Fary Khan, MBBS, MD, FAFRM (RACP)1,2,3, Bhasker Amatya, MD, MPH1, Kate Drummond MBBS, MD, FRACS3,4 and Mary Galea, PhD, BAppSci (Physio), BA, Grad Dip Physio, Grad Dip Neurosci1,3
From the 1Department of Rehabilitation Medicine, Royal Melbourne Hospital, 2School of Public Health and Preventive Medicine, Monash University, 3Department of Medicine (Royal Melbourne Hospital), The University of Melbourne and 4Department of Neuroscience, Royal Melbourne Hospital, Parkville, Victoria, Australia
OBJECTIVE: To evaluate effectiveness of a multidisciplinary rehabilitation program for persons following definitive primary brain tumour treatment in a community cohort.
METHODS: The brain tumour (glioma) survivors (n = 106) were allocated either to the treatment group (n = 53) (intensive ambulatory multidisciplinary rehabilitation), or the waitlist control group (n = 53). The primary outcome – Functional Independence Measure (FIM), measured ‘Activity’ limitation; secondary measures included Depression, Anxiety Stress Scale, Perceived Impact Problem Profile and Cancer Rehabilitation Evaluation System. Assessments were at baseline, 3 and 6 months after program completion.
RESULTS: Participants were predominantly women (56%), with mean age 51 years (standard deviation 13.6) and median time since diagnosis of 2.1 years. Intention-to-treat analysis showed a significant difference between groups at 3-month in favour of multidisciplinary rehabilitation program in FIM motor subscales: ‘self-care’, ‘sphincter’, ‘locomotion’, ‘mobility’(p < 0.01 for all); and FIM ‘communication’ (p < 0.01) and ‘psychosocial’ subscales (p < 0.05), with small to moderate effect size (r = 0.2–0.4). At 6-month follow-up, significant improvement in the treatment group was maintained only for FIM ‘sphincter’, ‘communication’ and ‘cognition’ subscales (p < 0.01 for all). No difference between groups was noted in other subscales.
CONCLUSIONS: brain tumour survivors can improve function with multidisciplinary rehabilitation, with some gains maintained up to 6 months. Evidence for specific interventions in the ‘blackbox’ of rehabilitation is needed.
Key words: brain tumour; rehabilitation; participation; quality of life; function.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Prof Fary Khan MBBS, MD, FAFRM (RACP), Director, Department of Rehabilitation Medicine, Royal Melbourne Hospital, Poplar Road, Parkville, Melbourne VIC 3052, Australia. E-mail: fary.khan@mh.org.au
Accepted Mar 31, 2014; Epub ahead of print Jun 4, 2014
INTRODUCTION
Primary brain tumours (BT) account for 2% of all cancers (1) and affect 7 per 100,000 population worldwide annually (2). The overall incidence of BT has increased, especially in patients over 60 years of age (3). In Australia, the estimated number of new cases of BT are approximately 1,400 per annum; and over 1,200 deaths annually (4). BT can be a source of disability and morbidity; associated with significant costs and socioeconomic implications with increased demand for health care, social and vocational services; and caregiver burden.
Current therapeutic advances in the treatment of BT have resulted in improved survivorship (5, 6). The mainstay of treatment is maximal safe surgical resection of the tumour followed by radiation therapy and chemotherapy as indicated (3, 6). These treatment regimens can produce adverse effects (7). Despite various treatment options, patients suffer significant medium to longer-term functional and psychosocial impairments that limit daily activity and participation (6, 7).
The International Classification of Functioning, Disability and Health (ICF) (8) framework defines a common language for describing the impact of disease at different levels. For example, BT related ‘impairments’ (headaches, seizures, neurocognitive dysfunction, paresis, dysphasia), can limit ‘activity’ (decreased mobility, inability to self-care) and ‘participation’ (work, family, social reintegration), and reduce life span (9). One study reported that a quarter of the adult survivors of childhood BTs experience visual and/or hearing deficits (23%), loss of sensation (21%) (10) and significantly lower muscle strength (grip, knee extension) and exercise tolerance compared with their matched counterparts (10). These disabilities can have a cumulative effect over time and cause considerable distress to the cancer survivors and their families, and reduce quality of life (QoL). Patients discharged back to the community are confronted by various ongoing concerns (relationship, employment, recurrence) in the medium to longer-term (11). Families often struggle to cope with new demands associated with increased care needs, inability to return to driving and work, financial constraints, marital stress and general limitation in patients’ participation.
BT is a complex and challenging condition requiring integrated multidisciplinary care and services. The UK National Service Framework for Long-Term Neurological conditions (12) (including BT), advocates 11 Quality-Requirements for integrated, life-long, person-centred care, and provides guidelines to explore the interaction between neurology, rehabilitation and palliative care services (neuropalliative-rehabilitation), in hospital and community. Needs must be defined so services can be matched for these individuals to optimise care. However, no studies currently utilize this proposed model of care in the BT population.
Although several studies evaluate functional outcomes for BT survivors from a multidisciplinary rehabilitation (MDR) perspective (7, 13–21), many are methodologically flawed (bias, lack of control group and blinding). A recent systematic review of MDR for BT (22) identified no randomized or controlled clinical trial (RCT/CCT) in this area. However, 10 observational studies of ‘poor’ methodological quality provided ‘weak evidence’ for short-term gains for impairment, psychosocial adjustment and QoL. No studies explored participation (social reintegration, return to driving, work) and none were in the Australian context. To date no clinical trials have evaluated effectiveness of MDR outcomes or comparisons of different methods of treatment in these persons. Therefore, the aim of this study was to evaluate the effectiveness of MDR in persons after BT treatment in an Australian community cohort. The effectiveness of rehabilitation in these survivors was expected primarily in the ‘activity’ domains and secondly in ‘participation’.
METHODS
Participants and setting
This prospective CCT was part of rehabilitation research program for BT survivors (gliomas), conducted at the Royal Melbourne Hospital (RMH), a tertiary referral centre in Victoria, Australia. The RMH MDR program provides intensive individualized treatment for BT survivors both in ambulatory and inpatient settings.
The recruitment process has been reported previously (11, 23). A clinical quality improvement audit at RMH identified 862 consecutive admissions for acute care between 2007–2011, with the International Classification Diseases (ICD) Code (C71) for BT (main diagnosis) incorporating all 10 sub-codes (C71.0–71.9) (excludes cranial nerves). These included same and multiday patients and those with recurrent admissions (details available from authors). The RMH Database was used for cross-indexing of diseases from HOMER using the Patient Administrator System of the Hospital Information Systems. The source of these patients was a pool of persons residing in the community, referred to the RMH from public and private medical clinics across greater Melbourne in Victoria. All patients were aged 18 years and over and fulfilled criteria for BT grading system (Grade I–IV) for gliomas as outlined by the WHO for Central Nervous System Tumours (24); and assessed by a surgeon/oncologist. The inclusion criteria for intensive MDR included: stable medical course, post BT surgery, radiotherapy and/or chemotherapy, assessed by a rehabilitation physician/neurosurgeon for presence of neurological deficits and ability to participate in therapy up to 2.5 h of interrupted therapy/day; and the clinical judgment of the assessing rehabilitation team. These participants resided in the community (area of greater Melbourne < 60 km radius), and were able to communicate in English. Those who had benign or metastatic BTs, significant co-morbidities or medically unstable, or psychiatric disorders (such as uncontrolled schizophrenia, actively suicidal/self-harm or physically aggressive (based on clinical judgement)) limiting participation in rehabilitation, those bed-bound and/or institutionalized in nursing homes were excluded.
The study was approved by the Royal Melbourne Hospital Ethical Committee (No. 2010.216) and informed consent was obtained from all the subjects.
Procedure
Group allocation. All eligible patients (n = 152) based on selection criteria were contacted by mail and invited to participate in this project by an independent project officer. A total of 106 subjects who consented were recruited for the study (Fig. 1). Consistent with usual care procedures, all patients were mailed an information package (containing standard BT education, information and support services details). After written informed consent, all participants were assessed and baseline data was obtained in a subacute clinical settings. All patients were allocated either to the treatment or control group by the treating team based on their clinical need. Attempts were made to ensure equal distribution of more aggressive BTs (based on histology) in each group. The treatment group (n = 53) received an individualized intensive ambulatory (centre-based) MDR, while the waitlist patients were the control group (n = 53), who continued with their usual activity in the community (see details below), monitored by their surgeon/oncologist and/or family doctors. The control group were informed that it could take between 2 to 3 months before they received intensive rehabilitation, consistent with current practice.
Assessment interviews. After group allocation all baseline assessments and interviews were completed using a structured format, by 3 independent experienced trained researchers over a 6-week period. These assessors (two physicians and a research officer) received a 3-day training session in cognitive and functional ability assessments examined and accredited by a national body (the Australian Rehabilitation Outcomes Centre). They were not in contact with the acute surgical/oncological or the treating rehabilitation teams. They did not share information about participants or assessments, and received separate and different clinical record forms at each interview. The information obtained included: demographic and disease-related information, cognitive and functional ability assessment and health-related QoL measures using standardized instruments (see measures). These interviews took approximately 40 min, with appropriate rest breaks. The assessors did not prompt participants, but provided assistance for those who had difficulty completing the questionnaires. All participants were evaluated at recruitment and at 3 and 6 months after completion of their rehabilitation program by the same three assessors. The assessors did not have access to previous assessments, treatment schedules or treating rehabilitation therapy team documentation. Participants were instructed to make no comments on whatever treatment they received in the time interval between examinations and only to report any concurrent illness or hospitalization. The control group was monitored in the community as per usual by their treating general practitioners/oncologists/surgeons. All assessments were secured and filed, and opened only at the time of entry into the database by an independent data entry officer.
Treatment schedules
Participants in the treatment group received comprehensive individualized MDR (for up to 6–8 weeks) over the study period. An assessment of each patient’s potential to benefit from MDR program was based on clinical features, individual need and accessibility to services, and made by a treating therapy team, who were not aware of the allocation of patients in the trial. They assessed these patients along with the usual referrals from the community referred by general practitioners, community health centres and other hospitals (general, surgical and oncology units) for a range of disabilities, consistent with current practice.
The MDR program included intensive treatment beyond symptomatic management of BT, which was individualized, achievable, time-based, functional, and goal-oriented with active involvement of patient (and family). The MDR incorporated a wide range of elements, such as education and health promotion for those mildly affected, to intensive mobilization and task reacquisition programs for the more severely affected patients. Consistent with existing practice at the RMH, MDR comprised half-hour blocks of therapy sessions (Social, Psychology, Occupational Therapy and Physiotherapy), 2 to 3 times per week for up to 8 weeks. A priori compliance with treatment was defined as participant attendance in > 80% of treatment sessions. Rehabilitation assessments for the treatment group were completed within one week of admission to the program. Structured weekly team case conferences assessed patient progress and goal setting. Adverse effects of rehabilitation were noted (falls, injury during treatment).
Measurement
The ICF (8) was used as a conceptual basis for choice of best outcomes for measurement.
BT-related information. This included: socio-demographic data, co-morbid conditions (diabetes, ischemic heart disease), presence of dysphasia; and BT lesion size, type, tumour grade and localization; and treatments received.
Measures of impairment. Visual Analogue Pain scale assessed pain (score range 0 to 10), with higher score indicating greater pain.
Measures of activity. Functional Independence Measure (FIM) (25) assessed the burden of care. The FIM has 18 categories: motor section with 13-items assessed level of function in 4 subscales: Self-care, Transfers, Locomotion and Sphincter control; and Cognition with 5 items. Participants rated each item on a scale of 1 to 7 (1 = total assistance, 2 = moderate assistance, 3 = maximal assistance, 4 = minimal assistance, 5 = needs supervision, 6 = modified independence, 7 = independent). The score reflects dependency in each area measured.
Measures of participation. Cancer Rehabilitation Evaluation System-Short Form (CARES-SF) (26), a self-administered 59-item measure, assessed cancer-specific rehabilitation need and QoL. Global scores indicated QoL with summary scores for the 5 domains: physical, psychosocial, medical interaction, marital and sexual function. The participant rated the degree to which a given problem applied during the 4 weeks before the survey. Scoring was based on a 4-point Likert scale, with higher scores indicating more difficulty or impairment.
Depression Anxiety Stress Scale-21 (DASS) (27), a 3 7-item self-report scale measured the negative emotional states of depression, anxiety and stress. Participants rated the extent to which they experienced each state over the past week on a 4-point Likert rating scale. Sub-scale scores were derived by totalling the scores, and multiplying by two to ensure consistent interpretation with the longer DASS 42-item version.
Perceived Impact of Problem Profile (PIPP) (28), a 23-item scale with 5 subscales: Mobility, Self-care, Relationships, Participation and Psychological Well-being, assessed the impact associated with BT. For each item, respondents were asked to rate ‘how much impact has your current health problems had on (item of function or activity)’, using a 6-point scale (‘no impact’ and ‘extreme impact’), with high scores indicating greater impact.
Statistical analysis
The FIM (motor) was the primary outcome for this study. The study was powered with 36 patients in each group needed for a 80% chance to detect a 3-point difference in FIM from baseline to 6 months in intervention versus control groups, assuming similar standard deviation (SD) change of 8.5 in both groups (two-sided alpha = 0.05). The primary analyses were conducted using analysis of covariance (ANCOVA), comparing the post-treatment scores (3 and 6 months follow-up) for the control and treatment groups, with the baseline score as a covariate. Mann-Whitney U tests compared change scores on each of the outcome measures (FIM, CARES-SF, DASS and PIPP) (baseline minus first and second post-treatment follow-up) for the treatment and control groups. Effect size statistics (r) were calculated and assessed against Cohen’s criteria (0.1 = small, 0.3 = medium, 0.5 = large effect) (29). Additional analyses were conducted comparing change scores on all measures. A p-value of < 0.05 was considered statistically significant. Analyses were on an intention-to-treat (ITT) basis, with patients assigned according to their initial allocation irrespective of their subsequent compliance to the protocol.
RESULTS
Of the 106 participants, 53 each were allocated to the treatment and control groups. Four participants in each group dropped out at the 3-month follow-up assessment (T2) and a further 13 dropped out (8 in intervention group and 5 in control group) at the 6-month assessment (T3) (Fig. 1). None in the control group required treatment during the study period. There was 96% compliance with treatment programme, as per the apriori compliance definition.
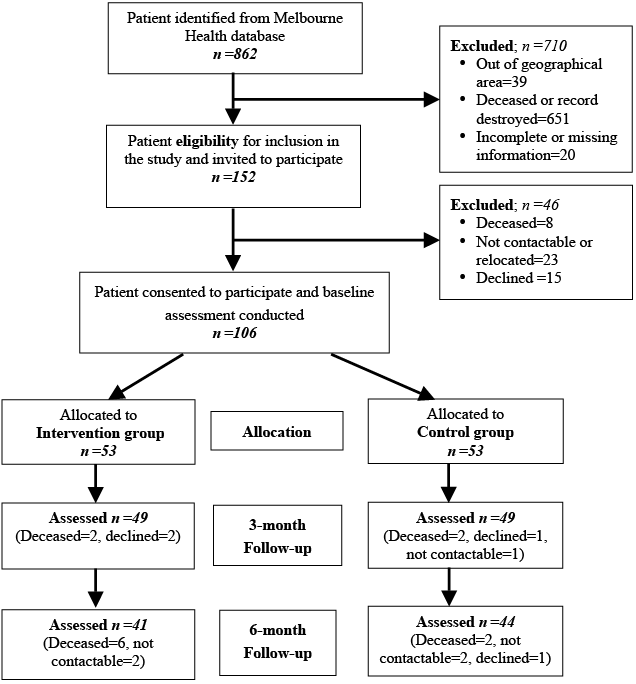
Fig. 1. Flow chart of recruitment process.
Baseline characteristics
Participants’ socio-demographic and clinical characteristics at baseline (T1) are summarized in Table I. Mean age of the participants was 51 years (SD 13.6) (range 21–77 years), most were female (56%) and median time since diagnosis was 2.1 years (interquartile range (IQR) 0.9–4.0). Although both groups were well matched for demographic and clinical characteristics, the control group had slightly longer disease duration (median 2.3 years, IQR 0.8–5.5 vs. 1.9 years, IQR 0.8–3.8 years) and higher grade tumours (21 vs. 16), compared with the treatment group; this however, was not statistically significant. Participants in both groups had high levels of functional independence (high Medical Research Council scores). Although BT-related symptoms (and pain/headache) were prevalent in both groups (Table I), the treatment group reported significantly higher ataxia/incoordination, dysarthria and visual problems. The mean duration of the rehabilitation program was 21 days (range 14–32 days). No adverse events were reported in either group. There was no significant difference between participants lost to follow-up and those who provided post-treatment results in terms of gender, age, BT duration and median scores for measures used.
|
Table I. Socio-demographic and clinical characteristics of participants (n = 106) |
||
|
Characterisitics |
Intervention group (n = 53) |
Control group (n = 53) |
|
Demographic factors |
||
|
Age, years, mean (SD) [range] |
53.1 (13.3) [21–77] |
49.6 (13.8) [28–74] |
|
Sex, female, n (%) |
31 (58.5) |
30 (56.6) |
|
Marital status, n (%) |
||
|
Married/Partner |
41 (77.4) |
40 (75.5) |
|
Single/Divorced/Separated/Widow |
12 (22.6) |
13 (24.5) |
|
Living status, n (%) |
||
|
Alone |
9 (17.0) |
9 (17.0) |
|
Partner/Family |
43 (81.1) |
44 (83.0) |
|
Education, n (%) |
||
|
Secondary |
24 (45.3) |
31 (58.5) |
|
Tertiary |
24 (45.3) |
18 (34.0) |
|
Smokers, n (%) |
9 (17.0) |
8 (15.1) |
|
Consumes alcohol, n (%) |
21 (39.6) |
24 (45.3) |
|
Clinical characteristics |
|
|
|
Disease duration, years, median, (IQR) |
1.9 (0.8–3.8) |
2.3 (0.8–5.5) |
|
WHO tumour grade (Gliomas)a (n = 96), n (%) |
||
|
Grade I |
10 (20.8) |
4 (8.3) |
|
Grade II |
12 (25.0) |
18 (37.5) |
|
Grade III |
10 (20.8) |
5 (10.4) |
|
Grade IV |
16 (33.3) |
21 (43.8) |
|
Treatment, n (%) |
||
|
Steriods during treatment |
36 (67.9) |
32 (60.4) |
|
Surgery, ≥ 2 surgery episodes (n = 105) |
15 (28.3) |
18 (34.6) |
|
Chemotherapy |
24 (45.3) |
27 (50.9) |
|
Radiotherapy |
33 (62.3) |
35 (66.0) |
|
Currently on medications (n = 99), n (%) |
23 (46.0) |
17 (34.7) |
|
Co-morbidities (n = 65), n (%) |
||
|
Hypertension |
16 (44.4) |
14 (48.3) |
|
Diabetes |
3 (8.3) |
2 (6.9) |
|
Depression |
4 (11.1) |
3 (10.3) |
|
Pain/headache, n (%) |
31 (58.5) |
28 (52.8) |
|
Pain score (n = 59), median (IQR) |
5 (3–7) |
3 (2– 5) |
|
Pain scoreb > 5, n (%) |
10 (32.3) |
6 (24.1) |
|
Limb weakness (MRC motor scale)c, median (IQR) |
||
|
Left upper limb |
4 (4–5) |
4 (4–5) |
|
Right upper limb |
4 (4–5) |
4 (4–5) |
|
Left lower limb |
4 (4–5) |
4 (4–5) |
|
Right lower limb |
4 (4–5) |
4 (4–5) |
|
Symptoms, n (%) |
||
|
Ataxia/incoordination* |
20 (62.3) |
14 (26.4) |
|
Seizures |
25 (47.2) |
20 (37.7) |
|
Paresis |
25 (47.2) |
14 (26.4) |
|
Cognitive impairment |
24 (45.3) |
20 (37.7) |
|
Visual impairment* |
25 (47.2) |
12 (22.6) |
|
Dysphasia (residual expressive) |
25 (40.5) |
17 (32.1) |
|
Dysarthria* |
18 (28.6) |
7 (17.0) |
|
Sensory-perceptual deficit |
14 (26.4) |
11 (20.8) |
|
Bowel/bladder dysfunction |
11 (20.8) |
10 (18.9) |
|
QoLd, median, (IQR) |
3 (2–3.5) |
3 (2–4) |
|
QoL score > 3, n (%) |
13 (24.5) |
16 (30.2) |
|
*p < 0.05. aWHO grading (gliomas): Grade I: slow growing, discrete, often surgical cure e.g., Astrocytic tumours; Grade II: slow growing but ability to invade adjacent normal tissue and higher grade of malignancy e.g., Oligodendrogliomas; Grade III: tumours actively reproducing abnormal cells that can infiltrate adjacent cells e.g., anaplastic oligodendroglioma; Grade IV: highly malignant and infiltrating into adjacent tissue e.g., Glioblastoma. bNo pain: 0, extreme pain: 10. cNo contraction: 0, normal power: 5. dDelighted: 0, terrible: 6. IQR: Interquartile range; MRC: Medical Research Council; QoL: quality of life; ROM: range of motion; SD: standard deviation; WHO: World Health Organisation. |
||
Outcome measurements change scores
Summary data for all outcome measures at different time periods are provided in Table II.
Short-term subjective outcomes. At 3 months post-treatment follow-up, Mann-Whitney U tests revealed a significant difference between treatment and control group participants in all FIM subscales: ‘self-care’, ‘sphincter’, ‘locomotion’, ‘mobility’, ‘communication’ (p < 0.01 for all), with small to moderate effect size (ES) (r = 0.3 to 0.4) and FIM ‘psychosocial’ subscale (p < 0.05, r = 0.2). There were no significant, short-term effects on other scores (Table II).
Longer-term subjective outcomes. At 6 months follow-up, compared to the control group, statistically significant improvement in the treatment group was maintained for the FIM ‘sphincter” (p < 0.01, r = 0.4) and ‘communication’ (p < 0.01, r = 0.5) subscales; and ‘psychosocial’ and “cognition’ (p < 0.01 for both), compared to control group. No difference between groups was noted in other subscales (Table II).
|
Table II. Summary of intention-to-treat analysis of outcomes of rehabilitation program |
|||||||||||||
|
Scales |
Intervention group |
Control group |
|||||||||||
|
T1 (baseline) (n = 53) Median (IQR) |
T2 (3-month) (n = 49) Median (IQR) |
T3 (6-month) (n = 41) Median (IQR) |
T1 (baseline) (n = 53) Median (IQR) |
T2 (3-month) (n = 49) Median (IQR) |
T3 (6-month) (n = 44) Median (IQR) |
Z values |
Effect size |
||||||
|
T1–T2 |
T1–T3 |
T1–T2 |
T1–T3 |
||||||||||
|
FIM Motor |
67 (61.5–75.5) |
85 (76.5–88) |
79 (67.5–86) |
70 (65–78) |
78 (78–78) |
74 (69–78) |
–3.13** |
–2.33* |
0.32 |
0.25 |
|||
|
Self-care |
32 (31–36) |
38 (30–41) |
36 (30–41) |
36 (32–36) |
36 (36–36) |
34 (32–36) |
–2.67** |
–1.90 |
0.27 |
0.21 |
|||
|
Sphincter |
11 (10–12) |
13 (13–14) |
14 (13–14) |
12 (10–12) |
12 (12–12) |
12 (11–12) |
–4.10** |
–4.05** |
0.41 |
0.44 |
|||
|
Locomotion |
10 (6–11) |
12 (10–13.5) |
11 (9–12) |
10 (10–12) |
12 (12–12) |
11.5 (10–12) |
–2.84** |
–0.89 |
0.29 |
0.10 |
|||
|
Mobility |
15 (15–18) |
20 (18–21) |
18 (15–20) |
15 (15–18) |
18 (18–18) |
18 (15–18) |
–3.01** |
–1.91 |
0.30 |
0.21 |
|||
|
FIM cognition |
25 (23–28.5) |
31 (27–33) |
31 (27.5–33) |
27 (23.5–29) |
30 (29–30) |
28.5 (26–30) |
–1.99* |
–3.09** |
0.20 |
0.34 |
|||
|
Communication |
10 (10–12) |
13 (12–14) |
13 (11.5–14) |
12 (10–12) |
12 (12–12) |
12 (10.3–12) |
–2.60** |
–4.86** |
0.26 |
0.53 |
|||
|
Psychosocial |
5 (5–6) |
6 (6–7) |
6 (5.5–7) |
5 (5–6) |
6 (6–6) |
6 (5–6) |
–2.05* |
–3.53** |
0.21 |
0.38 |
|||
|
Cognition |
10 (8–11) |
12 (8–13) |
12 (10–13) |
10 (9–11) |
12 (11–12) |
11 (10–12) |
–0.09 |
–2.51** |
0.01 |
0.27 |
|||
|
DASS |
|||||||||||||
|
Total |
20 (6–30) |
12 (2–27) |
8 (1–24) |
16 (4–23) |
4 (1–11) |
6 (2–21.5) |
–0.53 |
–0.98 |
0.05 |
0.11 |
|||
|
Depression |
8 (2–14) |
4 (0–12) |
2 (0–12) |
6 (0–11) |
2 (0–4) |
3 (0–9.5) |
–0.33 |
–1.9 |
0.03 |
0.21 |
|||
|
Anxiety |
4 (0–7) |
0 (0–4) |
0 (0–4) |
2 (0–6) |
0 (0–2) |
0 (0–2) |
–0.76 |
–0.23 |
0.08 |
0.02 |
|||
|
Stress |
6 (2,14) |
6 (0–9) |
4 (0–11) |
6 (0–15) |
2 (0–8) |
2 (0–8) |
–0.46 |
–0.19 |
0.05 |
0.02 |
|||
|
PIPP |
|||||||||||||
|
Total |
63 (48–80.5) |
57 (47–76) |
58 (39.5–91) |
45 (35–59.5) |
38 (33–51) |
35.5 (28.3–58) |
–0.40 |
–0.37 |
0.04 |
0.04 |
|||
|
Psychological |
3.6 (2.7–4.8) |
3.3 (2.5–4.4) |
3.8 (2.4–4.6) |
3 (2.1–4.6) |
2.4 (1.6–3.2) |
2.2 (1.4–2.9) |
–0.67 |
–0.98 |
0.07 |
0.11 |
|||
|
Self-care |
1.5 (1–2.5) |
1.3 (1–2) |
1 (1–2.9) |
1 (1–1.4) |
1 (1–1.3) |
1 (1–1.3) |
–1.03 |
–0.44 |
0.10 |
0.05 |
|||
|
Mobility |
2.8 (2–3.5) |
2.6 (2–3.6) |
2.4 (1.6–4.2) |
1.8 (1–2.6) |
1.6 (1–2.2) |
1.7 (1–2.4) |
–1.11 |
–0.86 |
0.11 |
0.09 |
|||
|
Participation |
3.4 (2.3–4.5) |
3.6 (2.2–4.4) |
3.4 (2–4.8) |
2 (1.4–3.7) |
2 (1.2–2.6) |
1.8 (1–3.2) |
–0.04 |
–0.04 |
0.00 |
0.00 |
|||
|
Relationship |
1.3 (1–2.3) |
1.5 (1–2.4) |
1.5 (1–3.3) |
1.3 (1–1.8) |
1 (1–1.5) |
1 (1–2.1) |
–0.36 |
–1.13 |
0.04 |
0.12 |
|||
|
CARES-SF (global) |
|||||||||||||
|
Physical |
1.3 (0.8–1.8) |
1 (0.5–1.6) |
1 (0.4–1.9) |
0.7 (0.3–1.1) |
0.3 (0–0.6) |
0.3 (0.1–1.2) |
–0.18 |
–0.13 |
0.02 |
0.01 |
|||
|
Psychological |
0.7 (0.4–1.3) |
0.6 (0.3–1) |
0.4 (0.1–1.1) |
0.5 (0.3–1.1) |
0.2 (0.1–0.6) |
0.3 (0.1–0.7) |
–1.02 |
–0.49 |
0.10 |
0.05 |
|||
|
Medical |
0 (0–0.5) |
0 (0–0.3) |
0 (0–0.6) |
0 (0–0.5) |
0 (0–0) |
0 (0–0) |
–1.07 |
–0.93 |
0.11 |
0.10 |
|||
|
Marital |
0.2 (0–0.8) |
0.2 (0–0.8) |
0 (0–0.8) |
0.2 (0–0.4) |
0 (0–0.2) |
0 (0–0.2) |
–1.08 |
–0.09 |
0.11 |
0.01 |
|||
|
Sexual |
0.3 (0–1.6) |
0.7 (0–2.9) |
2 (0–2.8) |
0.3 (0–1.3) |
0 (0–1.3) |
0.4 (0–1.3) |
–0.66 |
–1.40 |
0.07 |
0.15 |
|||
|
Overall |
0.8 (0.5–1.2) |
0.7 (0.4–1.1) |
0.6 (0.3–1.3) |
0.5 (0.3–0.8) |
0.3 (0.1–0.5) |
0.3 (0.1–0.7) |
–0.10 |
–0.42 |
0.01 |
0.05 |
|||
|
*p < 0.05-value (2-tailed); **p < 0.01-value (2-tailed). CARES-SF: Cancer Rehabilitation and Evaluation System short form; DAS: Depression Anxiety Stress Scale; FIM: Functional Independent Measure; PIPP: Perceived Impact of Problem Profile; SD: standard deviation; IQR: interquartile range. |
|||||||||||||
DISCUSSION
To our knowledge this is the first clinical trial evaluating efficacy of an ambulatory MDR program for BT population (gliomas) following definitive treatment in an Australian community cohort. The participants were with established impairments and functional disability, with median time since diagnosis of 2 years. These results provide some support for MDR for functional gain and psychosocial adjustment after BT treatment, consistent with other reports (6, 13–16, 18, 30, 31). The treatment group compared with the control group, showed improved ‘activity’ at 3 months following MDR and psychosocial gains were maintained at 6-month follow-up. There were no changes in other outcome measures (DASS, PIPP and CARES-SF Global scores) at both time-points. The participants in this study were similar to those in other studies with respect to demographic and clinical characteristics (13–15, 18, 32). The rehabilitation program provided standard treatment and management in accordance with existing BT care guidelines (33, 34).
It is not surprising that targeted MDR in BT survivors improved activity (self-care, mobility, continence) in the shorter-term (3 months) as they benefit from intensive reconditioning, exercise and task re-acquisition strategies. Many also showed improvement in psychosocial interactions, communication and cognitive abilities (problem solving, memory), with gains maintained at 6 months follow-up. This may be due to cessation of radio/chemotherapy regimens for those with more aggressive tumour types and improved fitness through structured and specific interventions within the MDR program. Participants in this study were complex in terms of disease severity, symptoms and co-morbidities (reflective of clinical practice) and presented with a range of survivorship issues, which required an individualized approach. Standardizing therapy was difficult, therefore ‘individualization’ of treatments was used (i.e., a described intervention provided by therapist X, e.g., a 30-min treatment session included a stretching and muscle strengthening protocol) (35). However, determining the effective dose, intensity, components and combination of treatment modalities in rehabilitation in the study population was not possible, and further research is needed.
With improved BT survival rates there is a growing acceptance of the longer-term factors impacting psychological functioning and QoL, however, these are often under-estimated (36, 37). Participants in this study did not show improvement in outcome measures assessing participation and QoL. This is not surprising as QoL is a difficult concept to measure, as many factors influence it. Survivorship issues (pain/headache, fatigue, low mood, psychosocial needs, the physical effects of treatment and consequences) can influence coping with cancer, attainment of previous levels of functioning (36), and negatively influence QoL (37). The optimum assessment tools for participation in BT are yet to be identified, and vary in different studies. Measures such as CARES-SF, PIPP, DASS have ‘ceiling’ effects, although they show clinical change with treatment but low statistical significance (ES). These issues have important implications for longer-term monitoring, education, health promotion, support and counselling of the BT patients (and their families) (22).
Rehabilitation in BT survivors is challenging as they can present with various combinations of disabilities (physical, cognitive, psychosocial, behavioural and environmental) (3, 9). The ICF (8) provides a useful framework for describing the impact of disease at the level of limitation in ‘activity and participation’. Our initial study (n = 106) (23) highlighted the patient-perspective of functional limitations due to BT, using the ICF domains and ‘linkage’ with ICF categories for relevant issues following definitive treatment. Participants identified many relevant ICF categories (88%), indicating a range of potential problems in: mobility, domestic life, inter-personal, family and intimate relations, and major life areas (economic self-sufficiency, remunerative employment). The most frequent issues identified (driving, recreation, and remunerative employment), reflected the socio-demographic characteristics and age distribution of participants (working age, educated, living with family) (23). Further, at 2 years following definitive treatment, these BT participants (n = 106) (11) showed that, despite good functional recovery over one-half reported pain (mainly headache), followed by impairments such as ataxia (44%), seizures (43%); paresis (37%), cognitive dysfunction (36%) and visual impairment (35%). This study also found that about 20% reported high levels of depression compared with only 13% in an Australian normative sample (11). Emphasis should be on a longer-term monitoring of maintenance of function and psychological sequelae in the community.
There were many challenges in conducting a clinical trial in a rehabilitation setting, similar to other reports (38, 39). This study has some potential limitations. First, randomisation of the participants was not possible due to ethical considerations; methodological issues included heterogeneous patient characteristics, multilayered treatments, interdependent components and individual interventions. Second, selection bias cannot be ruled out as participants are a selective cohort listed on a single database held at single tertiary institution (RMH) who agreed to participate in research projects. This may limit generalizability of findings. However, all eligible participants on the database were contacted, irrespective of their demographic or disease status, and the study cohort covered a wide geographical population in Victoria representing a wider sample of BT survivors in the community. Comparison and generalisability of these results is difficult, larger sample sizes in different settings are needed to confirm these findings. There was no statistical difference in any of the study variables between participants who completed post-treatment assessment and those lost to follow-up. We acknowledge that other factors may have impacted depression and QoL in BT participants and were not studied. More research into ongoing pain and other outcomes is needed. To reduce potential bias the treating therapists and assessors were blinded. The assessors were independent of the rehabilitation or acute hospital teams. Important outcomes such as impact on carers and families and analysis of costs associated with care were beyond the scope of this study. The impact of other different components of MD rehabilitation modalities and interventions is unknown.
This study highlighted challenges associated with conducting research in the ‘real world’ setting of a tertiary public hospital with finite resources. It was difficult to recruit participants as many were still undergoing radio/chemotherapy post-surgery, the mortality rates for aggressive BTs was high, and transport was an issue for those residing further away from our facility. There were no patient referral protocols for treating medical/surgical staff for an integrated neuro-rehabilitative-palliative approach (40). This required education of various treating teams and integration of existing services that operated in ‘silos’, with fragmented service delivery. The control group were informed of the wait time for rehabilitation services as per usual practice, and were not unduly disadvantaged. Operationally, it was beyond the resources of our hospital to provide therapy for this many patients simultaneously. Rehabilitation is an expensive intervention. The implications of this study include triaging and prioritizing the BT survivor who needs targeted rehabilitation input.
Rehabilitation for BT survivors is challenging due to high mortality rates. The condition is often progressive in nature, with an uncertain prognosis, and multifaceted physical, psychological and cognitive disabilities, and participatory limitations that require an integrated interdisciplinary approach (4). This study provides some evidence to support MDR for improved ‘activity’ in BT survivors in the shorter-term. More research in the effectiveness of ‘specific’ rehabilitation interventions and participation domains is needed. The MDR for BT survivors should be considered by treating clinical teams to improve disability management. Further, emphasis on outcome-orientated research to explore service models and strategies to implement individualized treatment and integrated MDR programs is needed to address survivorship issues in BT.
ACKNOWLEDGEMENT
We are grateful to all participants with primary brain tumour in this study. We thank Ms L Oscari, Dr K Mackenzie and Dr I Rajapaksa for patient assessments; Dr L Ng for assistance with ethics submission and T Khan for data entry.
The authors declare no conflicts of interest.
REFERENCES
