Lucy Dodakian, MA, OTR/L, Jill Campbell Stewart, PT, PhD and Steven C. Cramer, MD
From the Departments of Neurology and Anatomy and Neurobiology, University of California, Irvine, USA
OBJECTIVE: To examine the neural correlates of motor imagery performed in conjunction with movement of the paretic arm after stroke.
DESIGN: Cross-sectional, cohort study.
SUBJECTS: Seven individuals in the chronic phase of stroke recovery (median (range): age: 58 years (37–73); time post-stroke: 9 months (4–42); upper extremity Fugl-Meyer motor score: 48 (36–64)).
METHODS: Participants actively moved the paretic/right arm under two conditions while undergoing functional magnetic resonance imaging. In the motor condition, pronation/supination movements were made in response to a visual cue. In the motor + imagery condition, the same movements were performed in response to a visual cue but the participants were instructed to imagine opening and closing a doorknob during performance of the movement.
RESULTS: For the motor condition, the anticipated motor network was activated and included left sensorimotor cortex and right cerebellum. For performance of the same movements during the motor + imagery condition, additional brain regions were significantly engaged including the left inferior parietal lobule and right dorsolateral prefrontal cortex.
CONCLUSIONS: The addition of motor imagery to movement may provide a practical, accessible way to modulate activity in both the planning and execution components of the motor network after stroke.
Key words: stroke; motor imagery; upper extremity; functional neuroimaging.
J Rehabil Med 2014; 46: 843–848
Correspondence address: Steven C. Cramer, MD, University of California, Irvine Medical Center, 200 S. Manchester Ave., Suite 206, Orange, CA 92868, USA. E-mail: scramer@uci.edu
Accepted Apr 8, 2014; Epub ahead of print Sep 2, 2014
Introduction
Stroke continues to be a leading cause of disability (1) with persistent motor deficits being one of the most common long-term sequela (2). An inability to incorporate the hemiparetic arm and hand into the performance of functional activities has a negative impact on overall function and quality of life (3, 4). Currently available interventions that address upper extremity function vary in their effectiveness (2) and may be optimal for only a subgroup of individuals recovering from stroke (5, 6). Novel interventions are needed that include an understanding of how the stroke-damaged brain responds to behavioral manipulations.
Task-oriented training is a key component of many stroke rehabilitation programs aimed at improving motor function (7). One way to improve motor function during task-oriented training may be through movement execution and repetition. Several recent studies have suggested that increased training intensity via high levels of task repetition is an important driver of motor recovery after stroke (8–10). A parallel strategy to improve motor function may be through task conditions that target motor planning and the cognitive aspects of motor control which are important for the learning or relearning of novel motor skills (11). Motor imagery provides one avenue to target motor cognition during movement training (12).
In a strict sense, motor imagery is defined as the activation of a mental representation of a motor action without overt movement (13, 14). Motor imagery practice has shown promise as an intervention in rehabilitation after stroke (14, 15). A variety of brain regions that support movement are activated during motor imagery in nondisabled individuals. A recent meta-analysis of imaging studies in nondisabled adults (16) identified several motor planning and movement execution regions on both sides of the brain that were consistently active during imagery including premotor, prefrontal, and parietal cortices, basal ganglia, thalamus, and cerebellum. While the literature on individuals recovering from stroke is less robust, similar brain regions have been reported as playing a role in motor imagery after stroke, specifically bilateral premotor, prefrontal, and parietal cortices (17–20). If the goal of motor imagery in individuals post-stroke is to improve motor function, motor imagery performed concurrently with movement execution may be particularly attractive as a tool for motor rehabilitation, especially in situations where the ability to use real objects during movement is limited. However, the neural correlates of motor imagery performed during movement execution after stroke have not been reported.
The purpose of this pilot study was to examine the neural correlates of motor imagery performed in conjunction with a movement execution task with the paretic arm after stroke. We hypothesized that motor imagery during movement execution, compared with movement execution alone, would lead to additional brain activation in regions that participate in motor planning.
Methods
Participants
The participants in the current analysis were part of a larger study that investigated the effects of a robot-based therapy on hand motor function (21). The study protocol included 15 1.5-h training sessions over 3 weeks that focused on paretic hand grasp and release using a 3-degree-of-freedom, active-assist, pneumatic robot (22). Motor imagery was not part of the training protocol for any participant. All training sessions were supervised by a physical or occupational therapist. Eight participants in this study completed the functional magnetic resonance imaging (fMRI) protocol included in the current analysis, which took place immediately after the completion of training. All participants provided written informed consent in accordance with the University Institutional Review Board.
Entry criteria for the study included age > 18 years, stroke at least 3 months prior that resulted in right-hand weakness, a minimum of 10° of range of motion in the right index finger metacarpophalangeal (MCP) joint, score of 2 to 10 out of 24 total points on the hand motor function section of the upper extremity Fugl-Meyer (UE FM) (23), and time to complete the 9-hole pegboard test (24) that was at least 25% longer in the paretic hand (right) compared with the nonparetic (left) hand. Individuals were excluded if they presented with apraxia (score > 2.5 on the Alexander scale (25)), reduced attention (score > 0 on National Institutes of Health Stroke Scale (NIHSS) (26) questions 1a–c), substantial sensory loss (right hand Nottingham sensory score (27) < 75% of normal), severe increase in muscle tone (modified Ashworth spasticity score (28) of 4 for the right elbow, wrist, or MCP), severe aphasia (score of 2 on NIHSS question 9), major depression (Geriatric Depression Scale (29) score > 8), or another diagnosis having a major effect on hand function.
Motor task
During fMRI, each participant completed two task conditions that involved the same movement, pronation/supination of the right forearm, in response to the same visual cue. In the motor condition, individuals moved the forearm in pronation/supination in concert with movements of a stick-figure hand shown in a video moving at a rate of 0.125 Hz. In the motor + imagery condition, individuals performed the same movement along with the same video, however, immediately prior to scanning, additional imagery instructions were provided that cued the participant to focus on the functional intent of the task. Specifically, participants were instructed to make the same movement as in the motor condition but to “Imagine you are turning a doorknob when you are doing the movement. Imagine feeling the doorknob in your hand, imagine turning it like you are turning a real doorknob, feel your hand turning the doorknob to open it, then turning it back to close the knob”. During both conditions, a non-actuated, magnetic resonance imaging (MRI) compatible splint was worn to provide wrist support, and the range of motion was self-determined.
The motor condition was practiced in the laboratory immediately prior to the MRI session. This practice session included the same arm splint, video, and posture used during fMRI in order to closely mimic testing conditions. The video alternated between periods of rest (30 s, red cues) and periods of pronation/supination (30 s, green cues). Participants completed a full video (length of an fMRI run) accurately before moving to the MRI. The motor + imagery condition was not practiced prior to fMRI; individuals were only provided instructions for this condition while in the MRI, just prior to task completion, to insure that the imagery component would not contaminate the motor condition scan.
Magnetic resonance imaging data acquisition
All brain imaging sessions were performed on the same 1.5 Tesla MRI scanner (Philips Healthcare, Best, Netherlands). First, a high-resolution structural image was acquired, which included 150 1-mm thick slices with no interslice gap (acquisition voxel size 1 mm × 1 mm × 1 mm). Next, a total of 6 functional runs were completed, two runs for the motor condition and two for the motor + imagery condition; two additional runs were focused on a grasp/release condition and are not included in the current analysis (see Takahashi et al. (21)). Functional MRI data were acquired using a block design while participants moved (pronation/supination) in response to a visual cue presented via video. Periods of movement (30 s) during which visual cues were green alternated with periods of rest (30 s) during which visual cues were red. Each functional run lasted 2 min 5 s, during which 50 brain volumes were collected (repetition time = 2,500 ms, echo time = 40 ms); each volume included 25 axial slices that were 5 mm thick with no interslice gap (acquisition voxel size 1.8 mm × 1.8 mm × 5 mm).
The data included in the current analysis were collected during the post-training assessment session (immediately following 3 weeks of hand training). The two functional runs for the motor condition were completed first, followed by the two runs for the motor + imagery condition, in all participants. An investigator observed participant movement during scanning to verify task compliance.
Data analysis
All functional imaging data was analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). The first two volumes from each run were discarded due to tissue non-saturation. Volumes from each run were realigned to the first volume and resliced to account for motion artifact. The mean image for each participant was then normalized to the standard Montreal Neurological Institute (MNI) echo planar image template in SPM. The normalization parameters were applied to all of the functional volumes for that participant, and the normalized images were resampled to 2 mm × 2 mm × 2 mm voxels. All MNI transformed images were visually inspected to confirm that no distortions were introduced by the spatial normalization process. Images were then spatially smoothed with an isotropic Gaussian filter (FWHM = 8 mm).
First-level statistical analysis was performed separately for each individual participant to determine regions of significant activation using a general linear model (30). To determine the regions active during each of the two conditions (motor, motor + imagery), movement was contrasted with rest (Move > Rest); both runs for each condition were weighted equally in all contrasts. For first-level analyses, the first derivative of head motion for all 6 directions, which was uncorrelated with stimulus presentation, was added as a regressor of no interest in order to account for the effect of head motion in the data.
The contrast maps for each participant and each condition were moved to a second-level group random effects analysis to identify brain regions that were active during movement across the group of participants. To determine brain regions active during each condition, a one-sample t-test was used on the Move > Rest contrasts separately for the motor and motor+imagery conditions. Next, a paired t-test was used to determine differences in brain activation between conditions (motor > motor + imagery; motor + imagery > motor). For group comparisons, statistical significance was set at p < 0.001 at the voxel level (magnitude of activation) and p < 0.05 at the cluster level (extent of activation), both uncorrected for multiple comparisons.
Results
Participants
A total of 8 participants completed both the motor and motor + imagery conditions during the post-training fMRI session. Imaging data from one participant had excess head motion artifact and was excluded from further analysis. Therefore, data from 7 participants were included in the analysis (Table I). All 7 presented with hemiparesis on the right side per entry criteria, with 6 individuals being right-hand dominant and one left-hand dominant. The group had a median age of 58 years (range 37–73), median time post-stroke of 9 months (range 4–42), and a median UE FM motor score of 48 (range 38–64) at the end of training (time point that corresponded with fMRI data in current analysis). All 7 participants demonstrated improvement over the 3 week course of training, showing a median increase in UE FM motor score of 8 points (range 3–12) and in the number of blocks moved with the paretic arm on the Box & Blocks test of 6 (range 0–10).
|
Table I. Participant demographics |
|||||
|
Age/gender |
Months post-stroke |
UE FM motor |
Box & Blocks |
SIS hand section |
Stroke location |
|
73/M |
10 |
43 |
9 |
1.8 |
Pons |
|
63/F |
7 |
64 |
26 |
4.0 |
Corona radiata, PLIC |
|
50/M |
42 |
48 |
5 |
2.2 |
Corona radiata |
|
61/M |
12 |
62 |
55 |
4.4 |
Pons |
|
44/F |
4 |
38 |
0 |
1.4 |
Corona radiata, putamen, PLIC, temporal lobe |
|
37/F |
8 |
38 |
9 |
2.4 |
Pons |
|
58/M |
9 |
63 |
62 |
4.2 |
Putamen, PLIC, insula |
|
UE FM: Upper Extremity Fugl Meyer (max score = 66); Box & Blocks: number of blocks moved in one minute with right hand; SIS: Stroke Impact Scale (score = mean of 5 items in hand subsection; max score = 5); PLIC: posterior limb of the internal capsule; M: male; F: female. |
|||||
Brain activation during task performance
All participants actively moved in pronation/supination during the Move epochs for all functional runs. One participant had small visible mirror movements in the left hand and 5 participants had visible movement of the foot or elbow at least one time. Brain activation during the motor and motor + imagery conditions is shown in Fig. 1 and Table II. For the motor condition, activation was focused in the motor system: left sensorimotor cortex and right cerebellum. For the motor + imagery condition, activation was overall greater and included the same regions as the motor condition, however, additional regions were significantly recruited (right inferior parietal lobule (IPL), left cerebellum). When a lower statistical threshold (p < 0.01) was used for exploratory purposes, left IPL and bilateral supplementary motor area were additionally found to be active during motor + imagery.

|
Table II. Location of significant clusters for each condition and their comparison |
||||||
|
Condition |
Brain region |
Volume |
Peak Z |
MNI Coordinates |
||
|
x |
y |
z |
||||
|
Motor |
R Cerebellum |
105 |
5.04 |
30 |
–56 |
–28 |
|
L Sensorimotor cortex |
32 |
3.40 |
–28 |
–24 |
52 |
|
|
Motor + imagery |
R Cerebellum |
38 |
3.92 |
38 |
–70 |
–26 |
|
R Sensory cortex |
37 |
3.65 |
62 |
–26 |
34 |
|
|
L Cerebellum |
37 |
3.64 |
–26 |
–56 |
–30 |
|
|
R Inferior parietal lobule |
70 |
3.59 |
48 |
–54 |
52 |
|
|
L Sensorimotor cortex |
42 |
3.43 |
–26 |
–26 |
58 |
|
|
Motor + imagery > motor |
R Dorsolateral prefrontal cortex |
68 |
4.14 |
46 |
22 |
38 |
|
L Inferior parietal lobule |
51 |
3.81 |
–56 |
–54 |
40 |
|
|
|
L Inferior parietal lobule |
37 |
3.77 |
–54 |
–38 |
50 |
|
All regions were significant at p < 0.001 (corresponding to Z > 3.09), uncorrected for multiple comparisons. Volume: number of 8 mm3-voxels in the cluster; Peak Z: peak Z-value within the cluster; R: right; L: left. Note that there were no significant clusters for the comparison motor > motor + imagery. |
||||||
The paired t-test between conditions revealed 3 significant clusters that were more active during the motor + imagery condition compared to the motor condition located in right dorsolateral prefrontal cortex (DLPFC) and left IPL (Fig.1, Table II). These two regions were not reported as being significantly active in the one-sample t-test of the motor + imagery condition alone. For both of these regions, the significant difference between conditions was driven by two factors: (i) little to no activation (IPL) or decreased activation (DLPFC) during the motor task, plus (ii) increased activation during the motor + imagery task (Fig. 2). Thus, while the absent or decreased activation in (i) and the increased activation in (ii) were not significant by themselves, the difference between them (as measured by the paired t-test) was significant. Note that when a lower statistical threshold (p < 0.01) was used for exploratory purposes, right IPL and right posterior cingulate cortex were also found to be more active during the motor + imagery condition. No brain regions were more active in the motor condition compared with the motor + imagery condition.
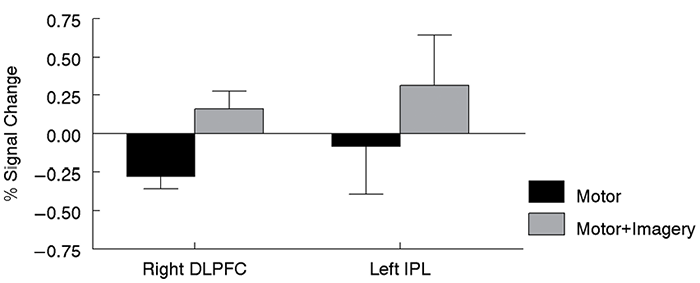
Discussion
To our knowledge, this is the first study to investigate the neural correlates of motor imagery done in conjunction with movement in individuals with hemiparesis after stroke. Motor imagery during movement activated similar brain regions as movement alone but also engaged additional regions previously shown to play a role in motor planning and motor imagery, specifically IPL and DLPFC. Task conditions that alter cognitive context (11) and modulate activation of the motor network during movement may be useful tools for rehabilitation. In young, nondisabled adults, conditions of motor task practice that benefit learning on a delayed retention test often show greater utilization of brain regions that support movement planning during practice (31, 32). Integration of motor planning via the addition of motor imagery to movement may provide a practical, accessible way to increase engagement of planning regions during movement after stroke.
Activation of the IPL and DLPFC during motor imagery has been commonly reported in nondisabled adults (14, 16). The IPL plays a role in the performance of goal-directed, grasping actions (33, 34). The addition of motor imagery to movement may increase the functional meaning of the movement and, therefore, lead to increased activity in this region. The DLPFC has been shown to play a variety of roles in the cognitive control of movement (35). The role of DLPFC for the control of movement may increase with age (36, 37) and after stroke (38). Activation of DLPFC during motor imagery done simultaneously with movement suggests an increased utilization of attentional resources to movement similar to motor imagery without movement. It cannot be determined from the current study whether increased activation in IPL and DLPFC would be beneficial or detrimental to the learning or relearning of motor skills after stroke. Task conditions that engage a larger component of the motor network may provide a level of challenge that benefits learning for some individuals and is overwhelming for others. However, the findings in the current study suggest that motor imagery during movement may provide a tool to modulate this activation during motor practice.
The functional intent of movement has been shown to have a behavioral impact on movement. In general, when movement includes a functional context (e.g., is goal-directed and involves a functional object), movement quality improves and is kinematically closer to that of nondisabled controls (39, 40). In individuals who lack the hand function to be able to physically interact with objects or during the performance of rote exercises, however, the ability to add functional context during movement can be limited. The addition of motor imagery to motor practice under these conditions may add some degree of functional meaning and, concordantly, have an effect on movement quality and neural activation. Additionally, the attentional valence and salience of motor practice are key factors in experience-dependent learning (41, 42). Motor imagery performed during movement may introduce increased attention and saliency in a manner that benefits learning and recovery. Based on the findings of the current study, future research could investigate the behavioral and neural effects of a period of motor imagery done in combination with motor task practice in individuals post-stroke.
There are a number of limitations and opportunities for future research in the current study. This was a small pilot study. While significant differences in activation between conditions were found, the small sample size may limit generalization of these results to individuals post-stroke who have a clinical presentation similar to the current patient cohort. Movement of the limbs during fMRI was not explicitly measured in the current study. Future research could benefit from quantitative measurement of movement to ensure compatibility between task conditions. The motor + imagery condition always followed the motor condition, an approach that was used to insure that brain events related to imagery did not in any way influence the motor condition, but which introduced a potential ordering effect that might have influenced results. The participants in the current study were all in the chronic phase of stroke recovery. Access to the motor system via motor imagery during moment may be different in the acute or subacute phases of stroke recovery.
In conclusion, motor imagery during movement increased brain activation in regions that support goal-directed, complex movement, IPL and DLPFC. The addition of motor imagery to movement may provide a practical, accessible way to modulate brain activation in both the planning and execution components of the motor network after stroke. Selective engagement of distinct components of the motor network via motor imagery during movement warrants further investigation.
Acknowledgments
This research was supported by the National Center of Research Resources, 5M011 RR-00827-29 and NS059909, US Public Health Service (SCC) and by grant T32 AR047752 from the National Institutes of Health (JCS). Steve Cramer has served as a consultant for GlaxoSmith Kline and MicroTransponder.
References
