Mark Laslett, PhD1, Michael Steele, PhD2, Wayne Hing, PhD1,3, Peter McNair, PhD1 and Angela Cadogan, PhD1
From the 1Health & Rehabilitation Research Institute, Auckland University of Technology, Auckland, New Zealand
2Department of Mathematical and Computing Sciences, Universiti Brunei Darussalam, Brunei, Griffith Graduate Research School, Griffith University, Gold Coast, Australia 3Faculty of Health Science & Medicine, Bond University, Queensland, Australia
OBJECTIVE: Measure changes in pain and disability of primary care shoulder pain patients over a 12-month period.
DESIGN: A non-randomized audit with repeated measures of pain and disability at 3 weeks, 3, 6 and 12 months.
Patients: Of 208 patients, 161 agreed to participate with 96.9%, 98.1%, 86.3%, 83.9% follow-up at 3 weeks, at 3, 6 & 12 months, respectively. Mean age was 44 years, mean symptom duration 3.6 months.
METHODS: Patients were treated with protocol driven corticosteroid injection and community based care. Primary outcome measure was the Shoulder Pain and Disability index (SPADI) questionnaire. Based on the SPADI and minimal clinically important difference (MCID), outcomes were categorized into: total recovery, 90% or more improved, better, unchanged and worse.
RESULTS: There was significant reduction of pain and disability at 3 weeks (p < 0.001), no change at 3 and 6 months and a significant reduction at 12 months follow-up (p < 0.001). Excellent outcomes were achieved by 32.9% and 45.3%, and a poor clinical outcome resulted for 32.8% and 14.9% at 3 and 12 months follow-up, respectively.
CONCLUSION: Though there was significant improvement at the 3 week and 12 month follow-up, 45% achieved an excellent outcome and a 16.7% of patients were the same or worse than baseline at 12 months.
Key words: shoulder pain; diagnosis; follow-up studies; primary health care; corticosteroid injection.
J Rehabil Med 2014; 46: 00–00
Guarantor’s address: Mark Laslett, 7 Baltimore Green, Shirley, Christchurch, 8061, New Zealand. E-mail: mark.laslett@xtra.co.nz
Accepted Apr 28, 2014; Epub ahead of print Aug 6, 2014
Introduction
Shoulder pain is a common and disabling complaint. The reported annual incidence of shoulder pain in primary care is 14.7 per 1,000 patients per year (1) with a lifetime prevalence of up to 70% (2). Recovery from shoulder pain can be slow and recurrence rates are high with 25% of those affected by shoulder pain reporting previous episodes, and 40 to 50% reporting persisting pain or recurrence at 12 months follow-up (3–5).
Guidelines for physiotherapy interventions exist (6) and there are broader recommendations and protocols available (7–19), but most general reviews are over 10 years old or refer to specific conditions or methods of treatment. With the exception of two guidelines (7, 18), recommendations are based on practice models in Europe and North America and are focused on management by general medical practitioners and surgical specialists. In New Zealand and Australia, initial contact with the health system for patients with musculoskeletal pain is usually a general medical practitioner (GP) or a physiotherapist in the private practice or acting as a triage clinician in a public hospital environment. There is limited evidence regarding the optimal diagnostic and therapeutic management protocol and no available data on the efficacy of the approach or outcomes associated with application of guideline informed principles (18, 17). The nature of care in the first 3 months after contact is likely quite heterogeneous and information about outcomes is lacking.
While outcomes of management of shoulder pain have been studied (5, 19–27), the heterogeneity of management protocols in these studies and in New Zealand primary care confound an understanding of reasonable expectations for recovery, need for imaging, the value of injection therapies, and surgery in the New Zealand environments. There is a need for basic data on outcomes associated with guideline informed community based care of shoulder pain presenting to primary care generally and in Australasia. The aim of this study was to document outcomes in shoulder pain patients over a 12-month period following a comprehensive and standardized diagnostic work up, management with community based care and a standardized protocol for steroid injection. Part 2 of this report presents predictive modelling for outcomes at 3 and 12 months follow-up.
methods
Consecutive patients presenting to their GP or physiotherapist for the first time with a new episode of shoulder pain were invited to participate in a diagnostic study during which they received a series of baseline diagnostic tests that included a standardised clinical examination, shoulder x-ray series, diagnostic ultrasound scan and diagnostic injections of local anaesthetic into the subacromial bursa (SAB) and acromioclavicular joint (ACJ). Those not reporting a positive anaesthetic response (PAR) (≥ 80% reduction of pain about 15 min post procedure) to the SAB and ACJ diagnostic injections, also received an intra-articular glenohumeral joint (GHJ) injection as part of a MR arthrogram procedure. These procedures are described in detail elsewhere (28–31). Following completion of the diagnostic tests, all participants were invited to participate in a 12-month follow-up study. Study approval was acquired from The New Zealand Ministry of Health Regional Ethics Committee and informed consent was acquired at an initial appointment prior to commencement of the follow-up study. Those patients reporting a PAR in post-injection pain intensity following one of the diagnostic injections were offered a therapeutic corticosteroid injection (CSI) to be delivered to the structure(s) identified by anaesthetic response using either ultrasound or fluoroscopic guidance.
Patients were followed up at 3 weeks, 3, 6 and 12 months. All follow-up assessments were carried out by the lead author (ML) except for two that were carried out by a co-author (AC). For patients receiving guided CSI procedures, the first follow-up assessment occurred approximately three weeks after the CSI procedure during which time firm instructions to engage in “relative rest”, i.e. no vigorous shoulder activity or high loads, was emphasized. At the 3 week follow-up, patients were referred back to the referring clinician for on-going management. All management after the three week follow-up was uncontrolled and followed usual community based conservative care in the form of physiotherapy (exercise, soft tissue and joint mobilisation, guidance on return to usual work and physical activities), corticosteroid injection or no treatment at all. Documentation of received treatments were based on patient self-reports only. No formal advice was given to patients by the researchers after the 3-week follow-up.
Outcome measures
Measures used at baseline, 3 weeks, 3, 6 and 12 months follow-up:
1. Shoulder Pain and Disability Index (SPADI) questionnaire (32,33). This questionnaire has 5 pain- and 8 function-related questions scored from 0 to 10 where 0 = no pain or disability and 10= maximum pain or disability. These scores generate pain and disability subscales and a total score, which were all percentalized.
2. Three 100-mm visual analogue scales (VAS) for pain intensity (pain at lowest, mean and worst in last 48 h) (34)
3. Fear Avoidance Beliefs Questionnaire (FABQ). This questionnaire generates “general”, “physical activity” and “work” subscales and a total score is also calculated. (35)
4. Short Form 8 item general health questionnaire (SF-8) with two subscales: Physical Component Score (PCS) and Mental Component Score (MCS) used to reflect two distinct dimensions of general health (36)
5. Global disability rating scale (33). This Likert scale has 7 options:
“No disability”, “Almost no disability”, “Minimal disability”, “Some disability”, “Moderate disability”, “A lot of disability”, and “Maximum disability” which were coded 1–7, respectively.
Data analysis
Baseline and all follow-up assessment data were acquired on standardized forms and data transferred to a SPSS version 20 database. All analyses were carried out using SPSS statistical software with the alpha level set to p < 0.05. To determine which baseline variables were significantly associated with SPADI results, correlations were calculated for continuous baseline variables and independent sample t-tests or 1-way ANOVAs were calculated for discrete baseline variables. A repeated measures ANOVA was used to determine if the SPADI scores changed significantly over the 5 time periods.
The minimally clinically important difference (MCID) for the primary outcome measure, the SPADI Total (%) score, was set at +/– 13 percentage points (37). Typically, patients seek complete recovery from pain and a return to normal activities, but this has been reported to be achieved in only 50% by 6 months (21). In this study we created categories enabling identification of those achieving clinically relevant outcomes. The MCID was used to identify those cases achieving less than highly satisfactory outcomes. The categories were based on changes in SPADI Total percentage scores between assessments:
1. Excellent outcome: Score reduced to zero between assessment points or improvement was 90% or more from baseline;
2. Better: Percentage reduction in score larger than the MCID but less than 90%;
3. Poor outcome: Change (increase or decrease) in score was less than the MCID or increase in score was more than the MCID.
All appropriate variables were considered for possible inclusion into predictive models of 12 month outcome, and were identified in two ways:
1. Those variables associated with SPADI Total score at 3 and 12 months (p ≤ 0.2)
2. All other variables in our dataset that measured a similar conceptual construct as variables identified in previous research (5, 21) as predictive of outcome at any time point were included. See Appendix I for full list of initial selection of variables.
All potential predictor variables were evaluated separately in univariate logistic regressions with the dependent variables being the three different clinical outcomes: excellent; better; poor. Statistical significance was set where p < 0.05.
Results
Two hundred and eight participants were enrolled in the diagnostic study and received baseline diagnostic tests. One hundred and sixty-one subjects (77.4%) agreed to participate in the follow-up study with 156 (96.9%) followed up at 3 weeks, 158 (98.1%) at 3 months, 139 (86.3%) at 6 months and 135 (83.9%) at 12 months (Fig. 1). There was no significant gender difference between those declining participation and those enrolling in the follow-up study (Pearson Chi-square p = 0.2). One way ANOVA of mean values for baseline age, SPADI subscale and total scores, SF-8 mental and physical component scores, mean and worst pain VAS scores, FABQ Work general and total scores were not significantly different between participants and non-participants (p > 0.1). Mean FABQ Physical Activity subscale and the VAS score for lowest pain intensity in the preceding 48 h were lower (p = 0.005 and 0.02, respectively) for those declining participation. Table I presents basic demographic profiles for included patients. Table II presents mean values for key outcome measures taken at baseline, 3 weeks, 3, 6 and 12 months. During the 12 month follow-up period, some patients were not followed up for a variety of reasons. Three patients withdrew because of time constraints, one patient withdrew because the researchers were not ‘doing anything’. The remainder of the cases where data are missing reflect patients failing to show for scheduled appointments and being unable to be contacted. In these cases multiple attempts over several weeks were made to contact patients and often contact was re-established.
Fig. 1. Diagram of flow of patients through diagnostic and follow-up studies. LA: guided local anaesthetic injection; CSI: corticosteroid injection; PAR: positive anaesthetic response, i.e. ≥ 80% relief of pain. Source participants derived from Cadogan 2011 (28).
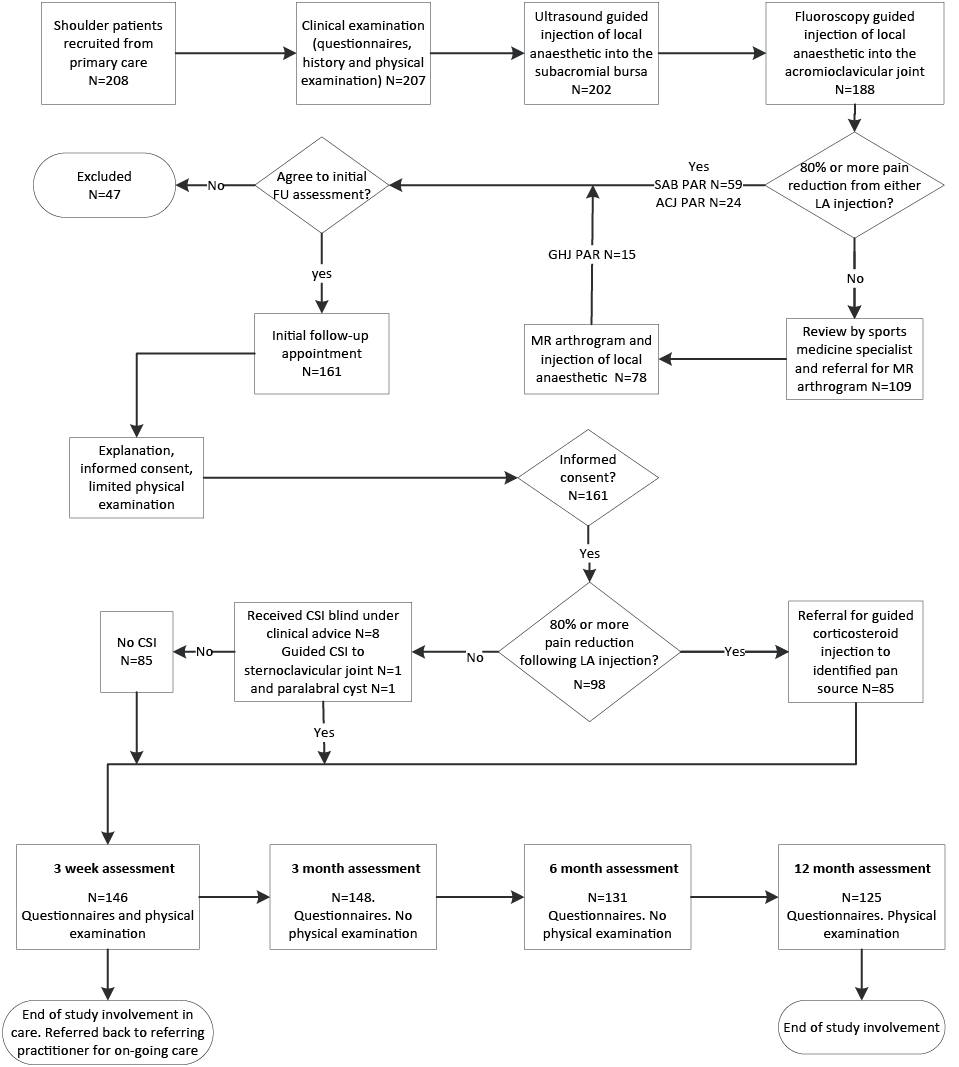
Table I. Demographic profile of patients included in shoulder pain follow-up study |
||||||
|
n (%) |
Missing, n |
Min–Max |
Mean (SD) |
||
Age, years |
161 |
0 |
18–81 |
44.00 (13.88) |
||
Duration of symptoms, days |
158 |
3 |
2–1,697 |
108.35 (193.88) |
||
SPADI Pain subscale, % |
161 |
0 |
0–100 |
50.56 (21.72) |
||
SPADI Disability subscale, % |
161 |
0 |
0–93 |
30.25 (21.10) |
||
SPADI Total, % |
161 |
0 |
0–94 |
38.03 (20.11) |
||
Pain intensity in last 48 h, 100 mmVAS |
||||||
Lowest |
157 |
4 |
0–66 |
7.30 (14.33) |
||
Mean |
157 |
4 |
0–84 |
36.35 (20.65) |
||
Worst |
159 |
2 |
0–100 |
62.42 (23.50) |
||
Global Disability Rating Score (1–7) |
161 |
|
1–6 |
3.64 (1.45) |
||
FABQ General Score, % |
155 |
6 |
0–74 |
25.11 (22.68) |
||
FABQ Physical Activity Score, % |
158 |
3 |
0–100 |
61.89 (23.37) |
||
FABQ Total (Work + Physical Activity Subscales), % |
158 |
3 |
0–78 |
27.01 (16.69) |
||
FABQ Work Score, %) |
156 |
5 |
0–82 |
38.48 (18.60) |
||
SF-8 Mental Component Score |
160 |
1 |
0–61 |
44.02 (8.64) |
||
SF-8 Physical Component Score |
160 |
1 |
0–66 |
52.38 (9.07) |
||
Male gender |
79 (49.1) |
0 |
||||
Right side affected |
88 (54.7) |
0 |
||||
Dominant right hand |
142 (88.2) |
0 |
||||
Diabetes |
– (0.0) |
0 |
||||
Heart disease |
3 (1.9) |
3 |
||||
Asthma |
29 (18.0) |
4 |
||||
Smoker |
31 (19.3) |
4 |
||||
Pain at night |
87 (54.0) |
7 |
||||
Unable to sleep on painful side |
87 (54.0) |
10 |
||||
Occupational demand on shoulder |
– (13.0) |
21 |
||||
Low |
52 (32.3) |
|
||||
Moderate |
50 (31.1) |
|
||||
High |
38 (23.6) |
|
||||
Sport/recreational demand on shoulder |
– (13.7) |
22 |
||||
Low |
28 (17.4) |
|
||||
Moderate |
37 (23.0) |
|
||||
High |
74 (46.0) |
|
||||
Description of current episode |
– (0.7) |
1 |
||||
Recurrent episode |
132 (82.0) |
|
||||
New episode |
28 (17.4) |
|
||||
Mechanism/cause of pain onset |
– (1.2) |
2 |
||||
Trauma |
57 (35.4) |
|
||||
Strain |
70 (43.5) |
|
||||
Repetitive/overuse |
16 (9.9) |
|
||||
Unknown/spontaneous |
16 (9.9) |
|
||||
Employment |
– (2.5) |
4 |
||||
In paid employment |
128(79.5) |
|
||||
Retired |
13(8.1) |
|
||||
Home with children/maternity leave |
2 (1.2) |
|
||||
Unable to work/sickness benefit |
1 (0.6) |
|
||||
Unemployed |
4 (2.5) |
|
||||
Student |
8 (5.0) |
|
||||
Other |
1 (0.6) |
|
||||
Min: Minimum; Max: Maximum; SD: standard deviation; SPADI: Shoulder Pain and Disability Index; VAS: visual analogue scale; FABQ: Fear Avoidance beliefs Questionnaire; SF-8: Short Form 8. |
||||||
Table II. Outcome measure scores from baseline to 12 month follow-up |
|||||
Baseline Mean (SD) |
3 week FU Mean (SD) |
3 Month FU Mean (SD) |
6 month FU Mean (SD) |
12 month FU Mean (SD) |
|
SPADI Pain % |
50.6 (21.72) |
21.9 (23.13) |
24.6 (25.42) |
24.2 (25.28) |
15.0 (21.34) |
SPADI Disability % |
30.2 (21.10) |
12.3 (17.89) |
12.8 (19.12) |
12.6 (18.13) |
7.0 (14.47) |
SPADI Total % |
38.0 (20.11) |
16.1 (19.22) |
17.5 (20.96) |
17.0 (20.16) |
10.1 (16.43) |
100 mm Pain VAS |
|||||
Lowest |
7.3 (14.33) |
4.7 (12.12) |
4.8 (11.46) |
3.6 (10.51) |
1.8 (7.26) |
Mean |
36.4 (20.65) |
13.2 (20.02) |
14.7 (20.41) |
15.0 (21.56) |
8.4 (17.65) |
Worst |
62.4 (23.50) |
25.3 (29.85) |
27.9 (30.48) |
29.2 (32.27) |
17.3 (28.13) |
Global Disability Rating (1–7) |
3.6 (1.45) |
2.4 (1.52) |
2.3 (1.57) |
2.4 (1.68) |
1.7 (1.23) |
Visual analouge scale (VAS) scores are the subjects rating from 0 to 100 in previous 48 h, where 0 = no pain and 100 = worst imaginable pain. FU: follow-up; SD: standard deviation. |
|||||
Of the 161 patients enrolled, 37%, 15% and 9% reported a PAR to diagnostic injection to the SAB, ACJ and GHJ, respectively. There was no significant gender difference between those reporting a PAR or negative anaesthetic response (NAR) (Pearson chi-square p > 0.3). One way ANOVA analyses of SPADI Total and subscales, pain intensity VAS scores, SF-8 PCS and MCS, Global Disability rating, and FABQ total and subscales for those reporting PAR from at least one structure injection, showed no significant differences from those reporting a negative anaesthetic response (NAR) (p > 0.1).
The majority of patients were managed with physiotherapy interventions (mostly exercise, mobilization and guidance on activity modification), and corticosteroid injection. Corticosteroid injection (CSI) was arranged at the initial interview by the principal author (ML) in accordance with reported PAR, i.e. ≥ 80% pain reduction criterion used to identify the source of pain (28). Fifty-five, 18 and 16 patients received guided CSI to the SAB, ACJ and GHJ, respectively. Of the 13 patients with both SAB and ACJ sources of pain, one received guided CSI to both structures, 4 and 6 received CSI to the ACJ and SAB respectively based on the clinical judgement of the lead author or Sports Medicine specialist, and two declined further injections. One patient received guided CSI into a paralabral synovial cyst and another, an injection into the sternoclavicular joint. After the 3 weeks follow-up, two patients were managed by rheumatology specialists. Other interventions used were: hydraulic distension combined with CSI to the glenohumeral joint (n = 4), aspiration followed by CSI to synovial cysts associated with labral pathology (n = 2), fenestration and barbotage to calcific tendinopathies in conjunction with CSI to the subacromial bursa (n = 2), and one patient received prolotherapy. By the 12 month follow-up assessment, 29 patients had been offered and 19 had undergone surgical intervention. At the 12-month assessment 5 were scheduled to have surgery. Insurance coverage for surgery was declined in 3 cases so it did not proceed, and one patient chose not to proceed because of clinical improvement. To determine if there were significant baseline differences between those patients offered or had received surgery compared with those not considered surgical candidates, one way ANOVA analyses were carried out on relevant measures (SPADI Total % and Pain and Disability sub-scales, pain intensity VAS scores, Global Disability Rating, FABQ Total and subscales, SF-8 Mental and Physical Component scores, and age). Only baseline FABQ Physical Activity subscale and Total scores were significantly higher for the surgical group (p > 0.02 and 0.03, respectively). However, with the exception of SF-8 mental component and physical component scores at 3 weeks, the physical component scores at 3 months and both component scores at 6 months, all scales of the SPADI questionnaire were significantly higher for the surgical group at 3 week, 3 month and 6 month follow-up (p < 0.05).
Physiotherapy treatment
Of the 152 patients where data are available, 76.6% received physiotherapy (mean number of treatments 6.9, (standard deviation (SD) 8.91)). During the 12-month study period, 39.5% received between one and 5 treatments, and 29.6% received 15 or more treatments. The maximum number of treatments was 55, with 3 surgical patients (2.6%) receiving more than 40 pre and post-operative treatments combined. The percentage subsidized by the universal Accident Compensation and Rehabilitation insurance scheme (ACC) was 90.1%.
Therapeutic outcomes
At baseline SPADI pain and disability sub-scales showed significant gender differences with females reporting higher scores (One way ANOVA, p = 0.009 and p < 0.001 respectively). These differences were not retained at subsequent follow-up assessments (p > 0.05). Repeated measures ANOVA analysis with 5 levels of SPADI scores (baseline, 3 weeks, 3 months, 6 months and 12 months) with gender and duration of symptoms as factors, and baseline pain intensity VAS (average), SF-8 PCS and MCS, FABQ (total%), GDR and age (years) as covariates, revealed a consistent pattern of change over time. For this reason, only the SPADI Total percentage score values are reported here. There was a significant reduction in SPADI total scores expressed as mean percentage values between the baseline assessment and all follow-up assessments (p < 0.001). Fig. 2 shows the pattern of change over 12 months for both genders, SPADI Total percentage scores, and for the SPADI subscales of pain and disability. The change in SPADI scores was significant between baseline and the 3 week assessment (p < 0.001), and between the 6 and 12 month assessments (p < 0.001), but non-significant between the 3-week and 6-months assessments (p > 0.1).
Fig. 2. Change in mean Shoulder Pain and Disability Index (SPADI) subscales and total percentage scores from baseline to 12 month follow-up (FU) assessments. Covariates appearing in the model are evaluated at the following values: Global Disability Rating at baseline = 3.68, FABQ (Fear-Avoidance Beliefs Questionnaire) Total (%) (Work + Physical Activity Subscales) at baseline = 37.84, Short Form 8 (SF-8) Physical Component Score at baseline = 43.68, SF-8_Mental Component Score at baseline = 53.42, SC: average VAS Score (0-100) in last 48 h (mm) at baseline = 36.58.
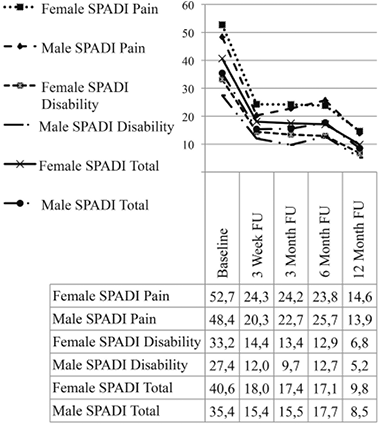
Fig. 3 presents data for proportions of change categories at 3 weeks, 3, 6 and 12 months. Thus at 12 months, 16.1% were lost to follow-up leaving a total sample size of 135. Using an intention-to-treat approach, 45.3% achieved an excellent clinical result, about 28.6% were improved more than the MCID but less than 90% better, 13.0% were not clinically different and 1.9% had worsened. The status of patients was generally poorest at the 3 month follow-up, with 63.3% of patients improved beyond the MCID and 34.8% either unchanged or worse (Fig. 4).
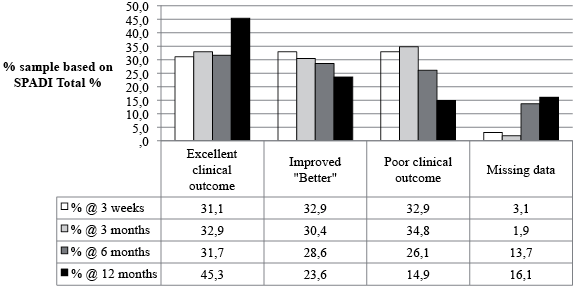
Fig. 3. Proportions of change categories in shoulder pain patients at follow-up assessments. SPADI: Shoulder Pain and Disability Index.
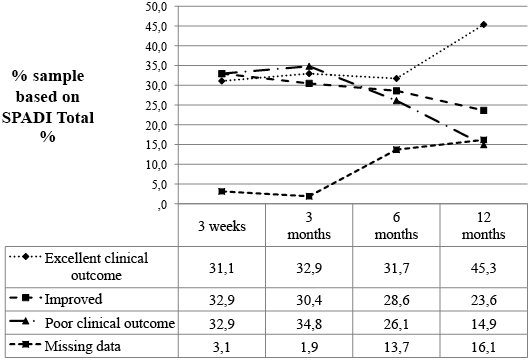
Fig. 4. Clinical outcomes based on Shoulder Pain and Disability Index (SPADI) Total % at follow-up assessments. Excellent clinical outcome: SPADI Total % reduced by 90% or more. Improved: SPADI Total % reduced more than MCID but less than 90% improvement. Poor clinical outcome: SPADI Total % not reduced by MCID (13 points) or worse.
A large number of variables were assessed by univariate logistic regression analyses for a relationship with 3 clinical outcomes (excellent, better, poor) at 3 and 12 months follow-up, and the results for key variables are presented in the appendix. Excellent outcomes at 3 months were associated with: Higher total SPADI scores at baseline, lower maximum reported pain VAS scores in the 48 h prior to enrolment in the study, lower pain scores with painful index tests carried out at the 3 week follow-up assessment, a PAR following diagnostic injection of local anaesthetic into the GHJ and the presence of subacromial bursa pathology(ultrasound imaging of thickening, fluid or calcification). Excellent outcomes at 12 months were associated with: Report of pain not being eased or ‘best’ when at rest, lower reported pain scores with painful index tests carried out at the 3 week follow-up assessment and a PAR following block of the ACJ. Only a PAR to diagnostic block to the SAB was associated with being classified as “Better” (improved beyond the MCID but less than 90% better) at 3 month follow up (p = 0.009). Being “Better” at 12 months follow up was associated with: A report of pain being eased at best when at rest, higher reported pain scores with painful index tests carried out at the three week follow up assessment, and a PAR to diagnostic block to the ACJ. A poor outcome at 3 month follow up was associated with: Higher FABQ Work subscale scores at baseline and higher reported pain scores with painful index tests carried out at the three week follow up assessment. A poor outcome at 12 month follow-up was associated with a lower SF-8 General Health subscale score at baseline and a PAR to diagnostic block of the ACJ.
Recurrences and re-injury rates
At each follow-up subjects were asked to recall and report recurrences of pain and re-injuries that may have occurred (Table III), with nearly half of all pain recurrences related to re-injury. Re-injury was defined as a significant traumatic event or a significant repetitive overload situation that the patient clearly connected to a rapid return or increase in the typical shoulder pain.
Table III. Number of cases reporting recurrences and re-injuries during intervals between follow-up assessments |
||||
Interval between 3 week and 3 month follow-up |
Interval between 3 month and 6 month follow-up |
Interval between 6 month and 12 month follow-up |
Total incidents/events |
|
Recurrence no re-injury |
10 |
12 |
19 |
41 |
Recurrence with re-injury |
8 |
2 |
9 |
19 |
Re-injury without recurrence |
1 |
7 |
4 |
12 |
No recurrence or re-injury |
122 |
115 |
103 |
N/A |
Totals |
141 |
136 |
135 |
72 |
N/A: not applicable. |
||||
Discussion
This study provides the first detailed report of outcomes for patients receiving community based primary care interventions for shoulder pain in a New Zealand environment. Management of patients throughout the follow-up period was broadly consistent with recommended guidelines (7, 17, 18) and with treatments recommended in a recent systematic review of studies of impingement syndrome (38–40), but were not directed. The outcomes at 12 months are broadly consistent with those established in previous reports (1, 3, 7). Also consistent with previous reports, recurrences were reported by 13.5% by the 3 month follow-up and 24% by the 12 month follow-up. However, in the current study about half of these recurrences were related to re-injury, so the spontaneous recurrence rate at 12 months was 14% in this study.
Corticosteroid anti-inflammatory injection is reported to be effective for short term relief of shoulder pain (41). In the current study, the majority of patients receiving corticosteroid injection were selected for this procedure based on a highly standardized criterion, i.e. ≥ 80% reduction of pain following a guided diagnostic injection into the subacromial bursa, acromioclavicular joint or glenohumeral joint. Though diagnostic block has been incorporated into the diagnostic work up in one previous study (42), to our knowledge, this is the first report of outcomes where such a stringent criterion for CSI has been used for the great majority of patients which were carried out within the first 3 weeks of the study. No other interventions had commenced until after the 3-week assessment.
Previous reports established a significant relationship between a shorter duration of symptoms at presentation and better outcome at 6 weeks and 6 months (43). This relationship was not supported by our data as there was no association between duration of symptoms prior to baseline assessment and the likelihood of an excellent outcome at 3 weeks or 3 months. These findings suggest that the structured timing of steroid injection carried out on 54% of the 156 patients assessed at 3 weeks was a factor in the observed rapid change, and may also account for the differences with previous reports (3, 43). It is noted that reports in 2009 and 2012 suggest that the added specificity provided by image guidance does not provide additional benefit in terms of accuracy of injectate placement or clinical outcome (44, 45). However, in 2008 we chose to use ultrasound or fluoroscopic guidance for diagnostic and therapeutic injections to assist in validation of injectate placement.
The profile of treatment in the current study differed substantially from the study by Kuijpers et al. (43) in that over 50% versus 12%, respectively, received corticosteroid injection and 77% versus 10%, respectively, received physiotherapy treatment. In addition, the great majority of CSI interventions occurred in the first 3 weeks. It seems reasonable to argue that the improvement observed in the first 3 weeks was related to corticosteroid injection independently of the duration of symptoms. The reason for the plateau in improvement between three week and three month follow-ups, and the subsequent significant improvement between the six month and 12 month follow-ups cannot be determined based on our data. Examination of these observations was beyond the design and scope of this study and should receive attention in future research. A proportion of patients received steroid injection after the 3 week assessment: 15 between the 3 week and 3 month assessments, 8 between 3 month and six month follow-ups and 9 patients between 6 and 12 month follow-up. The contribution of these interventions on the average clinical course could not be distinguished from improvement over time and the community-based interventions (mostly physiotherapy) employed in the majority of cases. Medication use during this period was not accurately assessed and it is also possible that during this period the use of oral medications may have had an effect on overall decline in pain and disability.
Patients who were offered or had received surgery for their shoulder problem were not significantly more painful or disabled at baseline, but did have elevated fear avoidance with regard to physical activity. However, significant differences emerged throughout the first 6 months of follow-up suggesting that failure to improve with conservative care or steroid injection were important factors in selection for surgical intervention.
Mean pain and disability generally (but not significantly) increased or stayed about the same between the 3 week and 6-month assessments and then significantly declined between the 6 and 12 month follow-up. This finding is contrasted to previous reports and opinion that suggest that reductions in pain and disability are not expected after 6 months (3, 22, 43). The reason for the difference in findings is unclear. It is important to acknowledge that during this period, some patients improved, some remained unchanged and others worsened. Some patients received surgical interventions (n = 17), which typically caused a major increase in pain for a variable amount of time in the post-surgery period.
The predictive value of findings from imaging studies such as x-ray, ultrasound and magnetic resonance imageing (MRI) was limited. Though the presence of SAB thickening, fluid or calcification is associated with an excellent outcome or being “better” at 3 months, it must also be remembered that those patients being offered guided corticosteroid injections into the bursa were those that had a positive response to guided diagnostic block to the SAB. Curiously, a PAR block to the SAB was not predictive of a good outcome. It is intended to examine this apparent paradox in future analyses. Another paradox that emerged needs further detailed examination: A positive response to anaesthetic block into the ACJ was associated with an excellent clinical outcome at 12 months, and a poor outcome at 12 months, suggesting that there are at least two different types of painful ACJ pathologies. The presence of ultrasound verified large or medium sized rotator cuff tear, MRI verified bursitis, MR verified labrum tears and x-ray verified pathology of any kind did not predict poor or good clinical outcomes.
The identification of data acquired at baseline or at three weeks post induction into the study for association with outcomes at 3 and 6 months, will inform the secondary analysis for potential predictive models and will be reported on in Part 2 of this report. Consistent with previous research on other painful musculoskeletal disorders, we have found that a poor outcome was associated with lower self-reported general health scores, and fear avoidance as it relates to work activities. In contrast, the instruments we employed for measuring other psychosocial factors were not strongly associated with 3 or 12 month outcomes.
Limitations
The current study was not a randomized controlled trial and cause/effect relationships are outside the scope of the design. Treatment methods by GPs and physiotherapists after the 3-week assessment were not standardized or controlled, so causal relationships between different oral medications or physiotherapy interventions and outcomes cannot be established. Nevertheless, the current design allowed us to evaluate outcomes associated with current practice in a pragmatic fashion. This would not have been possible with a more rigid design that did not allow clinicians the option to make appropriate treatment choices based upon the individual patient. In general the main treatment choices reflected nationally based guidelines.
Conclusion
Patients managed in a manner broadly consistent with guidelines for shoulder pain in primary care, demonstrate improvement in pain and disability over a 12-month period. However, a moderate proportion still reported pain and disability at 12 months and many reported recurrence of pain during this period. While 30% show dramatic improvement with a structured corticosteroid injection protocol at 3 weeks follow-up, mean pain and disability stayed rather static over the next few months, but improved in the 6–12 months period, regardless of symptom duration at presentation. There is evidence to support the hypotheses that a corticosteroid injection is a valuable treatment modality for selected patients in the short term. The prolonged course of recovery for many with shoulder pain requires further investigation to determine whether improvements in diagnostic and/or treatment processes may reduce recovery time, reduce costs and result in more rapid return of shoulder function. Part two of this report will present results of multivariate predictive modelling for clinical outcomes.
The authors declare no conflicts of interest.
References
