Zhen Chen, MD, PhD1, Yanhui Yang, MD, PhD2, Ge Chen, MD, PhD3, Maobin Wang, MD1 and Weiqun Song, MD, PhD1
From the 1Department of Rehabilitation Medicine of Xuanwu Hospital, 2Department of Radiology of Xuanwu Hospital, 3Department of Neurosurgery of Xuanwu Hospital, Capital Medical University, Beijing, China
OBJECTIVE: To investigate the impact of ventriculoperitoneal shunting during clinical rehabilitation of chronic normal pressure hydrocephalus patients with disorders of consciousness following aneurysmal subarachnoid haemorrhage.
DESIGN: Cross-sectional study.
Patients and methods: Thirty-five patients with disorders of consciousness following aneurysmal subarachnoid haemorrhage who had undergone ventriculoperitoneal shunting for chronic normal pressure hydrocephalus were compared with 16 matched controls with no ventriculoperitoneal shunting. Data from clinical examinations, rehabilitation assessments and computed tomography scans (to exclude other diseases that can cause ventricular enlargement) were analysed. All the patients with disorders of consciousness underwent neurorehabilitation. Consciousness was measured on the Glasgow Coma Scale. The cella media index was calculated as the change in size of the lateral ventricles (prior to ventriculoperitoneal shunting and/or rehabilitation, and 1 and 3 months after shunting and/or rehabilitation). The short-term outcome of treatment was assessed at 3 months using the Glasgow Outcome Scale.
RESULTS: Twenty-four out of 35 patients with disorders of consciousness recovered gradually after ventriculoperitoneal shunting and rehabilitation. There was a significant difference in the Glasgow Coma Scale between ventriculoperitoneal shunting and control groups at both 1 and 3 months (F = 19.29, p < 0.01). Significant differences were also observed between the 2 groups in the cella media index at 1 and 3 months (F = 15.03, p < 0.01). The Glasgow Outcome Scale of the ventriculoperitoneal shunting group was significantly higher than that of the control group (p < 0.01, r = 0.55) 3 months after shunting and/or rehabilitation.
CONCLUSION: Chronic normal pressure hydrocephalus during rehabilitation is a serious and previously unrecognized medical condition, which influences consciousness in patients following an aneurysmal subarachnoid haemorrhage. However, the condition can be treated by ventriculoperitoneal shunting, which helps some patients with disorders of consciousness to regain consciousness.
Key words: disorders of consciousness; rehabilitation, normal pressure hydrocephalus; aneurysmal subarachnoid haemorrhage; ventriculoperitoneal shunting.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Zhen Chen, International Collaboration on Repair Discoveries (ICORD), University of British Columbia (UBC), 818 West 10th Avenue, Vancouver, Canada, V5Z 1M 9; and Department of Rehabilitation Medicine of Xuanwu Hospital, Capital Medical University, Beijing, China, 100053. E-mail: zchen@icord.org, jenniferchen2009@gmail.com; Weiqun Song, Department of Rehabilitation Medicine of Xuanwu Hospital, Capital Medical University, Beijing, China, 100053. E-mail: songwq66@vip.163.com
Accepted Apr 15, 2014; Epub ahead of print Jul 30, 2014
INTRODUCTION
Aneurysmal subarachnoid haemorrhage (aSAH) is the primary cause of 85% of cases of subarachnoid haemorrhage (1). Although increasing numbers of patients with aSAH survive after injury as a result of improvements in neurological techniques and early detection, approximately half of survivors have residual chronic neurological deficits (2), including disorders of consciousness (DOC), such as coma and vegetative state (VS) (3). Coma is defined as a state of unconsciousness in which a person fails to respond normally to painful stimuli, light or sound; does not initiate voluntary actions; and cannot be awakened (4). In VS a person may appear to be awake (displaying spontaneous eye opening and sleep/wake cycles), but is unable to consciously feel, speak, hear or move (5). Individuals who experienced DOC after aSAH are often transferred from a stroke or neurosurgical unit to a rehabilitation unit once they are medically stable. Helping patients with DOC to recover consciousness is the primary goal for the medical team during clinical rehabilitation.
Physiatrists have the dual challenges of using passive rehabilitation methods with these aSAH patients to maintain their range of motion, stimulate sensory afferents and improve cardiovascular and respiratory function, in addition to preventing and treating secondary complications.
Hydrocephalus is an important neurological complication occurring after aSAH (6, 7). Patients who develop hydrocephalus typically have poorer short- and long-term outcomes and longer periods of hospitalization (8). Hydrocephalus was first described in 1928 by Bagley (9), and is broadly defined as an excess of cerebrospinal fluid (CSF) in the ventricular system. It can present with headache, nausea, vomiting, gradual decline of intellectual and motor activity, incontinence, coma, or death (10). The time course of hydrocephalus is described using the terms “acute”, “subacute” and “chronic”. Acute hydrocephalus usually occurs within 3 days, sub-acute within 4–14 days, and chronic for over 14 days (11). There are 2 categories of hydrocephalus: high pressure (CSF drainage pressure using a lumbar catheter > 180 mmH2O) and normal pressure (80–180 mmH2O). Chronic normal pressure hydrocephalus (CNPH) is defined as pressure between 80 and 180 mmH2O lasting 14 days or longer after the original haemorrhage. Acute neurological treatment of aSAH often lasts for 2–3 weeks, after which patients are usually transferred to a rehabilitation unit at Xuanwu Hospital, Capital Medical University, Beijing, China. CNPH therefore usually occurs during rehabilitation; however, the incidence of CNPH is unknown. The incidence rate of acute hydrocephalus after aSAH is between 11% and 30% (7, 12–14), and 8.9% of these patients subsequently undergo ventriculoperitoneal shunting (VPS), i.e. permanent cerebrospinal fluid diversion, for treatment of chronic hydrocephalus (15).
Data from 3521 cases of aSAH in the Cooperative Aneurysm Study showed that a poor level of consciousness was one of several factors that had a significant correlation with hydrocephalus (16). However, this study only reported acute and sub-acute hydrocephalus at the time of aneurysm surgery, and did not examine the relationship between DOC after aSAH and chronic hydrocephalus during rehabilitation. In addition, there is controversy among physicians about the treatment of normal pressure hydrocephalus, especially CNPH, as some regard it as “arrested” hydrocephalus (17) and do not consider it necessary to provide further treatment. There is currently no literature about the short-term outcome for DOC patients with CNPH after VPS and rehabilitation, with regards to consciousness and the objective degree of recovery.
The aim of this study was to clarify the relationship between DOC after aSAH, and CNPH by analysing the data of patients admitted to our institution over a period of 7 years. The impact of CNPH on clinical and radiological presentation was examined using data from the Glasgow Coma Scale (GCS), Glasgow Outcome Scale (GOS) and cella media index (CMI) in clinical rehabilitation.
METHODS
A consecutive series of 78 patients with DOC following aSAH, defined as spontaneous subarachnoid haemorrhage without other brain injury, was prospectively selected between January 2004 and January 2011 at the Departments of Rehabilitation Medicine and Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China. Exclusion criteria were: (i) non-aSAH, such as trauma, arteriovenous malformation rupture, vasculitis; and (ii) pre-existing neurological disease. Twenty-seven patients were excluded due to the presence of other diseases, high-pressure hydrocephalus, or missed follow-up. The 51 subjects eligible for this study were divided into 2 groups: 35 who underwent VPS in a VPS group (16 males, 19 females; 5 coma patients, 30 VS patients) and 16 who did not undergo VPS surgery in a control group (7 males, 9 females; 2 coma patients, 14 VS patients). The patients in the control group did not undergo VPS as their families declined the procedure due to fear of the risks and/or because they could not afford the cost of the operation.
This study was approved by the ethics committee of Xuanwu Hospital, Capital Medical University. Informed consent was obtained from the families of the DOC patients. The study procedures were in accordance with the guidelines of Xuanwu Hospital, Capital Medical University and the Declaration of Helsinki.
Procedure
Physiatrists assessed the consciousness and objective degree of recovery of all DOC patients with the GCS and GOS. Computed tomography (CT) scans were used to investigate the patients’ brain injuries when they were transferred to rehabilitation, and every 2–4 weeks during rehabilitation treatment. Deterioration of consciousness is an important clinical characteristic in patients with DOC presenting with hydrocephalus. If there were no apparent changes in consciousness, CT scans were used to clarify the diagnosis. Progressive enlargement of the ventricular system, shown on repeated CT scans, is key to diagnosis of hydrocephalus (8). Clinical diagnosis of hydrocephalus was based on the following characteristics: diagnosis of CNPH by an experienced neuroradiologist, who reviewed the CT scan images and calculated the width of the third ventricle (III) and CMI (B/A, where A is the largest width of the outer layer of the skull and B is the width of the lateral ventricles in the same layer). Values of the CMI above 0.25 and values of III above 7 mm were considered pathological (18). Lumbar puncture was used to measure ventricular pressure to distinguish normal or high-pressure hydrocephalus and to help in selecting the pressure of the shunt used for VPS. All 51 subjects fulfilled the clinical criterion of presumed CNPH. The opening pressure of the lumbar puncture ranged from 80 to 180 mmH2O. Patients were enrolled in the study from 3 weeks to 3 months after aSAH, when they were transferred to rehabilitation.
If CNPH was confirmed, the physiatrist consulted with a neurosurgeon about VPS, which is the most common treatment for hydrocephalus. The programmable valve VPS system usually connects the right ventricle with the peritoneal space, with the aim of avoiding injury to the language centres on the left side of the brain. Shunts are usually equipped with reservoirs that are used for transiently increasing output and for testing the patency of flow. After shunt implantation the resumption of rehabilitation is usually prompt. Patients are typically observed for 2–3 days postoperatively, before returning to rehabilitation.
The patient’s medical history was collected from medical records on admission. The level of patient’s impaired consciousness was assessed with the GCS (19). Repeated CT scans were used to measure the CMI and to confirm the effect of VPS. The 2 measures were evaluated at 3 time-points: (i) baseline (prior to VPS or the start of rehabilitation); (ii) 1 month after VPS and/or rehabilitation; and (iii) 3 months after VPS and/or rehabilitation. The overall outcome of the patients was assessed with the GOS (20) 3 months after VPS and/or rehabilitation.
Statistical analysis
Statistical analysis was performed using SPSS (Version 20, SPSS Inc., Chicago, IL, USA). The subjects’ clinical characteristics were reported as means (standard deviation (SD)) for continuous variables and as percentages for categorical variables. The between-subject variables were VPS, which had 2 levels (VPS, no VPS), and the within-subject variables were time, which had 3 levels (baseline, 1 month, 3 months). Analysis of variance (ANOVA) with repeated measures on the second factor was used to compare GCS and CMI between treatment and control groups. A Mann-Whitney U test was used to determine differences in GOS between the 2 groups 3 months after VPS and/or rehabilitation. Correlations between GCS and CMI were investigated using Spearman’s correlation coefficient.
RESULTS
Fifty-one DOC patients with aSAH were included in the analysis. Of these, 35 underwent the VPS operation. Baseline patient characteristics are shown in Table I. There were no significant differences between the 2 groups at baseline in terms of age, sex, time since aSAH, admission GCS and CMI.
|
Table I. Patients’ baseline demographic and aneurysmal subarachnoid haemorrhage (aSAH) characteristics |
|||
|
VPS treatment group (n = 35) |
Control group (n = 16) |
p-value |
|
|
Age, years, mean (SD) |
59.94 (11.88) |
58.19 (16.45) |
0.67 |
|
Males, n (%) |
16 (45.71) |
7 (43.75) |
0.50 |
|
Length since aSAH, days, mean (SD) |
47.43 (19.61) |
57.56 (29.27) |
0.22 |
|
Admission GCS, mean (SD) |
6.74 (2.75) |
6.31 (1.99) |
0.58 |
|
CMI |
0.37 (0.03) |
0.36 (0.04) |
0.23 |
|
SD: standard deviation; aSAH: aneurysmal subarachnoid haemorrhage; GCS: Glasgow Coma Scale; CMI: cella media index. |
|||
Effects of ventriculoperitoneal shunting on Glasgow Coma Scale
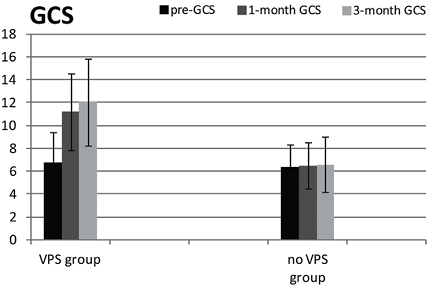
There were significant differences between the VPS and control groups on the GCS (F = 19.29, p < 0.01; Fig. 1); 24 out of 35 DOC patients in the VPS group gradually gained consciousness, whereas no patients in the control group regained consciousness. Although all patients in the VPS and control groups had a low GCS score when they transferred to rehabilitation treatment (mean 6.74 (SD 2.75); 6.31 (SD 1.99), respectively), there were significant differences at 1 month (mean 11.20 (SD 3.40), 6.50 (SD 2.03), respectively; p < 0.01) and 3 months (mean 12.03 (SD 3.87), 6.56 (SD 2.42), respectively; p < 0.01) after VPS and/or rehabilitation. Two patients in the VPS group regained consciousness at 1 month after VPS, but their GCS decreased from 15 to 13 at 3 months after VPS even though they did not lose consciousness again.
Effects of ventriculoperitoneal shunting on cella media index
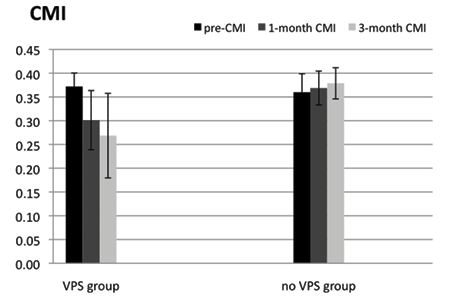
There was also a significant difference in the CMI of patients after aSAH with and without VPS (Fig. 2).
CMI was also significantly affected by VPS, as seen in the VPS treatment and control groups (F = 15.03, p < 0.01; Fig. 2). In the VPS treatment group patients’ ventricles shrank significantly and, concurrently, Mean CMI decreased significantly (by 18.8% and 27.9%, respectively, at 1 month (0.30 (SD 0.06)) and 3 months (0.27 (SD 0.09)) after VPS and rehabilitation compared with baseline (0.37 (SD 0.03)). However, in the control group there were no significant changes in CMI at 1 month and 3 months after rehabilitation compared with baseline.
Outcome by Glasgow Outcome Scale at 3-months
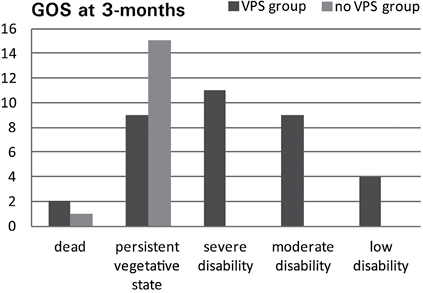
There were significant differences in GOS level between the VPS treatment group (median = 3, n = 35) and the control group (median = 2, n = 16); U = 98.5, Z = –3.93, p < 0.01, r = 0.55. Patients in the VPS treatment group had more favourable short-term outcomes at 3 months than those in the control group (Fig. 3). Two patients in the VPS group and 1 in the control group died because their aneurysms ruptured again within 3 months.
Correlation between cella media index and Glasgow Coma Scale
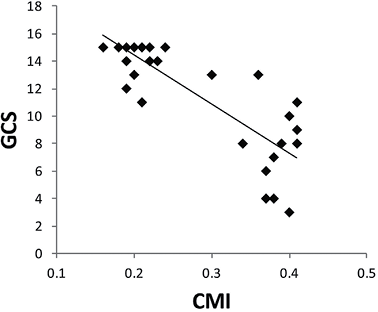
There was a strong, negative correlation between the size of the lateral ventricle (as measured by CMI) and statement of consciousness (as measured by GCS) in the VPS group (Fig. 4) (r = –0.73, n = 35, p < 0.01), with high values for the size of the lateral ventricle associated with lower values for the statement of consciousness.
DISCUSSION
This study found that CNPH impacts on the consciousness of DOC patients with aSAH; however, VPS may help to reverse this situation, improving patients’ short-term outcome during rehabilitation. To our knowledge, this is the first study of this size to exhibit this relationship during clinical rehabilitation.
Hydrocephalus foreshadows a poorer prognosis in patients with aSAH (13). However, the exact mechanism by which hydrocephalus develops after aSAH is poorly understood. Experimental studies have found that erythrocytes, proteins and fibrin of blood components increase the outflow resistance of the CSF through the subarachnoid space or the arachnoid granulations, which may lead to hydrocephalus (14, 21).
The pathogenesis of brain damage caused by hydrocephalus is multi-factorial. There are 2 main theories; first, that local dysfunction is caused by stretched axons and decreased periventricular blood flow and, secondly, that local dysfunction is caused by reduced extracellular fluid movement. At the simplest level, enlarging ventricles causes damage by stretching the periventricular axons. Swollen dendrites, axons and synaptic degeneration have been observed in ultrastructural studies of the human hydrocephalus cortex (22, 23). In addition, decreased periventricular blood flow, which is more pronounced in the deep grey matter and periventricular white matter than in remote sites in patients with subarachnoid haemorrhage has been found by CT perfusion (24). Furthermore, movement of extracellular fluid is reduced because the extracellular spaces of grey matter are compressed after hydrocephalus. A magnetic resonance imaging (MRI) study of extracellular fluid tracer movements in hydrocephalic rats found less spread in the ipsilateral cortex and preferential accumulation of tracer in oedematous white matter compared with non-hydrocephalic controls (25). A recent study on subarachnoid haemorrhage-induced acute hydrocephalus in rats found that ependymal deficits and periventricular iron deposition may lead to increased periventricular brain injury (26). The imbalance of fluid outflow may disturb the delicate extracellular environment of neurons, thereby impairing their function and resulting in DOC.
To-date most studies have not distinguished between high-pressure hydrocephalus and normal pressure hydrocephalus. From a clinical management viewpoint, CNPH is referred to as “arrested” hydrocephalus (17). The major problem is whether CNPH causes gradual brain damage, since the enlarged ventricles appear to reach a new pressure balance and do not significantly increase in size. Although the pressure in the ventricles is normal, according to Laplace’s law, the larger the vessel radius, the greater the wall tension required to withstand a given internal fluid pressure (27). Lumbar puncture was used to measure the inside pressure, which was normal; however, the radius of the ventricles increased, therefore the tension (the pressure on the outer wall of the ventricles) was increased. The increased pressure on the preventricular brain tissue may lead to an intracranial crisis, resulting in DOC (3). Furthermore, Sklar et al. have demonstrated changes in brain elasticity, which may be central to the development of ventricular enlargement despite normal pressure (28). The present study has demonstrated that VPS may reverse this damage by establishing new circulation of CSF and decreasing the pressure on the ventricular wall, which may result in a reduction in the size of the ventricles.
Recovery of consciousness is rare after 3 or 4 weeks of coma (29, 30), although “irreversible coma” is usually declared after a period of 12 weeks. The mean duration of prolonged unconsciousness in our patients in the VPS treatment group was approximately 7 weeks. In the present study, 68.6% of patients showed improvements in consciousness after VPS during the first 3 months of rehabilitation, which is higher than in other studies of DOC patients with post-traumatic hydrocephalus (31). This may be due to the shorter time period until shunting since the original brain injury in our study. However, more than 30% of DOC patients still did not recover consciousness after VPS; thus, it would be better if we could evaluate changes in consciousness before and after a protocol of continuous cerebrospinal fluid drainage via spinal catheter in order to determine which patients would benefit from VPS (32).
In addition, it is recommended that aSAH patients with DOC have a follow-up CT scan and clinical evaluation every 2–4 weeks during their rehabilitation programme to rule out CNPH, although we did not detect any change in their awareness, because changes in clinical symptoms may be too minor to be detected. The present study indicates that, 2 and 3 months after VPS treatment, CNPH was found in 70.7% and 16.1% of patients, respectively.
Minimally conscious state (MCS) is another common DOC similar to coma and VS. People with MCS have a severely altered consciousness in which they demonstrate clearly discernible behavioural evidence of consciousness, but do not demonstrate consistent functional levels (33). GCS subscales include eye opening, verbal communication and verbal command following instructions; thus the GCS scale in patients with MCS would be equal or greater than 12. However, in our study the baseline GCS score is less than 12; therefore we did not include individuals with MCS in this study.
Study limitations
The non-randomized (observational) nature of this study may have introduced bias. It was not feasible to perform a randomized controlled trial (RCT) due to the high costs (more than US$ 12 million per Phase III clinical trial funded by the National Institute of Neurological Disorders and Stroke before 2000 (34)) and the medical ethics surrounding our research question. In addition, observational studies can produce similar results to RCTs (35, 36), and are important in exploring topics for which an RCT may not be feasible. The results of this study provide the important insights that DOC patients with aSAH may develop the secondary complication of CNPH, which may prevent recovery of consciousness, and that VPS can reverse it effectively. However, not all physicians understand NPH very well; a survey by Conn from Yale University School of Medicine, demonstrated that almost one-third of physicians had never heard of NPH (37). Moreover, our study did not include CNPH that developed later than 3 months after aSAH, therefore the outcome for this group after VPS is not known. Since this was a single-centre study, our results may not be generalizable to other populations. A multiple-centre RCT may help further to determine the efficacy of this treatment on consciousness rehabilitation. Finally, the Coma Recovery Scale and the Disability Rating Scale are more appropriate measures to use in the post-acute phase; however, we used the GCS to compare changes in consciousness from the acute to chronic phases in both groups, which enabled us to identify changes over time within a single measure.
Conclusion
Recovery from brain injury after aSAH is determined not only by the primary injury, but also by the development of CNPH, which has an important role in determining recovery of consciousness and achievement of maximal functional abilities in DOC patients during and after rehabilitation. VPS can minimize the devastating effects that result from this condition, and some DOC patients can regain consciousness. Further multi-centre double-blind randomized control trials with larger sample sizes are needed to confirm the results obtained in this study, as well as to understand better the long-term outcomes in DOC patients with CNPH after VPS.
ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China (grant numbers 81171024 and 30770714), the Natural Science Foundation of Beijing (grant number 7102075) and the Ministry of Organization of the Beijing government (grant number 20071D0501800243).
The authors declare no conflicts of interest.
REFERENCES
