Anne-Cathrine Clarke-Jenssen, RPT, MSc1, Anne Marit Mengshoel, RPT, PhD2,
Yndis Staalesen Strumse, MD, PhD3 and Karin Øien Forseth, MD, PhD3,4
From the 1Department of Orthopaedics, Oslo University Hospital, Rikshospitalet, 2Department of Health Sciences,
Faculty of Medicine, University of Oslo, 3Section for Climate Therapy and 4Department of Rheumatology,
Oslo University Hospital, Rikshospitalet, Oslo, Norway
OBJECTIVE: To study the long-term effects on symptoms and physical function of a 4-week rehabilitation programme for patients with fibromyalgia, and to determine whether there are any differences if this programme is applied in a warm or cold climate.
METHODS: A total of 132 patients with fibromyalgia were randomized to a rehabilitation programme in a warm or cold climate, or to a control group without intervention. Assessments were performed before and after intervention, and after 3 and 12 months. The main outcome measures were pain, measured by tender point count (TPC), and physical function, measured with the 6-min walk test (6MWT).
RESULTS: There was no difference in any outcome variables at baseline. Persistent reduction in pain measured by TPC occurred only in the warm climatic setting. Mean difference (95% confidence interval (CI)) in TPC between warm and cold climate groups 1 year after the intervention was –1.7 (–2.9 to –0.5) and between the warm climate and the control group –2.2 (–3.3 to –1.0). Three months after the intervention the mean difference between the warm and cold climate groups in pain distribution (McGill mannequin) was –12 (–20 to –5) and between the warm climate and the control group –11 (–18 to –3). There were comparable improvements in physical function (6MWT) between the 2 intervention groups and the control group. The mean difference (95% CI) in 6MWT 1 year after the intervention between the warm climate and the control group was 33 (7–59) m. The corresponding value between the cold climate and the control group was 29 (3–55) m. Grip Strength (95% CI) was increased by 4.6 kg (2.3–6.4) in the warm climate and by 3.2 kg (0.9–5.5) in the cold climate compared with the control group 1 year after the intervention.
CONCLUSION: A rehabilitation programme for fibromyalgia may have a long-term effect on pain, as measured by TPC and pain distribution, when applied in a warm climatic setting, and may improve physical function regardless of the climatic setting.
Key words: fibromyalgia; rehabilitation; climate; warm climate; randomized controlled study.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Anne-Cathrine Clarke-Jenssen, Department of Orthopedics, Oslo University Hospital, Rikshospitalet, NO Oslo, Norway. E-mail: tclarke@ous-hf.no
Accepted Feb 18, 2014; Epub ahead of print Apr 29, 2014
INTRODUCTION
Fibromyalgia (FM) is a chronic, generalized pain condition with additional symptoms, such as fatigue, sleep disturbance, muscle stiffness, depression, anxiety and cognitive dysfunction (1). Functional capacity is usually impaired in patients with FM and associated with limitations in activities of daily living (ADL) (2). The aetiology and pathogenesis of FM is not yet fully understood, but an important factor may be abnormalities in pain processing within the central nervous system (CNS), leading to central sensitization associated with hypersensitivity and allodynia (3).
There is no known cure for FM. To improve symptoms and function, the European League Against Rheumatism recommends combining pharmacological and non-pharmacological therapies (4). Non-pharmacological therapies commonly comprise exercise, patient education and cognitive behavioural therapy (CBT) (5). Häuser et al. (6) reviewed the efficacy of these non-pharmacological therapies and found short-term improvements in pain, fatigue, depression and quality of life in patients with FM, and long-term improvements in physical function.
Studies of the efficacy of a rehabilitation programme comprising exercise, patient education and CBT given in a warm climate for patients with inflammatory rheumatic diseases have been reviewed by Forseth et al. (7). They concluded that there was low-to-moderate evidence that such a rehabilitation programme given in a warm climatic setting could have positive impact on symptoms and physical function. According to Hafstrøm & Hallengren (8), these benefits may be due partly to the climate and partly to the change in environment, as well as to the treatment. Aikmann (9) found an association between decreased temperature and increased relative humidity, on the one hand, and increased pain and stiffness in patients with arthritis, on the other hand. Thus, rehabilitation given in a warm climate may modify pain, which, in turn, may enhance the patients’ ability to be more physically active and better tolerate the exercise programme prescribed (7). Whether similar effects occur in patients with FM has been studied to a lesser extent.
In an effect study by Zijlstra et al. (10), a group of patients with FM received a 2.5-week treatment programme in a warm climate, including thalassotherapy, exercise and group education. A control group at home received no specific treatment. The warm climate group showed improvement in pain (tender point count; TPC) lasting for 3 months and improvement in physical function (6-min walk test; 6MWT) lasting for 12 months. Whether this improvement was due to the treatment or to the climatic influence is not known.
In a pilot study, Forseth & Mengshoel (11) found a reduction in pain (TPC) and improvement in physical function (6MWT) 3 months after a 4-week rehabilitation programme for patients with FM in a warm climate. However, as this was an uncontrolled study, the results are uncertain, and do not address the climatic effect.
To our knowledge, the long-term effect of the same rehabilitation programme given either in a warm or a cold climate for patients with FM has not been studied. Thus, the aims of the current study were, firstly, to examine the long-term effects of a 4-week rehabilitation programme on pain and physical function in patients with FM compared with a group receiving usual care at home and, secondly, to examine whether these potential effects differ when the same rehabilitation programme is given in a warm or a cold climatic setting.
MATERIAL AND METHODS
Design
A randomized, controlled study design was applied. Patients were randomly assigned by 1:1:1 to either a 4-week rehabilitation programme in a Mediterranean country (Scandinavian Rehabilitation Centre in Antalya, Turkey), to the same programme in Norway (Skogli Rehabilitation Centre, Lillehammer), or to a control group staying home with usual care. Randomization was performed by a statistician at the Biostatistics Centre at Rikshospitalet, who was otherwise unrelated to the study. To generate a random allocation sequence, a random-number table was used and the patients were stratified according to age and gender. Patients were randomized after inclusion but before the baseline data were collected. Intervention groups were examined before and after the intervention and after 3 and 12 months. The control group was examined at baseline and after 3 and 12 months. The intervention groups were examined at the rehabilitation venues immediately before and after the intervention. The other examinations were carried out at Rikshospitalet, Oslo University Hospital. Two experienced physiotherapists (PT) performed all the physical tests and the same PT examined the same outcome measure each time. These PTs travelled with the groups to the different rehabilitation venues, performed the pre-test on the first 2 days, and returned to perform the post-test on the last 2 days of rehabilitation.
Sample size was calculated according to Aalen (12). To detect a clinically relevant difference of 50 m (13), with a standard deviation of 75 m, in the 6MWT, a power of 80% and a significance level of 0.05, 36 patients were needed in each group. Allowing for an attrition rate of 20%, 44 patients were included in each group.
Material
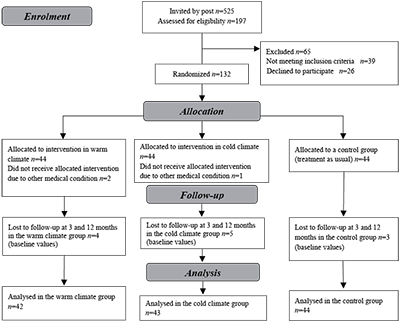
Patients were recruited from an outpatient clinic for rheumatic patients at Martina Hansen’s Hospital in the neighbouring county of Oslo. All patients with FM admitted to the clinic between 2004 and 2007, a total of 525 patients, received a written invitation to participate in the study. Those who responded to the invitation, a total of 197 patients, were invited to attend a screening test. Patients were included consecutively according to the inclusion criteria until the pre-determined number of 132 participants was reached.
Inclusion criteria were: fulfilling American Collage of Rheumatology’s (ACR) classification criteria of 1990 for FM (14); age between 18 and 60 years; independent in activities of daily living; capable of participating in a light exercise group on land and in warm water; and understanding written and oral Norwegian.
Exclusion criteria were: serious physical or psychiatric diagnoses; alcohol or drug abuse; being pregnant or breast-feeding; and receiving more than 50% disability pension.
During the first days of the intervention, two further patients were excluded because of other medical conditions (rheumatoid arthritis and venous thrombosis) and one due to drug abuse. These patients should not have been included in the study as they did not fulfil the inclusion criteria; they were therefore omitted from the analysis. Thus, the total number of participants analysed was 129 (warm climate group 42; cold climate group 43; control group 44). Patient characteristics are shown in Fig. 1.
Fig. 1. Flow diagram.
Intervention
The rehabilitation programme followed international recommendations for non-pharmacological treatment for patients with FM. The programme consisted of aerobic exercise on land and in warm water, stretching, relaxation and patient education. The intervention programme was group-based and was applied 5 days a week for 4 weeks. The content of the rehabilitation programme is described in Table I. The same rehabilitation programme was applied in a warm and a cold climate, and the same PT conducted the exercise and patient education classes in both locations. This PT travelled with the patients to the intervention locations and stayed there for the whole treatment period. Previous studies have shown that patients with FM have diminished working capacity and experience activity-induced pain, especially at the beginning of an exercise programme (15, 16). Thus, the programmes started at a low level, progressed slowly and the patients rested between exercise classes. Individual treatment was not given. The control group had no intervention and was treated “as usual”. The patients travelled as a group to and from the rehabilitation locations.
|
Table I. Rehabilitation programme for both intervention groups |
|||
|
Type of intervention |
Frequency of intervention |
Content |
Intensity of intervention |
|
Walking classes |
Daily for 45 min |
Walking outdoors on partly uneven and slightly gradient terrain |
Moderate intensity (slightly out of breath) |
|
Stretching |
Daily for 15 min after the walking |
All the main muscle groups |
Without causing pain |
|
Exercise groups in warm water |
Alternately 2 or 3 times a week for 45 min |
Emphasis on aerobic exercise |
Moderate intensity (slightly out of breath) |
|
Exercise groups on land |
Alternately 2 or 3 times a week for 45 min |
Emphasis on body awareness, |
Moderate intensity of the strengthening exercise |
|
Relaxation groups |
Two times a week for 45 min |
Hold-relax technique |
|
|
Patient education |
Once a week |
The topics were: update on FM, update on pain, self-efficacy and physical activity |
|
|
Subsequent discussions in smaller groups |
Once a week |
The same topics as patient |
|
|
Resting |
Daily 1 h × 2 |
||
|
The intervention programme was given 5 days a week for 4 weeks. The patients were not receiving individual treatments. |
|||
Climate
The interventions were performed in September in Norway and in October in Turkey. The mean daytime temperature (standard deviation; SD) in Norway during the intervention was 8.5°C (19 to –1°C) and the number of sunny days was 16. In Turkey the mean daytime temperature (SD) was 26°C (31–22°C) and the number of sunny days was 25.
Outcome measures
Outcome measures were chosen to detect changes in symptoms and physical function.
Primary outcome measures. TPC was used to assess pain and was tested according to the description in the ACR classification criteria (14). Up to 18 well-defined points are registered as positive when a 4 kg/cm2 pressure is perceived as pain. TPC has been used in many clinical studies of patients with FM (17), and is useful as an outcome measure for pain in these patients (18). TPC is sensitive to change in non-pharmacological studies of patients with FM (19).
The 6MWT is a clinically relevant measure of a patient’s functional capacity. The patient walks as far as possible in 6 min under standardized conditions, and the walking distance is measured in m (20). The 6MWT is recommended for use in clinical research and clinical examination when planning treatment for patients with FM (2). It is often used in exercise studies for patients with FM (17) and has been used in studies that are relevant for comparison with ours (10, 11, 21). The 6MWT has been tested for validity and reliability with these patients (22). The patients performed the 6MWT once before the baseline test in order to become familiarized with the test (23).
Secondary outcome measures. The Fibromyalgia Impact Questionnaire (FIQ) is a disease-specific questionnaire evaluating the health status of patients with FM (24). The questionnaire comprises 10 subscales of disability and symptoms, and the total score ranges from 0 to 100. The higher the score the greater impact the disease has on daily life. The FIQ has been tested for validity and reliability for patients with FM in Sweden (25).
The pain mannequin from the McGill’s Pain Questionnaire was used to assess pain distribution (26, 27). The patient was asked to circle painful areas experienced during the previous week. The number of circles was counted (e.g. fingers, hand, wrist, underarm, elbow, upper arm and shoulder) on the left and right sides and on the front and back of the body. The highest possible score is 70.
The Arthritis Self-Efficacy Scale (ASES) is a questionnaire designed to assess dimensions of self-efficacy for pain, physical function and symptoms in patients with arthritis (28). Each dimension is calculated and total scores range from 10 to 100; the higher score the better the self-efficacy. The ASES has been tested for validity and reliability for Swedish patients with FM (29).
The Short-Form Health Survey 36 (SF-36) is a generic questionnaire assessing health-related quality of life (HRQoL), which comprises 8 subscales ranging from 0 to 100 after a recalculation, with a higher score indicating a better quality of life (30). The scores build 2 composite scores; the physical and the mental components. SF-36 is recommended as outcome measure in clinical studies of patients with FM (31).
The Hospital Anxiety and Depression Scale (HADS) is a 14-item questionnaire measuring self-reported anxiety and depression (32). The 2 dimensions are calculated separately, and possible scores range from 0 to 21, with a higher score indicating the patient is more seriously affected. Scores above 11 are considered pathological. HADS has been used in a number of FM clinical trials (31).
A Baseline hydraulic hand dynamometer (Irvington, NY 10533, USA) was used to assess grip strength. In this test the patient sits with a 90° flexion in the elbow and the assessor supports the dynamometer. The patient grips as hard as he or she can, and the mean value of 3 attempts in pounds is calculated (33). Grip strength is recommended as an outcome measure in studies of patients with FM (2).
The Leisure Time Physical Activity Index (LTPAI) is a questionnaire designed to estimate the number of hours of physical activity during leisure time in the previous week undertaken by patients with FM. The number of hours they perform light, moderate and strenuous physical activity is noted, and the total hours of physical activity is calculated. The LTPAI is valid and reliable for patients with FM (34).
Statistics
All data were scanned via Teleform to Access and transported to the Statistical Package for the Social Sciences (SPSS), version 18. Descriptive statistics were used to calculate demographic data and baseline values. The demographic data are presented as numbers and percentages or means (SD). Normally distributed continuous data of the outcome variables are presented as means and 95% confidence intervals (CI), and non-normally distributed as medians and 25th–75th percentiles. Missing data in the follow-up tests were replaced with baseline values according to the principle of intention to treat (12). According to the distribution, the within-group comparisons were either analysed with paired-samples t-test or two related samples test (Wilcoxon). The between-group comparisons of the change scores were analysed by one-way analysis of variance (ANOVA) with post-hoc test Tukey’s Honestly Significant Difference (HSD) and the group variable was used as factor. The change score from baseline was used due to uneven distribution of some of the post scores.
Ethics
The study protocol was approved by the research committee at Martina Hansen’s Hospital, where the patients were recruited, and the invitations to participate in the study were signed by the senior doctor in the Department of Rheumatology. The regional ethics committee and the Norwegian Social Science/Data Inspectorate approved the study and written informed consent was obtained from all participants.
RESULTS
The patients’ baseline characteristics and medication are given in Table II. The 3 groups were comparable for all variables, apart from a significant difference in the mean years of symptoms of fibromyalgia between the participants in the warm climate group and the control group.
The effects on primary and secondary outcome variables are shown in Tables III and IV, respectively. Clinical response is expressed as mean difference from baseline at 3 months (16 weeks) and 12 months (52 weeks) after initiation of the 4-week rehabilitation programme.
Mean differences for within-group comparison (paired samples
t-test/Wilcoxon) and between-group comparison (ANOVA with post-hoc test Tukey’s HSD) are shown in Tables III and IV. Differences between the intervention in a warm and a cold climate were found only for variables measuring aspects of pain and physical activity.
|
Table II. Baseline characteristics of the fibromyalgia patients receiving treatment in a warm or a cold climate, or in the control group |
||||
|
All participants (n = 129) |
Warm climate (n = 43) |
Cold climate (n = 42) |
Control group (n = 44) |
|
|
Female, n (%) |
119 (92) |
37 (88) |
40 (93) |
42 (96) |
|
Age, mean (SD) |
45 (9) |
46 (9) |
46 (8) |
45 (9) |
|
Years of education, mean (SD) |
13 (3) |
13 (3) |
13 (3) |
13 (3) |
|
Years of symptoms of fibromyalgiaa, mean (SD) |
14 (10) |
17 (12) |
13 (9) |
12 (9) |
|
BMI, mean (SD) |
28 (4) |
29 (5) |
27 (4) |
28 (4) |
|
Working full-/part-timeb n (%) |
74 (58) |
28 (68) |
21 (48) |
25 (58) |
|
Medication at baseline, n (%) |
||||
|
Analgesics |
93 (72) |
27 (65) |
34 (78) |
32 (74) |
|
NSAIDs |
39 (30) |
9 (22) |
16 (37) |
14 (32) |
|
Anti-depressives |
34 (26) |
13 (31) |
10 (23) |
11 (25) |
|
Tranquillizers |
9 (7) |
2 (5) |
5 (12) |
2 (5) |
|
Sleeping medication |
28 (22) |
7 (17) |
15 (35) |
6 (14) |
|
Prednisolone |
8 (6) |
4 (10) |
4 (9) |
0 (0) |
|
aSignificant difference between the warm climate group and the control group. bThe remainder of the patients were on sick leave, on rehabilitation money or waiting for disability pension. SD: standard deviation; NSAIDs: non-steroidal anti-inflammatory drugs; BMI: body mass index. |
||||
Symptoms
The group receiving treatment in a warm climate improved significantly in pain measured by TPC and McGill mannequin at 3 and 12 months compared with the cold climate group and the control group (Tables III and IV). The mean difference (95% CI) in TPC between the warm and cold climate groups 1 year after the intervention was –1.7 (–2.9 to –0.5) (p = 0.002). The corresponding values between the warm climate and the control groups was –2.2 (–3.3 to –1.0) (p < 0.001). Three months after the intervention the mean difference between the warm and cold climate groups in pain distribution (McGill mannequin) was –12 (–20 to –5) (p < 0.001) and between the warm climate and the control group –11 (–18 to –3) (p < 0.002). VAS pain measures the intensity of pain, and this was reduced by 1.2 (2.2–0.1) (p = 0.023) 3 months after the intervention in the warm climate compared with the control group only. These results indicate that rehabilitation in a warm climatic setting may have a pain-relieving effect on some aspects of pain in patients with FM.
Absence from treatment was 5% (114 of the 2,296 treatments offered) in the Mediterranean group and 9% (208 of the 2,296 treatments offered) in the Norwegian group, and the main reason for absence was temporarily increased pain.
|
Table III. Responses in primary outcome variables tender point count (TPC) and 6-min walk test (6MWT) within and between groups (warm climate (n = 42), cold climate (n = 43) and control (n = 44)) at 16 and 52 weeks after initiation of rehabilitation |
|||||||
|
Baseline values |
Change from baseline |
||||||
|
16 weeks within-groups |
16 weeks between-groups |
p-value |
52 weeks within-groups |
52 weeks between-groups |
p-value |
||
|
TPC |
|||||||
|
Warm climatea |
17.0 (14.0 to 18.0) |
–3.3 (–4.3 to –2.3) |
< 0.001 |
–2.0 (–2.8 to –1.1) |
< 0.001 |
||
|
Cold climatea |
18.0 (16.0 to 18.0) |
–0.7 (–1.4 to –0.1) |
0.027 |
–0.3 (–0.9 to 0.3) |
0.531 |
||
|
Control groupa |
17.5 (15.0 to 18.0) |
–0.3 (–0.8 to 0.2) |
0.349 |
0.2 (–0.5 to 0.8) |
0.575 |
||
|
Warm climate vs cold |
–2.6 (–3.8 to –1.3) |
< 0.001 |
–1.7 (–2.9 to –0.5) |
0.002 |
|||
|
Warm climate vs control |
–3.0 (–4.2 to –1.7) |
< 0.001 |
–2.2 (–3.3 to –1.0) |
< 0.001 |
|||
|
Cold climate vs control |
–0,4 (–1.7 to 0.8) |
0.698 |
–0.5 (–1.6 to 0.7) |
0.614 |
|||
|
6MWT (m) |
|||||||
|
Warm climate |
517 (493 to 541) |
48 (34 to 61) |
< 0.001 |
38 (25 to 51) |
< 0.001 |
||
|
Cold climate |
527 (503 to 550) |
39 (26 to 52) |
< 0.001 |
34 (20 to 48) |
< 0.001 |
||
|
Control group |
504 (481 to 526) |
12 (–3 to 26) |
0.108 |
5 (–14 to 24) |
0.620 |
||
|
Warm climate vs cold |
8 (–14 to 31) |
0.659 |
4 (–22 to 31) |
0.922 |
|||
|
Warm climate vs control |
36 (14 to 59) |
0.001 |
33 (7 to 59) |
0.009 |
|||
|
Cold climate vs control |
28 (5 to 50) |
0.011 |
29 (3 to 55) |
0.025 |
|||
|
Baseline values are shown as mean (95% confidence interval (CI)) unless stated otherwise. Baseline values of all outcome measures are comparable between the groups. Changes from baseline are shown as mean difference from baseline (95% CI) as within-group comparison (paired-samples t-test/Wilcoxon) or between-group comparisons (analysis of variance (ANOVA) with test Tukey’s Honestly Significant Difference (HSD). aMedian (25th–75th percentile). Significance is shown in bold. |
|||||||
Physical function
Both intervention groups showed some improvements in physical function (6MWT and grip strength) at 3 and 12 months after treatment compared with the control group (Tables III and IV). The mean difference (95% CI) in 6MWT between the warm climate group and the control group 1 year after the intervention was 33 (7–59) m (p = 0.009). The corresponding values between the cold climate and the control groups was 29 (3–55) m (p = 0.025). The mean difference (95% CI) in grip strength 1 year after the intervention between the warm climate group and the control group was 4.6 kg (2.3-6.4) (p < 0.001), and between the cold climate and control group was 3.2 kg (0.9-5.5) (p = 0.003). The results of 6MWT and grip strength test indicate that a 4-week rehabilitation programme may result in some improvements in physical function in patients with FM, regardless of the climatic setting.
|
Table IV. Responses in secondary outcome variables within and between groups (warm climate (n = 42), cold climate (n = 43) and control (n = 44)) at 16 and 52 weeks after initiation of rehabilitation |
|||||||
|
Baseline values |
Change from baseline |
||||||
|
16 weeks within-groups |
16 weeks between-groups |
p-value |
52 weeks within-groups |
52 weeks between-groups |
p-value |
||
|
VAS pain |
|||||||
|
Warm climate |
6.6 (6 to 7.3) |
–1.1 (–1.6 to –0.5) |
< 0.001 |
–0.2 (–0.7 to 0.4) |
0.583 |
||
|
Cold climate |
6.9 (6.3 to 7.5) |
–0.7 (–1.4 to –0.1) |
0.035 |
–0.3 (–1 to 0.3) |
0.365 |
||
|
Control group |
6.6 (6 to 7.2) |
0.1 (–0.5 to 0.8) |
0.678 |
–0.3 (–0.9 to 0.4) |
0.408 |
||
|
Warm climate vs cold |
–0.3 (–1.4 to 0.7) |
0.755 |
0.1 (–0.9 to 1.2) |
0.949 |
|||
|
Warm climate vs control |
–1.2 (–2.2 to –0.1) |
0.023 |
0.1 (–0.9 to 1.1) |
0.968 |
|||
|
Cold climate vs control |
–0.9 (–1.9 to 0.2) |
0.124 |
0 (–1 to 1) |
0.997 |
|||
|
Pain mannequin |
|||||||
|
Warm climatea |
22 (15 to 33) |
–8 (–13 to –4) |
< 0.001 |
0.2 (–7 to 3) |
0.288 |
||
|
Cold climatea |
26 (20 to 33) |
4 (0 to 8) |
0.079 |
2 (0 to 5) |
0.099 |
||
|
Control groupa |
23 (16 to 30) |
2 (–2 to 7) |
0.432 |
4 (–1 to 8) |
0.094 |
||
|
Warm climate vs cold |
–12 (–20 to –5) |
< 0.001 |
–4 (–11 to 3) |
0.121 |
|||
|
Warm climate vs control |
–11 (–18 to –3) |
0.002 |
–5 (–13 to 2) |
0.392 |
|||
|
Cold climate vs control |
2 (–6 to 9) |
0.837 |
–1 (–8 to 6) |
0.772 |
|||
|
Physical activity, h |
|||||||
|
Warm climate |
7.4 (4.4 to 10.4) |
–1.2 (–3.4 to 1) |
0.292 |
0.5 (–1.3 to 2.2) |
0.610 |
||
|
Cold climate |
4.4 (3.2 to 5.6) |
2.9 (1 to 4.8) |
0.004 |
0.2 (–1 to 1.6) |
0.744 |
||
|
Control group |
4.4 (3.3 to 5.5) |
–0.5 (–1.7 to 0.8) |
0.458 |
1.3 (0 to 2.5) |
0.053 |
||
|
Warm climate vs cold |
–4 (–7.1 to –1) |
0.006 |
0.2 (–2.2 to 2.7) |
0.973 |
|||
|
Warm climate vs control |
–0.7 (–3.8 to 2.3) |
0.840 |
–0.8 (–3.3 to 1.7) |
0.721 |
|||
|
Cold climate vs control |
3.3 (0.3 to 6.4) |
0.027 |
–1 (–3.5 to 1.4) |
0.577 |
|||
|
ASES function |
|||||||
|
Warm climate |
77 (72 to 83) |
5 (2 to 9) |
0.007 |
1 (–2 to 4) |
0.577 |
||
|
Cold climate |
70 (63 to 76) |
7 (3 to 12) |
0.003 |
7 (2 to 13) |
0.009 |
||
|
Control group |
72 (66 to 77) |
0 (–3 to 4) |
0.872 |
4 (0 to 8) |
0.034 |
||
|
Warm climate vs cold |
–2 (–8.9 to 5) |
0.782 |
–6.5 (–13.5 to 0.5) |
0.076 |
|||
|
Warm climate vs control |
5.2 (–1.7 to 12) |
0.182 |
–3.6 (–10.5 to 3.4) |
0.448 |
|||
|
Cold climate vs control |
7.1 (0.3 to 14) |
0.040 |
2.9 (–4 to 9.8) |
0.578 |
|||
|
Grip strength, kg |
|||||||
|
Warm climate |
26.8 (23.6 to 29.6) |
4.1 (2.3 to 5.9) |
< 0.001 |
2.3 (0 to 3.6) |
0.033 |
||
|
Cold climate |
25.9 (23.6 to 28.6) |
1.8 (0.5 to 3.2) |
0.014 |
0.9 (–0.5 to 1.8) |
0.172 |
||
|
Control group |
24.6 (22.3 to 27.3) |
0 (–0.9 to 0.9) |
0.982 |
–2.7 (–3.2 to –1.4) |
< 0.001 |
||
|
Warm climate vs cold |
5 (0 to 10) |
0.070 |
2.3 (0 to 4.6) |
0.383 |
|||
|
Warm climate vs control |
9 (4 to 14) |
< 0.001 |
4.1 (1.8 to 6.4) |
< 0.001 |
|||
|
Cold climate vs control |
4 (–1 to 9) |
0.132 |
1.8 (0.5 to 4.1) |
0.003 |
|||
|
Baseline values are shown as mean (95% confidence interval (CI)) unless stated otherwise. The baseline values of all outcome measures are comparable between the groups, except for hours of physical activity. Changes from baseline are shown as mean difference from baseline (95% CI) as within-group comparison (paired-samples t-test/Wilcoxon) or between-group comparisons (ANOVA with post-hoc test Tukey’s Honestly Significant Difference (HSD)). aMedian (25th–75th percentile). Significance is shown in bold. VAS: visual analogue scale; ASES: Arthritis Self-Efficacy Scale. |
|||||||
The ASES functional sub-scores revealed some improvements in both intervention groups 3 months after the intervention, but a significant difference was found only between the cold climate group and the control group (p = 0.040).
There was an increase in physical activity (LTPAI) in the cold climate group compared with both the warm climate group and the control group (p ≤ 0.027) (Table IV) 3 months after the intervention, demonstrating that the difference in effects according to climatic setting may go in either direction.
There were no significant changes in SF-36, HADS or other sub-scores of ASES and FIQ. There were no adverse effects at the end of the intervention.
DISCUSSION
In this study only rehabilitation in a warm climatic setting led to long-term improvements on some aspects of pain (TPC and McGill mannequin). This result is in agreement with those of similar studies. Zijlstra et al. (10) found a statistically significant improvement in pain measured by TPC compared with a control group 3 months after an intervention in a warm climate. Forseth & Mengshoel (11) found a similar result in their pilot study. However, it is not known whether these improvements were due to the rehabilitation programme, the warm climate, or a combination of these factors. The rehabilitation programmes in our study were as identical as possible and, as the improvements seen on TPC and the McGill mannequin were found only in the warm climate group, one might assume that this improvement was mainly due to the climatic setting.
TPC measures a single aspect of pain; tenderness, and its extent. There was no reported minimal clinically important difference (MCID) of TPC, but the Philadelphia Panel defines a clinically important benefit to be a 15% change relative to a control group (35). In our study the reduction in TPC after 12 months was 15% in a warm climate compared with a control group, which may be of clinical significance.
Pain is regarded as the main symptom in FM and there are few pharmacological options for pain reduction. Interventions targeted to improve all aspects of pain for the longer term would be of importance for these patients. Thus, rehabilitation in a warm climate may be a supplement to other treatments for patients with FM.
The main reason for absence from the exercise classes was temporarily increased pain. The increase in pain in the intervention groups was transitory and participants showed no adverse effects at the end of the rehabilitation. Previous studies have shown that patients with FM often experience activity-induced pain especially at the beginning of an exercise programme (2, 15, 16); thus, it may be a subject of discussion as to whether this pain qualifies as an adverse event or a symptom of the disease only. Absence from the exercise classes in this study were almost twice as high in the Norwegian group compared with the Mediterranean group, which may indicate that it is easier to commence an exercise programme in a warm climate for patients with FM.
The therapeutic effect of a warm climate is not fully understood. It appears that subtropical climates confer less pain and stiffness, and less fear of increased pain during exercise (9, 36). Furthermore, heat is thought to increase the elasticity of tendons, muscles and other soft tissues (7). A number of studies have suggested a link between low levels of vitamin D
in patients with FM and chronic pain, but only uncontrolled studies have found benefits of vitamin D treatment, and the association between FM and vitamin D deficiency remains inconclusive (37).
This study found a long-term effect of a rehabilitation programme on physical function regardless of climatic setting. Both treatment groups improved in terms of the 6MWT and grip strength measurement compared with the control group, and the improvement was maintained after 1 year. This concurs with results from similar studies in different climatic settings. Zijlstra et al. (10) found a statistically significant improvement in physical function compared with a control group 3 months after an intervention in a warm climate. In an uncontrolled study Forseth & Mengshoel (11) found a statistically significant improvement in physical function within the group 3 months after a rehabilitation programme in a warm climate. Rehabilitation under different climatic settings has also been shown to be beneficial in other diagnoses. Staalesen Strumse et al. (21) demonstrated comparable improvements in the 6MWT within both treatment groups 3 months after an intervention for patients with rheumatoid arthritis.
The MCID for a 6MWT might differ somewhat according to the diagnosis and baseline values of participants in the study. Perera et al. (13) found an MCID of 50 m in older adults, while an improvement of 24–45 m was assessed as of clinical importance in patients with pulmonary fibrosis according to du Bois et al. (38). Our study found a statistically significant improvement in the 6MWT after 3 and 12 months in both intervention groups. The improvement in the warm climate group was 48 (34–61) m (Table III) 3 months after the rehabilitation, which may be judged to be of clinical importance. The improvements between the intervention groups and the control group 1 year after the intervention, which was in the range of 29–33 m, is the subject of discussion; however, our results may contribute to the conclusion that, independent of climatic factors, exercise and patient education may be beneficial for improving physical function in patients with FM.
In concordance with other studies of patients with FM, the present study indicates that exercise and physical activity of low-to-moderate intensity is safe for most patients with FM (17). There is a general consensus that adults should undertake moderate-to-intensive aerobic physical activity for a minimum of 30 min daily in order to promote and maintain health (39). It is important that patients with FM undertake the recommended level of physical activity in order to obtain the above-mentioned health benefit.
Physical activity, measured as the number of hours that the patients were active, showed greater improvements in the Norwegian group. However, this group was less physically active at the beginning of the study and, in spite of the improvement, the group did not reach the physical activity level of the Mediterranean group 3 months after the intervention. One year after the intervention there was no increase in the amount of physical activity for the participants in spite of their good intentions to continue with the increased amount of physical activity they had been undertaking during the intervention. This limited compliance with physical activity is in accordance with other studies of patients with FM (40). How compliance with beneficial physical activity can be improved in these patients is an important issue that should be addressed in future studies.
Double-blinding is the gold standard in RCT, where both the patients and the assessors are blinded. In exercise studies where the control group has no intervention and the intervention groups are in different climatic settings, it is not possible to blind the participants. As one-third of the participants in this study spent 4 weeks in a sunny climate that inevitably resulted in a tan, it was not possible to blind the assessors as to which group the patients had been allocated. However, the assessors did not have access to prior assessments and were blinded in that respect. The lack of blinding may have had an influence on both patients and testers, as they could have had a preconception of the effect of the intervention. The lack of blinding may lead to overoptimistic estimates of intervention effect for subjectively assessed outcomes (41). Three patients were excluded before the intervention started because they did not fulfil the inclusion criteria, and their data were not analysed. According to the Consort Statement and the intention-to-treat analysis, these data should have been part of the analysis and this is a shortcoming of the study.
Many exercise studies that compare two intervention programmes use different physiotherapists and different treatment programmes. This may have different impact on the patients and contribute to confounding factors. In this study we controlled for these factors by using the same therapist and the same programme at both locations, which strengthened the internal validity of the study. Although we took great care to make the interventions as similar as possible, there may still be confounding factors that are difficult to control for. The overall experience of the change in the environment may have a greater impact on the patients in a Mediterranean setting than in a Norwegian setting.
In conclusion, this RCT demonstrates pain reduction in patients with FM up to 1 year after a 4-week rehabilitation programme in a warm climatic setting, and some improvements in physical function persisting 1 year after a comparable rehabilitation programme for patients with FM in both warm and cold climatic settings.
ACKNOWLEDGEMENTS
The authors would like to thank the patients who participated in the study, Tone Bråthen and the staff at Section for Climate Therapy, Oslo University Hospital, Rikshospitalet for initiating and supporting the study, Torhild Garen for assistance with data input, Catarina Norèn and Kristine Risum for testing the patients, and Pia Foss for leading the intervention.
The study was financed by the Section for Climate Therapy, Oslo University Hospital, Rikshospitalet. The writing of the article was supported by the Norwegian Fibromyalgia Association.
REFERENCES
