, the RTNS significantly (p = 0.003) increased the mean claudication distance from the baseline value of 66 m (SD 19) to 133 m (SD 37). The RTNS also significantly (p = 0.007) shortened the mean F-wave minimal latency, from 48.3 ms (SD 1.7) to 46.5 ms (SD 1.4), and the mean increased velocity (p = 0.009), from 53.3 m/s (SD 2.0) to 55.5 m/s (SD 1.9), calculated based on the measured distance from the stimulus site to the T12 spinous process (mean 1,122 mm (SD 59)). However, neither persistence, chronodispersion, nor amplitude changed significantly with RTNS (p = 0.35, 0,065 and 0.73, respectively) (Table II and Fig. 2). The latency of the CMAP evoked in the AH by tibial nerve stimulation at the knee was also unchanged with RTNS (13.0 ms (SD 1.3) vs 13.1 ms (SD 1.2); p = 1.0000).
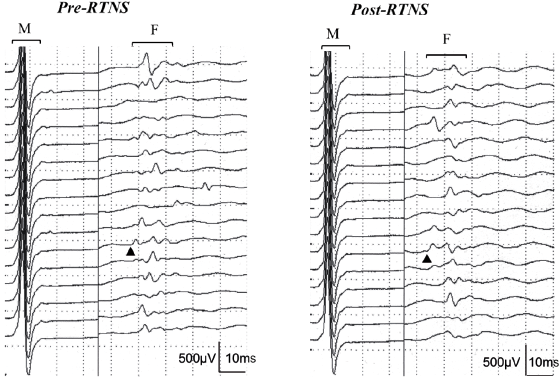
Fig. 2. Raster mode display of 16 consecutive traces showing M responses and F-waves recorded from the abductor hallucis before (left) and after (right) conditioning repetitive tibial nerve stimulation at the ankle applied with an intensity 20% higher than the motor threshold at a rate of 5/s for 5 min in the same patient. Analyses of 100 traces showed a change from 47.6 to 43.9 ms in F-wave minimal latency (arrowheads) and from 49.2 to 53.8 m/s in F-wave conduction velocity with conditioning stimulation. RTNS: repetitive tibial nerve stimulation.
In the control group, RTNS did not significantly alter any of the F-wave measures (minimal latency (p = 0.65), velocity (p = 0.72), chronodispersion (p = 0.093), persistence (p = 0.93) and amplitude (p = 0.75) (Table II)).
|
Table II. Comparison between pre- and post-repetitive tibial nerve stimulation (RTNS) data |
|||||||
|
LSS group |
Control group |
||||||
|
Pre-RTNS Mean (SD) |
Post-RTNS Mean (SD) |
p-value* |
Pre-RTNS Mean (SD) |
Post-RTNS Mean (SD) |
p-valuea |
||
|
Claudication distance, m |
66 (19) |
133 (37) |
0.003 |
NA |
NA |
NA |
|
|
F-wave |
|||||||
|
Persistence, % |
72.3 (5.0) |
68.3 (6.0) |
0.35 |
90.7 (3.3) |
90.8 (3.6) |
0.93 |
|
|
Minimal onset latency, ms |
48.3 (1.7) |
46.5 (1.4) |
0.007 |
44.8 (1.0) |
44.8 (0.9) |
0.65 |
|
|
Conduction velocity, m/s |
53.3 (2.0) |
55.5 (1.9) |
0.009 |
58.8 (1.3) |
58.8 (1.3) |
0.72 |
|
|
Chronodispersion, ms |
12.7 (6.2) |
14.4 (7.5) |
0.065 |
7.5 (2.8) |
9.1 (3.8) |
0.093 |
|
|
Peak to peak amplitude, μV |
361 (43) |
404 (88) |
0.73 |
346 (28) |
348 (20) |
0.75 |
|
|
aWilcoxon signed-rank test. NA: not applicable; LSS: lumbar spinal stenosis; SD: standard deviation. |
|||||||
ICC values in measuring F-wave latencies showed very high levels of reliability; 0.998 for the LSS group and 0.996 for the control group.
DISCUSSION
In LSS patients, exacerbation of symptoms on walking, and relief of symptoms in flexion or sitting are common findings (4, 5). Severity and functional impairment in such patients are usually quantified by measuring maximal walking distance at which the patients can no longer continue walking, due to increasing leg symptoms (absolute claudication distance) (6). To more objectively document the neurogenic claudication in LSS, dynamic electrophysiological studies were previously conducted showing evidence of transient conduction slowing and/or block at a radicular level by using spinal cord evoked potentials, F-wave (8), H-reflex (9, cortical somatosensory evoked potentials and motor evoked potentials with transcranial magnetic stimulation (10).
In contrast, our study has documented the beneficial effects of RTNS on neurogenic claudication, confirming an earlier observation reported by Tamaki et al. (3), which has received little attention since. In our series of central LSS patients, RTNS at the ankle with submaximal intensity at a rate of 5/s for 5 min doubled their claudication distances. Our baseline F-wave data obtained prior to RTNS showed a significantly reduced F-wave persistence and increased F chronodispersion in the LSS group compared with the control group, indicating that motor nerves were already diseased in LSS patients. The novel finding reported here is that the favourable effect of RTNS on neurogenic claudication was associated with partial improvement in F-wave conduction; i.e. the F-wave minimal latency became slightly, but significantly, shorter, and the velocity faster, in LSS patients, but not in age- and sex-matched healthy volunteers. Since the F-wave results from the backfiring of antidromically activated anterior horn cells (5), our results for F-wave latency change with RTNS at the ankle, in conjunction with motor conduction remaining unchanged below the knee in the LSS group, probably reflect transient improvement in conduction slowing in proximal motor axons at the motor root level. This latency shift of the fastest F-waves may have resulted from a preferential effect of RTNS with submaximal intensity on fast-conducting larger neurones, although an increase in F chronodispersion with RTNS did not reach statistical significance (p = 0.065).
Neurogenic claudication in LSS is believed to mainly have an ischaemic basis. The rapidly reversible nature of the symptoms supports this view. Measurements of cerebrospinal fluid pressure or epidural pressure (11) in LSS patients have demonstrated an increase in intraspinal pressure while standing upright and walking. Experiments on nerve compression in animals (12) and humans (13) showed evidence of compression-induced focal nerve ischemia. Animal experiments on neurogenic claudication also revealed a reduction in nerve blood flow and/or venous congestion of the nerve roots at the site of compression, indicating an ischaemic component of the pathology. In LSS, therefore, a posture-related increase in compression would cause neural and microvascular compromise of the cauda equina already deprived and vulnerable, giving rise to neurogenic claudication.
Viewed in this light, we speculate that the beneficial effect of RTNS on neurogenic claudication and F-wave conduction shown in this study may be explained on the basis of improved oxygenation of the cauda equina, which is inadequate in LSS. In fact, Takahashi et al. (14) demonstrated in dogs an increase in the blood flow in the lumbar spinal cord and the nerve roots ipsilateral to electrical stimulation of the sciatic nerve. Accumulated evidence also supports vascular effects induced by repetitive peripheral nerve stimulation, causing improved tissue oxygenation (15). Taken together, these findings imply that an anti-ischaemic effect of RTNS may, in part, account for the current results of an increase in claudication distance accompanied by an increase in F-wave conduction velocity. Although the exact mechanisms for neurovascular effects of RTNS remain unclear, these observations may have value in providing a new therapeutic modality for neurogenic claudication and in adapting electrophysiological investigations to this clinical phenomenon in LSS. The technique could be carried out at home for LSS patients with a portable stimulator running on lithium batteries, which allows modulation of both amplitude and frequency, in order to reduce claudication symptoms. This new therapeutic modality for LSS is indicated for these patietnts, unless they have progressive neurological loss or they find the level of disability unacceptable without surgical intervention.
Study limitations
This study has several limitations in drawing generalized conclusions from the data. First, the sample size was relatively small. A larger sample size would be more representative. In addition, the sample was biased by excluding from subsequent analyses 3 patients in whom RTNS had no effect on neurogenic claudication. Secondly, there is no control group with LSS who did not undergo RTNS. Thirdly, treadmill testing would be more appropriate to standardize the walking speed between the first and the second walking period. Fourthly, F-wave latencies were measured twice by one investigator with a high level of intra-observer agreement, so that inter-observer agreement was not confirmed. Fifthly, repeated F-wave studies were not conducted to investigate how the duration of the effects of RTNS on F-waves. Sixthly, we chose the stimulus intensity, duration, frequency and number of stimuli for RTNS similar to those originally described by Tamaki et al. (3), while the stimulus parameters most effective on neurogenic claudication and F-wave conduction remain to be elucidated. Seventhly, we measured the tibial nerve motor conduction time below the knee before and after RTNS in only 4 of the 12 LSS patients. Nevertheless, this study contributes to the reassessment of a useful technique that currently receives little attention, and thereby, will at least provide some basis for further refinement in its practical application to LSS treatment.
Conclusion
A conditioning RTNS at the ankle with a square-wave pulse of 0.3 ms in duration and 20% higher than the motor threshold in intensity delivered at a rate of 5/s for 5 min had a beneficial effect on neurogenic claudication and F-wave conduction in central LSS patients. RTNS significantly increased claudication distance and the F-wave conduction velocity in the LSS group, but not in the control group. Although the underlying mechanisms remain unclear, this phenomenon may have practical value in providing a new therapeutic modality for LSS.
References
