Fang Li, MD, PhD1, Yuedi Wu, MD2 and Li Xiong, BS2
From the 1Department of Rehabilitation, Huashan Hospital, Fudan University and 2Department of Rehabilitation, The First Rehabilitation Hospital of Shanghai, Hangzhou Road, Shanghai, China
OBJECTIVES: To evaluate the reliability of a new scale, the Triple Spasticity Scale (TSS), for assessing spasticity in stroke, through measurement of affected elbow flexors and ankle plantar flexors of hemiplegic patients with stroke, and to compare the new scale with commonly used scales.
DESIGN: Cross-sectional study.
SETTING: Inpatients at a rehabilitation hospital.
Patients: Seventy-one inpatients with hemiplegic stroke.
Main outcome measures: TSS, Modified Ashworth Scale (MAS) and Modified Tardieu Scale (MTS).
RESULTS: Test-retest reliability for TSS total score was good (intraclass correlation coefficient (ICC) = 0.905~0.918). Inter- rater reliability for TSS total score was also good (ICC = 0.778~0.885). Spearman’s correlation coefficient demonstrated significant correlation between the TSS and MAS, in both elbow flexors and plantar flexors (r = 0.840~0.946, p = 0.000), and between the TSS and MTS, in both elbow flexors and plantar flexors (r = 0.715~0.795, p = 0.000). There were small, but significant, correlations between the scores for increased resistance and dynamic muscle length in these 2 muscles (r = 0.307~0.564, p = 0.000~0.009).
CONCLUSION: The TSS has good test-retest reliability and inter-rater reliability in measurement of muscle tone. This new scale provides an alternative for measuring spasticity, which avoids some of the shortcomings of previous scales.
Key words: spasticity; reliability; scale.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Fang Li, Department of Rehabilitation, Huashan Hospital, Fudan University, Shanghai, China. E-mail: fangl@fudan.edu.cn
Accepted Apr 3, 2014; Epub ahead of print Jun 19, 2014
INTRODUCTION
Upper motor neurone syndrome (UMNS) has both “positive” and “negative” signs. Negative signs are weakness, paralysis, impaired dexterity and fatigue. Positive signs are spasticity, spastic co-contraction, associated reactions, enhanced primitive reflexes and spastic dystonia (1). Spasticity is characterized by a velocity-dependent increase in the excitability of tonic and phasic muscle stretch reflexes (2). The defining characteristic of enhanced tonic muscle stretch reflexes is excessive resistance of the muscle to passive stretch, whereas hyperactivity of phasic stretch reflexes refers to exaggerated tendon jerks and clonus. Clonus is characterized by repetitive, rhythmic contractions observed in one or more muscles of a single limb segment or multiple limb segments (3). In UMNS, muscles with dystonia also appear to have stretch sensitivity (4). Muscle tone, referring to resistance to passive stretch, has neural and non-neural components (5). The neural components arise from their reflex activity (and/or dystonia), whereas the non-neural components arise from rheological properties intrinsic to muscle and other soft tissues. Non-neural resistance is caused by inertia, elasticity and viscosity of the body part that is moved (6). Several studies have shown that the muscle properties are altered following central nervous system lesions (7, 8). Resistance coming from muscle depends on the length of the muscle and the rate of change of muscle length. The length-dependent components are proportional to coefficients termed elasticity (or stiffness) (9). The velocity-dependent components are proportional to coefficients termed viscosity (or damping) (9). Inertia is an external force that counteracts the muscle. Measurement of spasticity should be aimed at neural components, rather than non-neural components that are usually not velocity-dependent (viscosity is an exception; however, it may be less in stroke patients than in controls (6)). Previous measurements were often criticised if they did not address the velocity-dependence of the stretch reflex, as spasticity is a velocity-dependent phenomenon. It is important to assess spasticity precisely so as to evaluate the effectiveness of different treatments and to choose the best option for each patient. For instance, patients with dominant neural components should be considered for treatment aimed at reducing the exaggerated stretch reflex, such as botulinum neurotoxin injection, whereas those with dominant non-neural components may benefit from other strategies, such as casting or stretch.
Commonly used clinical tools for assessment of spasticity/muscle tone are the Ashworth/Modified Ashworth Scale (AS/MAS), Tardieu/ Modified Tardieu Scale (TS/MTS), Composite Spasticity Index (CSI), etc. (10, 11). The validity of the MAS in terms of spasticity assessment is questionable, as it does not address the velocity-dependent phenomenon, but is a sum of neural and non-neural components to passive movement (12–14). However, a significant positive correlation has been found between the AS scores and neural components in stroke patients, whereas no consistent correlation has been found between AS scores and non-neural components (6). Results concerning reliability of the MAS remain equivocal (15–20).
The TS has been regarded as a better option than the AS for assessing spasticity (9), as it measures and compares the muscle reaction (known as dynamic muscle length or angle difference) to passive stretch at both slow and fast speeds, which agrees more closely with Lance’s definition (21). In the earlier TS version, 3 speeds were necessary: a slow speed below which the stretch reflex would be induced; a fast speed corresponding to the limb segment falling under the influence of gravity; and a very fast speed to trigger the stretch reflex as strongly as possible (22). A later version (MTS) of the scale has only 2 speeds, 1 “slow” and 1 “fast” (23). The MTS is unique in assessing spasticity, and the dynamic muscle length of MTS is in agreement with the dynamic stretch reflex threshold (DSRT), which is a laboratory measurement of spasticity (9). However, the MTS does not address passive resistance created by neural components when comparing the angle difference. In addition, the reliability of MTS in both paediatric and adult populations is inconclusive (15, 16, 24–27).
Spasticity is considered to be a segmental reflex elicited by muscle stretch, which is processed abnormally in related cord segments, ultimately generating excessive drive on segmental alpha motor neurones that innervate the very muscles being stretched (28). The mechanism of abnormal processing is complicated, such that the stretch resistance from neural components may have different sources. For example, primary endings of the muscle spindle are known to be velocity sensitive, but secondary spindle endings have static length sensitivity (29), which may generate resistance to passive stretch. Neural resistance to passive movement, which is not emphasized in the TS/MTS, is a source of task restriction.
The CSI was used to measure spasticity in patients with stroke and cerebral palsy (11, 30). An earlier version of the CSI includes 3 subsections: tendon jerks and clonus were scored to measure phasic stretch reflex excitability, whereas resistance to manual stretch was scored to measure tonic stretch reflex excitability. A modified version of the CSI includes only 2 subsections: tendon jerks and resistance to stretch (30). The CSI used in neurological patients has shown validity and good reliability for measurement of spasticity (31, 32). However, the scale may have some limitations. First, although the resistance to manual stretch applied by a rater at a moderate speed is closely related to the clinical concept of muscle tone, the CSI does not address the velocity-dependent phenomenon. Secondly, tendon reflex may not be easily elicited from some overactive muscles in the common patterns of upper motor neurone dysfunction. For example, the pronator teres and pronator quadratus are responsible for the pronated forearm, and pectoralis major, latissimus dorsi, teres major and subscapularis are responsible for the adducted/internally rotated shoulder, etc.
Evaluation of spasticity includes biomechanical laboratory measures and clinical scales. It has not been possible to date to replace clinical scales with laboratory measures. The objective of this study was to design a new scale, the Triple Spasticity Scale (TSS), to evaluate spasticity in 3 ways, while attempting to avoid the shortcomings of previous scales. The aim of this comprehensive scale is to use threshold and supra-threshold resistance measures together to provide an insight into spasticity, scoring the severity of the spasticity so as to make it comparable. A further aim is to investigate the inter-rater and intra-rater reliability of the TSS in measuring spasticity and to analyse the relationships between TSS and MAS, and between TSS and MTS, by using an adequate sample of patients with hemiplegia. In designing the study protocol, factors influencing stretch reflex were taken into account so as to minimize their effects.
Methods
Participants
A total of 71 post-stroke hemiplegic inpatients admitted to our hospital were included in the study. The patients’ demographic and clinical variables, including age, gender, time since stroke, paretic side, and lesion type (ischaemic or haemorrhagic), are shown in Table I. All of the participants fulfilled the following inclusion criteria: hemiparesis due to a unilateral single clinical stroke, with at least 1 positive sign of upper motor neurone syndrome (exaggerated tendon jerks, spasticity, co-contraction, associated reaction and increased flexor reflex) and able to give informed consent. Exclusion criteria were: additional neurological conditions, ongoing treatment with muscle relaxants or antibiotics, major joint pathology (e.g. joint surgery or rheumatoid arthritis), emotional lability, and inability to accomplish simple commands. The study was approved by the local research ethics committee of the hospital and all participants provided informed written consent.
The TSS and MAS were rated by 1 physiatrist and 1 physiotherapist, both of whom were trained in applying these 2 scales. Before the start of the study, the raters were instructed about the measurements and study procedures by a professor, to ensure that the definitions were uniformly understood.
|
Table I. General data for the hemiplegic patients (n = 71) |
|
|
Patient data |
|
|
Age, years, mean (SD) |
62.3 (15.01) |
|
Gender, male/female, n |
50/21 |
|
Time since stroke, months, mean (SD) |
14.8 (26.03) |
|
Affected side, left/right, n |
38/33 |
|
Lesion type, ischaemic/haemorrhagic, n |
55/16 |
|
SD: standard deviation. |
|
Design
Elbow and plantar flexors were chosen for testing because flexed elbow and equinus foot are common patterns of UMNS in the upper and lower limbs (4). The elbow flexors and ankle plantar flexors of each participant were assessed twice (test and re-test) with the TSS and MAS by rater 1 (the physiatrist). The re-test was conducted 1 day after the initial test. The participants were first measured with the MAS and then with the TSS. For each participant, both measurements were conducted between 07.00 h and 08.30 h. In addition, patients were measured during their initial assessment by 1 physiatrist and 1 physiotherapist (inter-rater). The results were entered into separate recording sheets. The 2 raters were blind to each other’s results.
Measurements
MAS. Participants rested for 10 min in the supine position, arms by their sides and head in a neutral position. The therapist examined the patient approximately 30 min after the doctor’s first measurement. When performing the stretch of the elbow flexors, the assessor kept the subject’s arm in a neutral position. When performing the stretch of the plantarflexors, the assessor kept the subject’s knee extended and controlled inversion of their ankle. The MAS was described as follows: during 2 repetitions of a passive motion within 1 s, resistance was measured on the following 6-point scale: 0 = no increased resistance; 1 = slightly increased resistance (catch followed by relaxation or minimal resistance at the end of the range of motion); 1+ = slightly increased resistance (catch followed by minimal resistance throughout less than half of the range of motion); 2 = clear resistance throughout most of the range of motion; 3 = strong resistance; passive movement is difficult; 4 = rigid flexion or extension.
TSS and MTS. The TSS includes 3 subsections shown in Table II. Increased resistance is graded in subsection 1, which is not the same as the AS (33). The extent of increased resistance is scored according to 2 stretches, 1 of which is very slow (r2, less than 5°/s) (6), and another is as fast as possible (r1). The rater compares the resistance between the 2 stretches according to his or her subjective perception and then scores the increased portion (r1–r2). The different combinations of r1–r2 are listed in Fig. 1. The rater used this figure to determine the type of resistance. Clonus is scored in subsection 2. In subsection 3, dynamic muscle length, also known as angle difference or Y value, is measured as follows: the rater rotates the joint first at a slow speed (less than 5°/s) through its full range of motion (described as R2). The rater then moves the joint as rapidly as possible in the same direction and through the same arc, and the angle of muscle reaction is recorded as R1 (24). The angles of R1 and R2 were measured with a universal goniometer placed near the joints. Dynamic muscle length is the angle difference between R1 and R2 (R1–R2), which is converted into 5 grades in the TSS described in Table II. In the meanwhile R1–R2 was recorded as MTS score. According to the measurement in the normal population, the full range of motion of elbow and ankle joints in the patients were regarded as 150° and 60° (34), respectively. Participants rest in the same standardized position as described for the MAS when performing the stretch.
|
Table II. Triple Spasticity Scale |
||
|
Subsection |
Grade |
Description |
|
Increased resistance between a slow stretch and a fast stretch (r1–r2) |
0 |
No increased resistance |
|
1 |
Mild increased resistance |
|
|
2 |
Moderate increased resistance |
|
|
3 |
Severe increased resistance |
|
|
4 |
Extremely severe increased resistance |
|
|
Clonus |
0 |
None |
|
1 |
Fatigable, refers to a clonus less than 10 s |
|
|
2 |
Infatigable, refers to a clonus greater than 10 s |
|
|
Dynamic muscle length (R1–R2) |
0 |
Angle difference between R1 and R2 is 0 |
|
1 |
Angle difference between R1 and R2 < 1/4 full range of motion |
|
|
2 |
Angle difference between R1 and R2 ≥ 1/4 and < 1/2 full range of motion |
|
|
3 4 |
Angle difference between R1 and R2 ≥ 1/2 and < 3/4 full range of motion Angle difference between R1 and R2 ≥ 3/4 full range of motion |
|
|
Total |
0~10 |
|
|
Slow stretch (less than 5°/s), fast stretch (as fast as possible). r1(r2) = 0, no resistance; r1(r2) = 1, mild resistance; r1(r2) = 2, moderate resistance; r1(r2) = 3, severe resistance; r1(r2) = 4, extremely severe resistance. The meaning of the overall score was interpreted based on clinical experience as mild (0–2), moderate (3–5), or severe spasticity (6–8) in the muscles in which clonus could not be elicited. The meaning of the overall score was interpreted based on clinical experience as mild (0–3), moderate (4–6), or severe spasticity (7–10) in the muscles in which clonus could be elicited. |
||
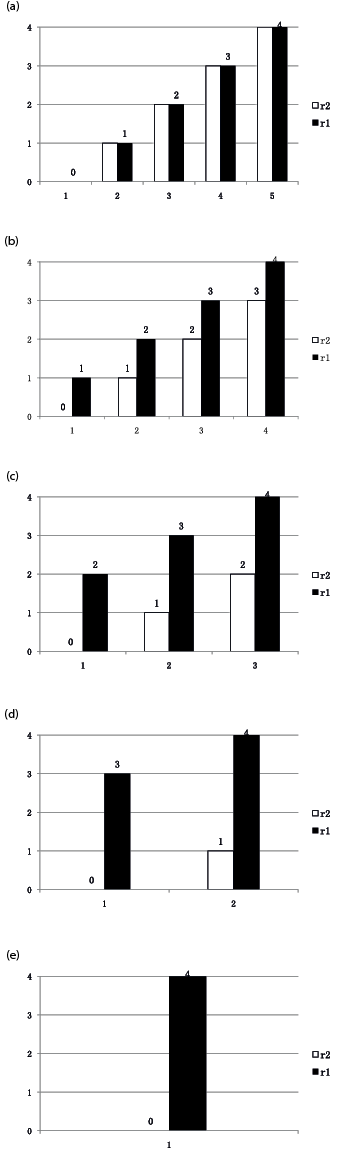
Statistical analysis
The assessments of TSS by 2 raters were used to determine the inter-rater reliability of the scale. The assessments by 1 rater (the physiatrist) 1 day apart were used to determine the test-retest reliability. The correlation was analysed either between the TSS and MAS, or between the TSS and the R1–R2 of the MTS. In addition, the correlation between the increased resistance (r1–r2, subsection 1) and dynamic muscle length (R1–R2, subsection 3) was also analysed. The single measures of intraclass correlation coefficient (ICC) were chosen as the test statistic of reliability (ICC; 2-way random, absolute agreement). The ICC reflected both the degree of correspondence and the degree of agreement between the scorings. Reliability was considered to be good if the ICC was greater than 0.75, or fair if the ICC was between 0.40 and 0.75. Spearman’s correlation coefficient was chosen as a test of correlation. A Spearman’s correlation coefficient of 0.61 or more was considered good (35). Data were analysed using SPSS 20.0 (Statistical Product and Service Solutions, SPSS Inc.).
Results
Seventy-one patients with a mean age of 62 years (50 males, 21 females) were included in the study. The mean time since onset of stroke was 14.84 months (standard deviation 26.03). The test-retest and inter-rater score distribution of the TSS of the study population are shown in Table III. Descriptive statistics of the TSS characteristics measured by rater 1 are shown in Table IV.
|
Table III. Test-retest score and inter-rater score distribution of the Triple Spasticity Scale (TSS) (n = 71) |
||||||||||||||||||||||
|
|
Initial test (rater 1) |
Retest (rater 1) |
Rater 2 |
|||||||||||||||||||
|
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
TN |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
TN |
|||
|
EF |
0 |
15 |
1 |
1 |
17 |
16 |
1 |
17 |
||||||||||||||
|
1 |
3 |
1 |
12 |
1 |
8 |
2 |
1 |
12 |
||||||||||||||
|
2 |
8 |
3 |
14 |
4 |
6 |
4 |
14 |
|||||||||||||||
|
3 |
1 |
3 |
1 |
2 |
7 |
3 |
3 |
1 |
7 |
|||||||||||||
|
4 |
1 |
10 |
11 |
1 |
1 |
7 |
2 |
11 |
||||||||||||||
|
5 |
8 |
8 |
1 |
3 |
2 |
2 |
8 |
|||||||||||||||
|
6 |
1 |
1 |
2 |
1 |
1 |
2 |
||||||||||||||||
|
7 |
0 |
0 |
||||||||||||||||||||
|
8 |
0 |
0 |
||||||||||||||||||||
|
TN |
15 |
12 |
14 |
6 |
12 |
11 |
1 |
0 |
0 |
71 |
17 |
12 |
13 |
10 |
12 |
5 |
2 |
0 |
0 |
71 |
||
|
PF |
0 |
10 |
10 |
10 |
1 |
11 |
||||||||||||||||
|
1 |
2 |
2 |
1 |
2 |
7 |
3 |
2 |
1 |
1 |
7 |
||||||||||||
|
2 |
1 |
3 |
4 |
1 |
2 |
1 |
4 |
|||||||||||||||
|
3 |
2 |
2 |
12 |
2 |
18 |
1 |
6 |
11 |
1 |
19 |
||||||||||||
|
4 |
5 |
6 |
2 |
13 |
1 |
1 |
5 |
5 |
1 |
13 |
||||||||||||
|
5 |
2 |
4 |
2 |
8 |
1 |
1 |
2 |
1 |
3 |
8 |
||||||||||||
|
6 |
1 |
1 |
6 |
8 |
2 |
3 |
1 |
6 |
||||||||||||||
|
7 |
2 |
2 |
2 |
2 |
||||||||||||||||||
|
8 |
1 |
1 |
1 |
1 |
||||||||||||||||||
|
TN |
12 |
5 |
3 |
23 |
10 |
7 |
10 |
1 |
0 |
71 |
13 |
7 |
11 |
23 |
12 |
5 |
0 |
0 |
0 |
71 |
||
|
EF: elbow flexors; PF: plantar flexors; TN: total number of assignments. No scores of 9 or 10 were recorded. |
||||||||||||||||||||||
|
Table IV. Descriptive statistics of the Triple Spasticity Scale (TSS) characteristics (rater 1) |
||
|
Elbow flexors |
Ankle plantar flexors |
|
|
Muscle tone, mean (SD), range |
1.37 (1.24) 0~4 |
1.46 (1.16) 0~4 |
|
Clonus, mean (SD), range |
0 |
0.51 (0.67) 0~2 |
|
R1–R2a, mean (SD, range |
24.51 (26.90) 0~90 |
11.69 (11.95) 0~50 |
|
Dynamic muscle lengthb, mean (SD), range |
0.85 (0.90) 0~3 |
1.15 (1.09) 0~4 |
|
Total score, mean (SD), range |
2.21 (1.82) 0~6 |
3.13 (2.02) 0~8 |
|
aoriginal angle difference, bGrade of the subsection. SD: standard deviation. |
||
The results for the inter-rater reliability and intra-rater reliability are shown in Table V. ICCs for the total score and subsection 1 (muscle tone) of elbow and plantar flexors were good, at between 0.750 and 0.973. For the subsection of dynamic muscle length, ICCs of elbow flexors and plantar flexors were fair to good, at between 0.536 and 0.810. In subsection 2, ICCs of ankle plantar flexors were good, at 0.934 and 0.985. The ICC of the elbow flexors could not be calculated because the clonus had not been elicited in the muscles.
|
Table V. Inter-rater and intra-rater reliability of the Triple Spasticity Scale (TSS) (n = 71) |
|||||
|
Muscle tone ICC (95% CI) |
Clonus ICC (95% CI) |
Dynamic muscle lengtha |
R1–R2b ICC (95% CI) |
Total score ICC (95% CI) |
|
|
Inter-rater reliability |
|||||
|
Elbow flexors |
0.972 (0.956~0.983) |
– |
0.701 (0.561~0.803) |
0.712 (0.575~0.810) |
0.902 (0.847~0.938) |
|
Plantar flexors |
0.908 (0.857–0.942) |
0.934 (0.896~0.958) |
0.536 (0.348~0.683) |
0.456 (0.251~0.622) |
0.750 (0.627~0.836) |
|
Intra-rater reliability |
|||||
|
Elbow flexors |
0.973 (0.957~0.983) |
– |
0.810 (0.712~0.877) |
0.800 (0.698~0.871) |
0.921 (0.876~0.950) |
|
Plantar flexors |
0.971 (0.954~0.982) |
0.985 (0.976~0.991) |
0.693 (0.550~0.797) |
0.715 (0.579~0.812) |
0.908 (0.856~0.941) |
|
aGrade of the subsection, boriginal angle difference. ICC: intraclass correlation coefficient (single measures); 95% CI: 95% confidence interval. |
|||||
In rater 1, Spearman’s correlation coefficients between the TSS and the MAS were 0.946 (p = 0.000) for the elbow flexors and 0.840 (p = 0.000) for the plantar flexors, respectively. In rater 1, Spearman’s correlation coefficients between the TSS and the MTS were 0.795 (p = 0.000) for the elbow flexors and 0.715 (p = 0.000) for the plantar flexors, respectively. The graphs of scatterplot indicating the correlation between the total scores of TSS and the MAS scores with a regression line are shown in Fig. 2, the correlation between the total scores of TSS and the MTS scores (angle difference) with a regression line are shown in Fig. 3.
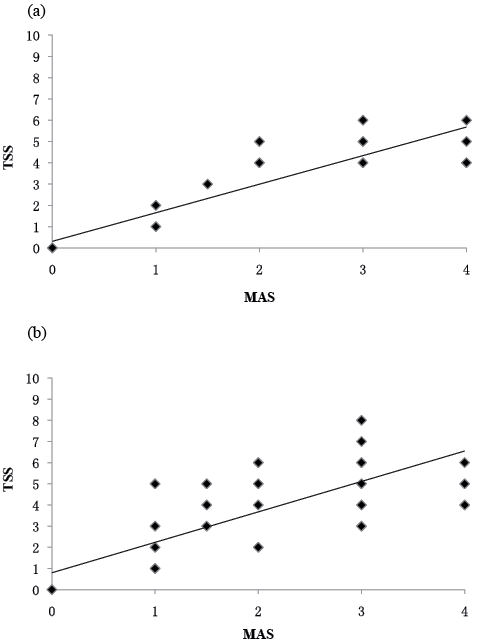
Fig. 2. Rate 1: correlation between the total scores of Triple Spasricity Scale (TSS) and Modified Ashworth Scale (MAS) scores for: (a) elbow flexors (r = 0.946, p = 0.000), (b) plantar flexors (r = 0.840, p = 0.000). There is some overlap in the scatterplot.
In rater 1, Spearman’s correlation coefficients between the scores of increased resistance (r1–r2, subsection 1) and the scores of dynamic muscle length (R1–R2, subsection 3) were 0.564 (p = 0.000) for the elbow flexors and 0.307 (p = 0.009) for the plantar flexors, respectively. The correlation between these 2 subsections with a regression line is shown in Fig. 4.
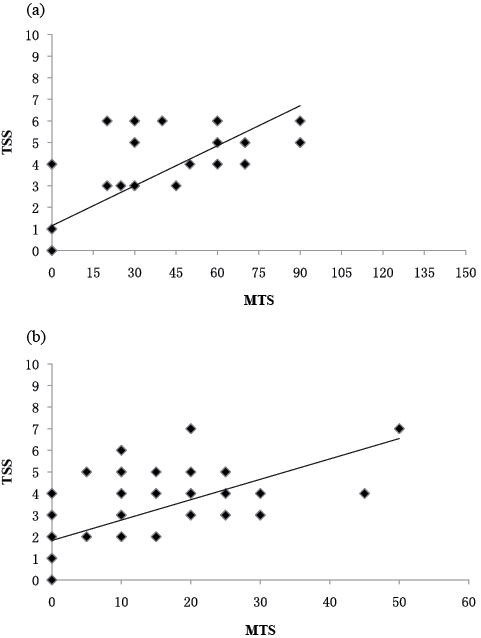
Fig. 3. Rater 1: correlation between the total scores of Triple Spasricity Scale (TSS) and Modified Ashworth Scale (MAS) scores for: (a) elbow flexors (r = 0.795, p = 0.000), (b) plantar flexors (r = 0.715, p = 0.000). There is some overlap in the scatterplot.
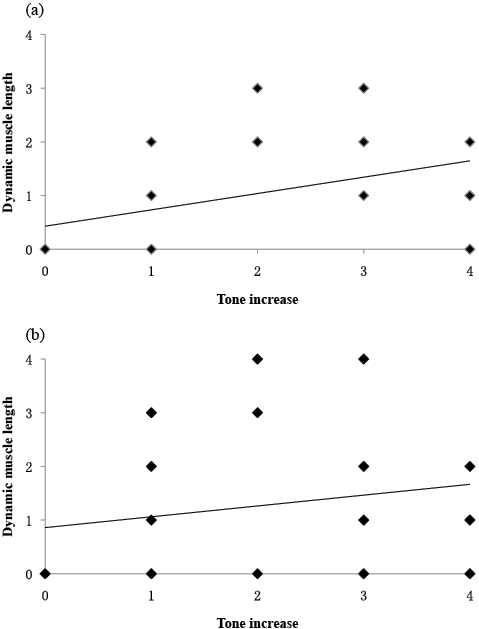
Fig. 4. Rater 1: correlation between the scores of increased resistance (subsection 1) and dynamic muscle length (subsection 3) for (a) elbow flexors (r = 0.564, p = 0.000), (b) plantar flexors (r = 0.307, p = 0.009). There is some overlap in the scatterplot.
Discussion
The TSS is a synthesis of different responses for measuring muscle reaction, which addresses the velocity-dependent phenomenon. It measures spasticity on the basis of tonic and phasic stretch reflexes, and evaluates passive resistance and dynamic muscle length. After collecting and analysing the data for the scale, we demonstrated that the TSS was reliable. Although the TSS includes 3 subsections, it is scored through several manual stretches, thus it takes a similar time to complete to that of the MTS.
The problem of measurement of spasticity is more related to the lack of validity of measures of resistance than to their lack of reliability. Indeed, the reliability problem is due to the fact that the definition of spasticity underlying these scales lacks validity. The ability to regulate muscle force may be impaired in stroke patients because of a narrowing of the limits of regulation of stretch reflex threshold (SRT) (9). The stretch threshold assessment indicates the joint angle at which motoneuronal recruitment starts, i.e. the specific muscle length or respective joint angle at which the stretch reflex begins to act (9). In healthy subjects, the range of spatial SRT regulation exceeds the biomechanical joint range, whereas in stroke patients, the range of spatial SRT regulation limits within the range and results in motor deficits (36). Thus, from this perspective, the SRT measure achieves content validity (37, 38). SRT is considered a more sensitive method than passive resistance, which is a supra-threshold response to measure spasticity (36). Angle difference (also named dynamic muscle length) measurement is a clinical way to reflect DSRT. The MTS is unique in measuring and comparing the muscle reaction to passive stretch at both slow and fast speeds. Patrick & Ada (39) stated that the TS differentiates spasticity from contracture, whereas the AS is confounded by it; however, the reliability of angle difference may be insufficient (15, 16). Although the TSS, including the measurement of angle difference, was shown to have good reliability, the reliability of subsection 3 alone was insufficient in our study.
AS/MAS measures the resistance perceived by the rater, which is a sum of neural and non-neural components. The TSS provided an alternative clinical assessment; monitoring of the force during slow and fast passive movements. The increased resistance, which was mainly composed of neural components, was velocity dependent (6). In the TSS measurement, the rater should carefully determine the change in increased resistance between 1 slow stretch and 1 fast stretch, and then score the change. A larger score for AS does not mean a larger score in subsection 1. The method we propose agrees with Lance’s theory that spasticity is velocity-dependent. The viscosity also increased with increasing velocity, but the force generated by neural components is mostly much greater than the force generated by viscosity (6).
The stretch speed is an important factor that should be treated with care. When performing a slow stretch, we suggest that it should take approximately 30 s in the elbow and more than 10 s in the ankle (approximately 5°/s) to make a full range of passive movement (6), since the stretch reflex could be elicited at speeds as low as 8°/s in hemiplegic patients (9). The fast stretch we performed was as rapid as possible, which might elicit a strong spinal stretch reflex.
We found that there was a significant correlation between subsection 1 (increased resistance) and subsection 3 (dynamic muscle length), although Spearman’s correlation coefficient was not good. Therefore, it is estimated that the DSRT measures correlates with the supra-threshold resistance measure. It is possible that a velocity-dependent increased tone at the muscle level is an adaptive mechanism to compensate for the loss of velocity-sensitivity at the reflex level, thus partly maintaining stability of posture and movement in rigidity. For example, increased tone in the quadriceps often plays a role in weight-bearing. Therefore, it had been recommended that SRT and resistance measures could be used together to gain more insight into adaptive mechanisms related to deficits of muscle tone at different neuromuscular levels (36). The design of TSS takes this into account. Furthermore, the combined effects of positive and negative signs lead to a net balance of muscle torque across individual joints and impairs 2-way motion of the limb segments (4), while SRT of a spastic muscle should play a role in active movement of its antagonist.
Spasticity can be measured with clinical scales or mechanical devices and recording instrumentation. Although clinical scales do not provide a precise measure of spasticity, they are still commonly used because they are inexpensive and easy to use. A new scale might be valuable if the superiority is verified, or deduced theoretically. The TSS borrows the ideas of previous measures. Therefore, the TSS can be considered as just another “modified” version of the current scales (e.g. CSI). In theoretical comparisons of the TSS with AS or CSI, the former is more in line with Lance’s definition. In comparing the TSS with the MTS, the former addresses the neural resistance of passive stretch, and makes grading more readable and comparable (the X and Y value of the MTS are not in the same sequence).
Since an understanding of the clinical meaningfulness of a scale necessitates that the scale can differentiate between different levels of severity, we classified the spasticity as mild, moderate or severe according to the TSS scores, as Levin et al. did for the CSI (30). The total scores of TSS should be different in various muscles according to whether clonus is elicited. When clonus exists, the score of ankle plantar flexors may be different from that of elbow flexors if the same level of spasticity existed in these muscles. A modified version of the TSS without the clonus subsection may be developed in future, but considering the impact of ankle clonus on pathological gait pattern, we are not willing to remove this subsection.
A good assessment tool is required for choice of treatment, and this is a further aim of our design. Distinguishing neural from non-neural components is clinically very important for a reasonable treatment. We might make a preliminary judgment according to the scores of the TSS and its subsections. Taking into consideration that changes in mechanical muscle-fibre properties might contribute to spastic muscle tone (40), we assume that the clinical situation will involve dominant neural components if there is a higher score on the TSS, and dominant non-neural components if there is a lower score on the TSS with a comparatively higher score on the r2 of subsection 1.
The good quantity of reliability estimates is observed in the sample size of 50 or more (41). Gwet (42) stated that one should use a sample size of 100, 44 or 25, depending on the error margin of 20%, 30% and 40%, if one anticipates that the raters will agree approximately 50% of the time. Few studies on the reliability of the MAS and MTS have previously reached the criteria of adequate sample size. In fact, we finally enrolled 71 participants in order to meet the requirements of sample size. For nominal and ordinal data, reliability should be tested with kappa statistics. A weighted kappa, which assigns less weight to agreement as categories are further apart, could also been used in such an instance (43). When such a weighting system was applied, the weighted kappa would be equivalent to the ICC (44). For continuous data, such as R1–R2, the reliability should be measured with the ICC. Therefore, the ICC was chosen as the test statistic.
Study limitations
The TSS can be considered as a “modified” version of the current scales, with the same disadvantages as these scales. Although we analysed the correlations between the TSS and the MAS, and between the TSS and the MTS, the MAS and the MTS are not gold standards but only the commonly used scales. Electromyography should be a better alternative for this standard. Spasticity may fluctuate over the course of a day due to personal and environmental factors (45), thus the assessments could be performed at different times within a day or a week. In making decisions about optimal treatment, the patients’ perception of spasticity plays an important role. Further research is needed into the correlation between the TSS scores and the subjective perception of patients. The present study has some further limitations. First, r1–r2 was scored according to the perception of a rater, thus the accuracy of this subsection partly relies on the experience of assessors. Secondly, we did not study the reliability of the TSS in assessing other joints or segments, although we believed that the scale was applicable; further studies are needed in this area. Thirdly, the memory of rater 1 might have interfered with the results of test-retest reliability because the retest was conducted only 1 day after the initial test. The time interval could be designed to be 1 week. However, the status of spasticity might be altered significantly because some of the patients enrolled were in the acute phase of stroke. Fourthly, unlike the collection procedure introduced by Mehrholz et al. (15), R1 and R2 were measured by 1 rater who performed the stretch and read the values simultaneously in our study. Therefore, influence of memory may also have existed.
Conclusion
The TSS provides good test-retest reliability and inter-rater reliability in the measurement of spasticity. This newly-designed scale offers an alternative method of measuring spasticity, and avoids some of the shortcomings of previous measurements. In addition, the TSS may provide insight into adaptive mechanisms and active movement related to deficits in muscle tone at different neuromuscular levels, as well as estimation of active function. The TSS can be used for muscles around joints in which stretch reflex can be elicited. When measuring spasticity with the TSS, variations may be found in the highest scores for different muscles.
Acknowledgements
The authors would like to thank the Fund of Shanghai Science and Technology Commission for financial support (11DZ1921304).
References
