Thilo O. Kromer, PhD1,3, Rob A. de Bie, PhD2,3,4 and Caroline H. G. Bastiaenen, PhD2,3,4
From the 1SRH University Heidelberg, School of Therapeutic Sciences, Department of Physiotherapy, Heidelberg, Germany, 2Department of Epidemiology, 3CAPHRI School for Public Health and Primary Care and 4Centre for Evidence-Based Physiotherapy (CEBP), Maastricht University, Maastricht, The Netherlands
OBJECTIVES: To investigate the effect of manual physiotherapy and exercises compared with exercises alone in patients with shoulder impingement syndrome one year after inclusion.
DESIGN: Randomized controlled trial.
SUBJECTS: Patients with shoulder impingement of more than 4 weeks.
METHODS: The intervention group received individualized manual physiotherapy plus individualized exercises; the control group received individualized exercises only. Both groups had 10 treatments over 5 weeks; afterwards all patients continued their exercises for another 7 weeks at home. Primary outcomes were the Shoulder Pain and Disability Index and Patients’ Global Impression of Change. The Generic Patient-Specific Scale was used as secondary outcome. Costs were recorded in a log-book.
RESULTS: Ninety patients were included in the study and 87 could be analyzed at 1-year follow-up. Both groups showed significant improvements in all outcome measures, but no difference was detected between the groups. Only costs differed significantly in favour of the control group (p = 0.03) after 5 weeks.
CONCLUSION: Individualized exercises resulted in lower costs than manual physiotherapy and showed a significant effect on pain and functioning within the whole group after one year. Exercises should therefore be considered as a basic treatment. Due to the progressive improvement that occurred during the follow-up period with individualized exercises further treatments should be delayed for 3 to 4 months.
Key words: shoulder impingement syndrome; manual therapy; physiotherapy; exercise therapy; rehabilitation; randomized controlled trial.
J Rehabil Med 2014; 46: 00–00
Guarantor’s address: Caroline H. G. Bastiaenen, Department of Epidemiology, Maastricht University, PO Box 616, NL-6200 MD Maastricht, The Netherlands. E-mail: chg.bastiaenen@maastrichtuniversity.nl
Accepted May 6, 2014; Epub ahead of print Sep 11, 2014
Introduction
Shoulder complaints are one of the most common musculoskeletal conditions seen by healthcare professionals (1–4), with an incidence of 9.5 per 1,000 patients. They are often recurrent in nature and do not necessarily resolve over time, and thus lead to a significant reduction in health (5, 6). Most patients with shoulder conditions presenting to primary care show clinical signs of subacromial impingement (4, 6), indicative of mechanical problems within the subacromial space causing pain and functional restrictions mostly during overhead activities (7).
Physiotherapy is therefore often prescribed for the treatment of subacromial shoulder pain (4, 8, 9). In the literature a positive short-term effect of physiotherapist-led exercises and manual physiotherapy on pain and functioning is suggested, but study results are inconsistent and often limited by poor methodological quality and small sample sizes (10–12). However, long-term results are scarce, and evidence for a sustained effect of the positive results of physiotherapeutic interventions seen in short-term follow-up is therefore even more limited.
This trial compared the effectiveness of individualized manual physiotherapy (IMPT) with an individualized exercise protocol (IEP) on pain and functioning in patients with clinical signs of shoulder impingement syndrome (SIS) and presents the results one year after inclusion. The 5-week and 12-week result of this trial have been published previously (13).
Methods
Participants
Participants were recruited by referral from general practitioners or orthopaedic surgeons to physiotherapy due to shoulder complaints. They were then screened by trained physiotherapists for eligibility. Patient who fulfilled the eligibility criteria were asked to sign informed consent, they underwent baseline assessment and were subsequently allocated to treatment groups in blocks of 6 using central randomization via the internet. To further guarantee allocation concealment, therapists were informed about allocation immediately before the first treatment. The eligibility criteria for this trial are described in detail in the published study protocol for this paper (14).
Interventions
The intervention group received examination-based, individualized manual physiotherapy (IMPT) plus an IEP; the control group received IEP only. Treatment was provided in 6 outpatient physiotherapy clinics by 12 trained physiotherapists with international qualifications for manual therapy according to the standard of the International Federation of Manipulative Physical Therapists (IFOMPT). Participants received 10 treatment sessions within 5 weeks. Shoulder log-books were used to record exercise frequency, sick leave (days off work paid by the employer), additionally prescribed medication intake, co-interventions, further diagnostic measures, costs for paid help (e.g. somebody who helps with house cleaning or grocery shopping), and over-the-counter medication. For all these measures (except for over-the-counter medication) a prescription, a referral, or a sick note from a physician is mandatory; these measures are then paid by the German heath system. Due to ethical considerations the use of analgesics and non-steroidal anti-inflammatory drugs was permitted and was also recorded in the log-book. A detailed description of the interventions is provided in the published protocol for this study (14) and the published short-term results of this trial (13); an overview of the key components is also given in Table I.
|
Table I. Key elements of interventions |
||
|
Both groups: Individualized exercise protocol |
Intervention group: Individualized manual physiotherapy |
|
|
Content |
Stretching and strengthening exercises for the shoulder, shoulder girdle, and the cervical and thoracic spine. |
Manual pain treatment, pain-reducing exercises, individualized education about the pathology and instructions for the most provocative ADLs to reduce pain events during the day. Manual mobilization of articular, muscular, or neural restrictions identified in the shoulder joint, the shoulder girdle, the cervical or thoracic spine. Interventions were based on clinical examination results and initially guided by a decision aid. Subsequent treatment decisions were based on retest results (test-retest-principle). |
|
Frequency |
2 supervised training sessions per week; 2–5 home training sessions per week. |
2 treatment sessions per week. |
|
Dosage |
2–3 sets with 10–20 repetitions; increasing resistance over time. |
Initial duration of mobilization techniques: 20–30 s. Subsequent dosages were based on retest results. Detailed information about test and assessment results, and therapy interventions was given. |
|
Instructions |
Detailed exercise instructions from the physiotherapist; booklet with pictures and written instructions. |
Detailed instructions on how to perform effective manual techniques at home to intensify their effect. |
|
Stopping rules |
Pain of more than 3/10 VAS or longer than 30 s after the exercises. |
Treatment intensity was limited by pain of more than 4/10 on a VAS. |
|
Monitoring |
Log-book records. |
Log-book records and structured reassessment process. |
|
ADLs: activities of daily living; VAS: visual analogue scale. |
||
Outcome measures
Primary outcome measures for the 1-year follow-up were the Shoulder Pain and Disability Index (SPADI) and Patient’s Global Impression of Change (PGIC). The SPADI is a shoulder-specific self-reported questionnaire measuring pain and disability (15). Subscales for pain and function are scored from 0 to 100, with higher scores reflecting higher pain/disability levels. The total SPADI score was calculated by averaging the score of the 2 sub-scales. The minimum clinically important change was considered as 11 points in the total SPADI score (16). PGIC was measured with an ordinal scale from 1 (much worsened) to 5 (much better). A rating of “slightly or much better” was defined as a successful result.
As a secondary outcome measure we used the Generic Patient-Specific Scale (GPSS), which assesses individual complaints and restrictions in a short and efficient way (17). Patients chose their 3 most difficult activities and rated the ability to perform them on an 11-point visual numeric rating scale (VNRS). A score of 10 at the right-hand end of the VNRS was defined as “I can perform the chosen function without difficulty”, and 0 at the left-hand end as “I am unable to perform the chosen function”. A mean score across all activities was calculated and a minimum change of 3 points was considered as a clinically important improvement (18, 19). In addition, all patients completed a modified version of the Fear Avoidance Beliefs Questionnaire (FABQ) and the Pain Catastrophizing Scale (PCS); 2 factors possibly influencing our main outcome measure.
Direct and indirect healthcare costs were assessed with the shoulder log-book. Direct costs included all diagnostic and therapeutic measures paid by the German healthcare system due to the patient’s shoulder complaints. Indirect costs included days of sick leave and paid help. Demographic data, including age, sex, height, weight, profession, sports activities, severity and duration of symptoms, and previous episodes of shoulder pain were also documented. Patients were assessed at baseline, at 5, 12, and 52 weeks after inclusion in the trial. Due to the nature of the intervention, therapists and patients could not be blinded. However, patients also acting as assessors were kept naive to their allocation.
Ethical approval. Ethical approval was granted by the ethics committee of the Ludwig-Maximilians-University Munich, Germany (project-no. 018-10). All patients gave informed consent.
Trial registration. Current Controlled Trials ISRCTN86900354.
Sample size and recruitment
Power calculation resulted in an estimated sample size of 90 participants (45 per group) to detect a 13-point difference in SPADI score. The assumed standard deviation was set at 20 points based on the results of other studies (20–23). Alpha was set at 0.05, statistical power at 80%, and a dropout rate of 15% was expected.
Data analysis
Descriptive statistics for demographic and clinical characteristics for both groups and the total group were used. Working hours per week, and sick leave were analyzed only for patients who were at work.
Differences after 5 and 12 weeks were calculated for between-groups comparisons and within-groups results according to the “intention-to-treat principle”. These results have been published previously (13).
Because of the unbalanced structure of our repeated-measures design and the assumed correlation of observations in longitudinal data-sets we used a linear mixed models approach for calculating differences between baseline and our final follow-up at 52 weeks. This method uses both fixed and random effects in the same analysis. It handles naturally unbalanced data as, for example, uneven spacing of repeated measures, and allows analysis of the relationship of predictor covariates with the dependent variable. It also accounts successfully for the observed pattern of dependences in those measurements. Appropriate covariates were identified in a univariable regression analysis and from literature. Before starting the analysis, the baseline SPADI score and all identified covariates were centred by subtracting the group mean.
In a first step a fixed effects model was run and in a second step random effects were added. Insignificant covariates were then stepwise removed from the model. Model fit was assessed with the help of the Bayesian Information Criterion (BIC) and the -2Log likelihood.
Costs recorded in the shoulder log-book were valued using published prices for medical costs. Productivity costs were calculated by applying the friction costs method (24). Depending on data distribution between-group differences in outcomes of total costs were analyzed by Student’s t-tests for unpaired observations or the Mann-Whitney U test.
Results
Recruitment process
A total of 188 patients were assessed for eligibility over an 18-month period. A final total of 90 participants were randomly assigned to either IMPT or IEP. After 1 year data were available for 87 patients; 44 patients in the IMPT group and 43 in the IEP group. This process is summarized in Fig. 1. No significant differences for baseline characteristics between groups were found, except for sports hours per week, overall duration of symptoms, total FABQ, and the FABQ activity subscale. Baseline data are shown in Table II.
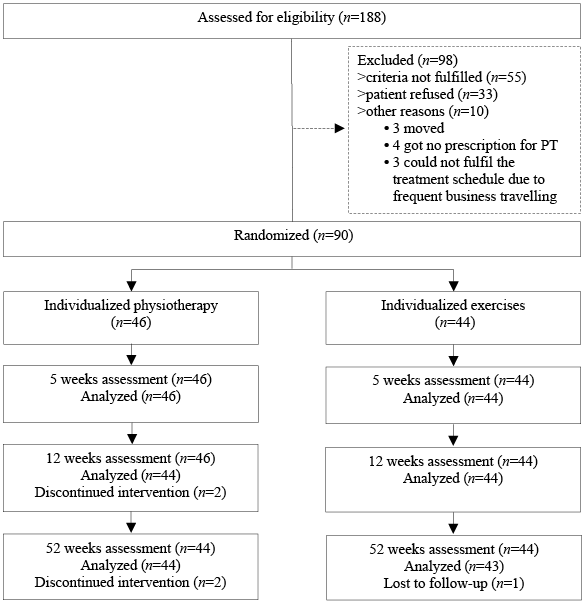
Fig. 1. Inclusion process. PT; Physiotherapists.
|
Table II. Baseline demographic data and baseline results of the questionnaires |
|||
|
Intervention (n = 46) |
Control (n = 44) |
Total group (n = 90) |
|
|
Age, years, mean (SD) |
50.1 (12.2) |
53.7 (9.9) |
51.8 (11.2) |
|
Gender, female, n (%) |
22 (47.8) |
24 (54.5) |
46 (51.1) |
|
BMI, mean (SD) |
25.3 (3.7) |
26.8 (4.3) |
26.0 (4.1) |
|
Working hours per weeka, mean (SD), [no. of patients] |
32.2 (13.8) [40] |
37.2 (10.7) [38] |
34.6 (12.6) [78] |
|
Days of sick leavea, mean (SD), [no. of patients] |
0.1 (0.6) [40] |
1.1 (4.1) [38] |
0.6 (2.9) [78] |
|
Sports hours per week, n (%) |
|||
|
0–2 h |
13 (28.3) |
21 (47.4) |
34 (37.8) |
|
3–5 h |
33 (71.7) |
23 (52.6) |
56 (62.2) |
|
Duration of the current episode, weeks, mean (SD) |
27.4 (28.4) |
40.8 (53.4) |
33.9 (42.8) |
|
Overall duration of shoulder pain, weeks, mean (SD) |
136.9 (198.5) |
71.3 (68.7) |
104.8 (152.6) |
|
Number of episodes during the last 12 months, n (%) |
|||
|
1–3 (including the current one) |
37 (80.4) |
38 (86.4) |
75 (83.3) |
|
> 3 |
9 (19.6) |
6 (13.6) |
15 (16.7) |
|
Pain score, mean (SD) |
5.2 (1.8) |
5.0 (1.8) |
5.1 (1.8) |
|
SPADI total score, mean (SD) |
39.7 (17.2) |
41.3 (17.0) |
40.4 (17.0) |
|
SPADI sub-score for pain, mean (SD) |
47.8 (18.8) |
49.6 (17.3) |
48.7 (18.0) |
|
SPADI sub-score for function, mean (SD) |
31.5 (18.6) |
32.9 (19.3) |
32.2 (18.9) |
|
GPSS score, mean (SD) |
4.1 (1.8) |
4.0 (1.7) |
4.0 (1.7) |
|
FABQ total score, mean (SD) |
36.4 (17.4) |
28.7 (16.7) |
32.7 (17.4) |
|
FABQ sub-score for physical activity, mean (SD) |
15.9 (4.1) |
13.3 (5.3) |
14.6 (4.9) |
|
FABQ sub-score for work, mean (SD) |
13.4 (10.3) |
10.8 (9.5) |
12.1 (9.9) |
|
PCS total score, mean (SD) |
12.4 (9.7) |
10.4 (7.1) |
11.4 (8.5) |
|
PET, mean (SD) |
8.4 (1.6) |
8.7 (1.3) |
8.5 (1.5) |
|
aOnly participants who are in employment. SD: standard deviation; BMI: body mass index; SPADI: Shoulder Pain and Disability Index; GPSS: Generic Patient-Specific Scale; FABQ: Fear Avoidance Beliefs Questionnaire; PCS: Pain Catastrophizing Scale; PET: Patients Expectancies of Treatment Outcome. |
|||
Shoulder log-books
From a total of 90 participants, 89 (98.9%) returned a complete log-book after 5 weeks and 85 (94.4%) after 12 weeks, of which 3 were incomplete. Within the follow-up period (weeks 13–52) 87 (96.7%) participants returned their log-books, from which another 4 in the intervention group were incomplete for analysis.
Additional medication, co-interventions and diagnostics
During the first 12 weeks more patients in the intervention group had additional treatments and diagnostic measures compared with the control group. However, co-interventions, especially the cortisone injections, had no significant influence on between-group comparisons (13).
After one year similar figures were found for additional interventions and diagnostics in both groups. However, 2 patients in the intervention group, but only 1 in the control group, underwent surgery. An overview of additional treatments and diagnostics is shown in Table III.
|
Table III. Number of patients receiving additional medication, co-interventions and diagnostics |
||||||||
|
Additional intervention |
Week 0–5 (n = 89) |
Week 6–12 (n = 85) |
Week 13–52 (n = 87) |
|||||
|
Intervention (n = 46) |
Control (n = 43) |
Intervention (n = 45) |
Control (n = 40) |
Intervention (n = 44) |
Control (n = 43) |
|||
|
Cortisone injection |
5 |
0 |
2 |
3 |
7 |
2 |
||
|
NSAIDs |
5 |
7 |
4 |
4 |
3 |
4 |
||
|
Cortisone injection + NSAIDs |
0 |
0 |
2 |
1 |
0 |
2 |
||
|
Physiotherapy (no. of treatment) |
0 |
0 |
11 (39) |
2 (12) |
10 (118) |
7 (150) |
||
|
Surgery (SAD) followed by rehabilitation |
0 |
0 |
0 |
0 |
2 |
1 |
||
|
Massage (no. of treatment) |
1 (1) |
2 (12) |
0 |
1 (3) |
0 |
2 (18) |
||
|
Soothing ointment |
1 |
1 |
1 |
2 |
0 |
2 |
||
|
Electrotherapy (no. of treatment) |
1 (5) |
0 |
0 |
0 |
0 |
0 |
||
|
X-ray |
1 |
0 |
0 |
0 |
0 |
0 |
||
|
MRI |
3 |
1 |
1 |
0 |
4 |
4 |
||
|
GP clinical assessment |
0 |
0 |
1 |
0 |
3 (9) |
1 |
||
|
Ultrasound |
0 |
0 |
0 |
1 |
1 |
1 |
||
|
NSAIDs: non-steroidal anti-inflammatory drugs; MRI: magnetic resonance imaging; GP: general practitioner. |
||||||||
Direct costs
During the first 5 weeks basic costs for the prescribed physiotherapy interventions differed between groups (intervention group: 188€ per patient for a prescription of manual therapy; control group: 171€ for a prescription of physiotherapy exercises). A total of only 21 (26.9%) patients were responsible for all additional costs, with 13 (16.7%) being in the intervention group. Total direct costs differed significantly (p = 0.03) in favour of the control group at 5 weeks. However, no differences could be found after 12 and 52 weeks, or for overall directs costs between groups. These results are shown in Table IV.
|
Table IV. Direct costs in Euros (SD) and between-group differences |
||||
|
Intervention Mean (SD) |
Control Mean (SD) |
Difference between groups |
||
|
Mean (SE) |
p- value |
|||
|
Week 0–5 (n = 89) |
209.3 (49.6) |
185.7 (50.4) |
23.6 (10.6) |
0.03* |
|
Week 6–12 (n = 84) |
30.4 (68.2) |
14.0 (28.3) |
16.4 (11.2) |
0.15 |
|
Week 13–52 (n = 87) |
167.9 (518.7) |
127.2 (447.0) |
40.6 (103.9) |
0.70 |
|
Week 0–52 (n = 84) |
408.5 (545.8) |
332.7 (472.2) |
75.8 (111.9) |
0.5 |
|
*p < 0.05. SD: standard deviation; SE: standard error. |
||||
Indirect costs
Indirect costs were analyzed only for patients who were in employment and could be calculated for 78 patients after 5 weeks, for 73 patients after 12 weeks, and for 75 patients after one year. Cost calculations for sick leave were based on the average daily working hours of the patient and the average hourly labour costs in Germany (25). Only a few patients were responsible for all days of sick leave (n = 10; female = 5, male = 5; mean (SD) age in years 54.2 (11.0)) and most of the costs during the evaluation period. Only 1 patient in the intervention group used paid help.
During the 5-week treatment period 7.7% (n = 6) of patients who were in employment (n = 78) were responsible for all days of sick leave. Only one patient from the intervention group had 12 sick days, compared with 5 patients from the control group with a total of 58 days. Similar results were found for weeks 7–12. During the 1-year follow-up 2 patients in the intervention and 3 in the control group were on sick leave. Between-group differences for sick leave and indirect costs were analyzed with the Mann-Whitney U test for non-normally distributed data (tested with the Kolmogorov-Smirnov Z). However, neither days of sick leave nor indirect costs differed significantly between groups at any time. These results are shown in Table V.
|
Table V. Days of sick leave and indirect costs in Euros (SD) for patients who were in employment |
||||||||
|
Week 0–5 (n = 78) |
Week 6–12 (n = 73) |
Week 13–52 (n = 75) |
Week 0–52 (n = 73) |
|||||
|
Intervention (n = 40) |
Control (n = 38) |
Intervention (n = 38) |
Control (n = 35) |
Intervention (n = 38) |
Control (n = 37) |
Intervention (n = 38) |
Control (n = 35) |
|
|
Days, no. of sick days (no. of patients) |
12 (1) |
58 (5) |
4 (1) |
41 (3) |
30 (2) |
66 (3) |
46 (3) |
165 (3) |
|
p-value |
0.82 |
0.25 |
0.60 |
0.13 |
||||
|
Total costs (no. of patients) |
838 (1) |
5,400 (5) |
571 (2) |
4,205 (3) |
2,954 (2) |
7,109 (3) |
4,363 (4) |
16,714 (7) |
|
Costs, mean (SD) |
20.9 (132.4) |
142.1 (528.0) |
15.0 (69.1) |
120.1 (401.0) |
77.7 (444.3) |
192.1 (741.5) |
114.8 (521.4) |
477.5 (1,292.0) |
|
Differences between groups mean (SE) |
–104.5 (76.4) |
–89.6 (58.9) |
–98.2 (121.6) |
–308.5 (201.7) |
||||
|
p-value |
0.18 |
0.14 |
0.42 |
0.13 |
||||
|
SD: standard deviation |
||||||||



Effectiveness analysis
SPADI total score. Over the 1-year period both groups improved significantly in total SPADI score, its sub-scores for pain and for function and the GPSS. These results are shown in Table VI–VII.
To identify the influence of group allocation, baseline SPADI and pain scores, overall duration of symptoms, the FABQ activity sub-score, the PCS total score, and of the time factor on our primary outcome measure, we included them into our mixed model analysis for the total SPADI score. During the analysis group allocation was kept in the model because of our primary research question. The final model included random intercepts for subjects and fixed effects for group allocation, duration of symptoms, baseline SPADI score and the time factor in weeks.
Subject heterogeneity accounted for part of the residual variability (estimated intra-class correlation 37.9%; Wald z = 4.11, p = 0.000). Group allocation did not significantly influence the result of our main outcome measure (p = 0.38, 95% CI = –7.45 to 2.85).
|
Table VI. Results after 5, 12, and 52 weeks |
||||||||
|
Outcomes |
Week 0 |
Week 5 |
Week 12 |
Week 52 |
||||
|
Intervention (n = 46) Mean (SD) |
Control (n = 44) Mean (SD) |
Intervention (n = 46) Mean (SD) |
Control (n = 44) Mean (SD) |
Intervention (n = 44) Mean (SD) |
Control (n = 44) Mean (SD) |
Intervention (n = 44) Mean (SD) |
Control (n = 43) Mean (SD) |
|
|
SPADI (0–100) |
39.7 (17.2) |
41.3 (17.0) |
23.5 (17.5) |
26.8 (17.8) |
16.1 (17.2) |
19.8 (19.5) |
15.3 (20.3) |
10.2 (15.2) |
|
Pain SPADI (0–100) |
47.8 (18.8) |
49.6 (17.3) |
29.8 (21.1) |
31.5 (18.8) |
20.1 (19.7) |
24.1 (21.7) |
17.7 (21.8) |
12.4 (16.9) |
|
Function SPADI (0–100) |
31.5 (18.6) |
32.9 (19.3) |
17.1 (15.0) |
22.1 (18.1) |
12.1 (15.4) |
15.5 (18.1) |
12.9 (19.4) |
7.7 (14.1) |
|
GPSS (0–10) |
4.1 (1.8) |
4.0 (1.7) |
7.1 (2.0) |
6.3 (2.0) |
7.3 (2.5) |
7.4 (2.0) |
7.9 (2.6) |
8.6 (1.8) |
|
Shoulder Pain and Disability Index; GPSS: Generic Patient-Specific Scale; SD: standard deviation. |
||||||||



|
Table VII. Results after 5, 12, and 52 weeks for within-groups comparison |
||||||||
|
Outcomes |
Difference within groups at 5 week |
Difference within groups between 5 and 12 weeks |
Difference within groups between 12 and 52 weeks |
Difference within groups between 0 and 52 weeks |
||||
|
Intervention (n = 46) Mean (SD) [95% CI] |
Control (n = 44) Mean (SD) [95% CI] |
Intervention (n = 44) Mean (SD) [95% CI] |
Control (n = 44) Mean (SD) [95% CI] |
Intervention (n = 44) Mean (SD) [95% CI] |
Control (n = 43) Mean (SD) [95% CI] |
Intervention (n = 44) Mean (SD) [95% CI] |
Control (n = 43) Mean (SD) [95% CI] |
|
|
SPADI (0–100) |
16.2 (18.2)** [10.8–21.6] |
14.4 (17.1)** [9.2–19.6] |
7.5 (12.3)** [3.7–11.2] |
7.0 (13.8)* [2.8–11.2] |
0.8 (18.0) [–4.6–6.3] |
9.4 (15.2)** [4.8–14.1] |
25.2 (21.5)** [18.7–31.7] |
31.5 (16.5)** [26.5–36.6] |
|
Pain SPADI (0–100) |
18.0 (20.2)** [12.0–24.0] |
18.0 (21.4)** [11.5–24.5] |
9.8 (15.2)** [5.2–14.4] |
7.4 (16.6)* [2.4–12.5] |
2.4 (18.1) [–3.1–8.0] |
11.3 (16.8)** [6.1–16.5] |
31.1 (22.5)** [24.2–37.9] |
37.4 (18.8)** [31.6–43.2] |
|
Function SPADI (0–100) |
14.4 (18.8)** [8.8–20.0] |
10.8 (15.8)** [6.0–15.6] |
5.1 (10.8)* [1.9–8.4] |
6.7 (12.6)** [2.8–10.5] |
–0.8 (19.3) [–6.7–5.1] |
7.6 (14.7)* [3.1–12.1] |
19.3 (23.0)** [12.3–26.3] |
25.7 (17.7)** [20.2–31.1] |
|
GPSS (0–10) |
3.0 (2.3)** [2.3–3.7] |
2.3 (2.2)** [1.6–3.0] |
0.3 (1.8) [–0.27– 0.81] |
1.1 (2.0)** [0.5–1.7] |
–0.6 (1.9) [–2.0–0.1] |
1.2 (2.1)** [0.5–3.6] |
3.9 (2.8)** [3.1–4.8] |
4.6 (2.8)** [3.9–5.3] |
|
*p = 0.01; **p = 0.001. SPADI: Shoulder Pain and Disability Index; GPSS: Generic Patient-Specific Scale; SD: standard deviation. |
||||||||
|
Table VIII. Total group numbers (percentage) of patients with a clinically important change for every outcome measure |
|||
|
Outcomes |
Week 5 (n = 90) n (%) |
Week 12 (n = 88) n (%) |
Week 52 (n = 87) n (%) |
|
Total SPADI score (> 10) |
51 (56.7) |
67 (76.1) |
72 (82.8) |
|
GPSS (> 2) |
39 (43.3) |
60 (68.2) |
68 (78.2) |
|
PGIC (slightly and much better) |
79 (87,8) |
81 (92.1) |
79 (90.8) |
|
PGIC (much better) |
42 (46.7) |
50 (56.8) |
67 (77.0) |
|
SPADI: Shoulder Pain and Disability Index; GPSS: Generic Patient-Specific Scale; PGIC: Patient’s Global Impression of Change. |
|||
PGIC and patients with a MCID after one year. The number of patients with a clinically important difference as defined a priori, and the number of patients who rated their treatment as a success increased progressively over the observation period (Table VIII). Because no differences between groups could be found at any follow-up point, numbers are given only for the total group.
Adverse events
One patient had a 12-point deterioration and another patient a 38-point deterioration after an accident involving the shoulder.
Discussion
This randomized controlled trial examined the long-term effect of individualized physiotherapy combined with individualized exercises in comparison with individualized exercises alone on pain and function in patients with clinical signs of shoulder impingement syndrome.
Both groups improved significantly during the 1-year follow-up period, but there were no differences between groups in terms of costs or any of the outcome measures. These results bring into question the additional benefit of individualized manual physiotherapy.
Direct and indirect costs
Patients from the intervention group underwent more additional interventions and diagnostics during the first 5 weeks of the intervention phase than did the controls. This finding was contrary to our expectations that the more intensive therapeutic contact and the more tailored education in the intervention group would have resulted in a reduction in additional measures. However, we could not completely control the influence of general practitioners and orthopaedic surgeons on these decisions. Although indirect costs did not differ significantly between groups, the difference between the absolute amounts of money was notable, with much higher costs in the control group (Table V), and this may therefore also influence therapeutic decisions. In comparison, the significant differences in direct costs at 5 weeks become less important.
Effectiveness analysis
SPADI score. Both groups showed a significant improvement in SPADI score over the follow-up period. However, our mixed models analysis showed that group allocation, and therefore the IMPT, had no influence on these results (p = 0.38).
While the intervention group showed no further improvement during the final follow-up period, a remarkable improvement was seen in the control group in total SPADI score. This development is difficult to explain. We can hypothesize that patients from the control group may have established a clearer association between exercising and improvement in complaints, and therefore a stronger belief in the effectiveness of their exercises. They may have then restarted their exercises more quickly when complaints recurred.
MCID after one year in the primary outcome measures. The number of patients with a clinically important improvement in total SPADI score increased progressively during the observation period, with a peak of approximately 83% (n = 72) for the total group in the final assessment. One may argue that patients with a high SPADI baseline score had a better chance of improving by more than 10 points than patients with a comparably low score at baseline. Therefore, when comparing these results with an analysis based on a minimum change of 30% of the initial score (instead of the absolute MCID of 11 that we defined a priori) (26), we can see that 87% (n = 76) of the total group showed a 30% improvement or more in the SPADI baseline score and 81% (n = 70) of 50% or more, respectively. For a concept that accounts for the baseline score, a large improvement in patients with high baseline scores is needed to reach this cut off, in contrast to patients with relatively low baseline scores. However, the similar results from both concepts and the ongoing improvement over time seen in our patient group both support the suggestion to delay further treatments until approximately one year after physiotherapy for SIS. This positive development is also very well reflected in the patients’ impression of change (PGIC), with 91% (n = 79) being “slightly and much improved” at one year. Although at first appearance results for the PGIC appear to remain unchanged over time, the positive development becomes obvious in the increasing percentages of patients scoring the development of their complaints as “much improved”, increasing from 47% (n = 42) at 5 weeks up to 77% (n = 67) after 1 year.
Comparisons with other studies. Few data are available about the additional effect of manual physiotherapy over exercises alone in patients with SIS. Earlier studies have reported short-term results, but none of them have presented long-term results (27–29). Our results suggest that IMPT is of no additional effect in the long term, but the significant and progressive improvement of both of our groups may support the positive effect of exercises in SIS, not seen in groups treated with sham or no treatment (30–32), and different from the natural course of shoulder complaints over time described in the literature (33). Based on this evidence we examined studies with a follow-up of 1 year or longer, comparing exercises that we used as the basic treatment in both of our groups, with other physiotherapeutic measures or surgery in patients with SIS. Engebretsen et al. (34, 35) compared exercises with shock-wave therapy, 2 clearly different types of interventions. Both of their groups showed a significant improvement in total SPADI, but no difference between groups after 1 year. Similar results were reported by Beaudreuil et al. (36), who compared a supervised dynamic humeral centring training with a supervised non-specific mobilization programme. Dorrestijn et al. (37) published a systematic review summarizing studies comparing physiotherapy or exercises with surgery; even between these interventions no differences in pain or functioning could be found in the long term. These results are confirmed in a randomized controlled trial by Ketola et al. (38). Interestingly, in most of the studies surgery was followed by an exercise programme which made it impossible to analyze the actual contribution of surgery to the results. Cummins et al. (39) followed a cohort of 100 patients treated with single corticosteroid injections and a 4-week period of physiotherapy followed by home exercises. Within a 2-year observation period 79% of a SIS group did not require surgery. The orthopaedic surgeon offered surgery as an appropriate intervention to many patients in our sample, even if patients had not had physiotherapy as an initial treatment beforehand. As we did not systematically collect data on recommendations made prior to inclusion in this trial, no clear statement can be made. Of the 3 patients who underwent surgery after the intervention phase, 1 had a total SPADI score of 12 points and a 19-point improvement from baseline. The decision regarding surgery in this case was perhaps based on reasons other than the objective functional status of the patient. Overall, it seems to be difficult to justify recommending surgery as an initial treatment for SIS, because this has not been shown to deliver better results than physiotherapy in the short and long term.
Since similar results after one year are seen with different exercise protocols, shock-wave therapy or surgery, it is debatable whether the planned intervention itself is solely responsible for the improvement. The question arises as to which other mechanisms, shared by all these interventions, contribute to the overall improvement. A recent systematic review by Chester et al. (40) identified low baseline disability and a short duration of symptoms as the 2 most important predictors for a good outcome in patients with musculoskeletal shoulder pain. This is in accordance with our mixed model results with duration of symptoms and the initial SPADI score as the 2 remaining baseline variables with a significant influence on outcome. At baseline our group started with a relatively low mean (SD) SPADI sub-score for function (32.2 (18.9)), but had a comparably long mean (SD) duration of symptoms (104.8 (152.6) weeks). The good overall improvement after one year may indicate that duration of symptoms may have had less influence on outcome than the baseline SPADI score; however, further research is needed to answer this question.
Strengths and limitations of the study. This study has sufficient power because of the low dropout rate and a standard deviation around the mean SPADI score below the standard deviation used for power calculation. In addition to a sound statistical analysis controlling for possible confounders, covariates and time, further data about additional medication, diagnostics, co-interventions and sick leave are given, enabling the reader to draw a comprehensive picture of the patient group. Both interventions are described in detail and can therefore be reproduced easily.
For ethical and practical reasons it was not possible for us to include a placebo group. We therefore could not analyze the contribution of the natural course of the shoulder disorder to the improvement. However, other studies found a significant difference between exercise treatment and placebo or no intervention. Due to the nature of our interventions and outcome measures it was not possible to blind either therapists or patients to the study protocol, but patients were kept naive to group allocation. Since there was no difference between groups, the influence of therapists’ beliefs about the applied treatments or the longer contact times in the intervention group seemed not to be relevant.
Implications for further research. Further research is required to allow a definite conclusion to be drawn about the effect of individualized manual physiotherapy in this context. For the sake of comparability, a standard procedure for the assessment of patients with shoulder pain is required. Furthermore, eligibility criteria should be set according to the clinical pattern instead of structure-based diagnoses. When the effect of different interventions is investigated in clinical trials, potential prognostic factors should be analyzed in order to clarify their importance and contribution to baseline scores and treatment effects. These factors could then be addressed therapeutically to reinforce or reduce their impact on outcome.
Clinical implications. Data from this study indicate that there is a chance of further improvements in pain and functioning over time after the intervention has ceased. In conclusion, after physiotherapy patients should be observed for about 3 to 4 month before another treatment is tested out.
Conclusion
To our knowledge this is the first study to present long-term results for an additional effect of individualized manual physiotherapy compared with exercises alone in patients with shoulder impingement syndrome. Although the results of this study suggest that additionally applied manual interventions are of no benefit, this must be confirmed by further research before a clear statement can be made. Exercises should be considered a basic treatment, because they are less expensive and carry less risk than, for example, shock-wave therapy or surgery. The cost for exercises is also less than for manual physiotherapy in the initial stages of treatment. In conclusion, due to the ongoing improvement over the follow-up period, it is advisable to wait for some time after the intervention to allow for possible improvement before considering further treatments, especially surgery.
References
