Anthony B. Ward, MD, FRCP1, Jörg Wissel, MD, FRCP2, Jörgen Borg, MD3, Per Ertzgaard, MD4, Christoph Herrmann, MD5, Jai Kulkarni, MD, FRCP6, Kristina Lindgren, MD7, Iris Reuter, MD8, Mohamed Sakel, FRCP9, Patrik Säterö, MD10, Satyendra Sharma, MD, FRCPC11, Theodore Wein, MD, FRCPC12, Nicola Wright, MSc13 and Antony Fulford-Smith, MB, BS, MRCGP13; on behalf of the BEST study group
From the 1North Staffordshire Rehabilitation Centre, Haywood Hospital, Stoke on Trent, UK, 2Neurological Rehabilitation, Department of Neurology, Vivantes Klinikum Spandau, Berlin, Germany, 3Department of Clinical Sciences, Karolinska Institutet, Rehabilitation Medicine, Danderyd Hospital, Stockholm, 4Department of Rehabilitation Medicine, Linköping University, Linköping, Sweden, 5Clinics for Neurological Rehabilitation and Early Rehabilitation, Asklepios Clinics Schildautal, Seesen, Germany, 6Manchester Royal Infirmary, Manchester, UK, 7Department of Neurology and Rehabilitation, Central Hospital, Karlstad, Sweden, 8Department of Neurology, Justus-Liebig University, Giessen, Germany, 9Kent & Canterbury Hospital, Canterbury, UK, 10Department of Rehabilitation Medicine, Sahlgren University Hospital, Göteborg, Sweden, 11Sunnybrook Health Science Centre, Toronto, 12Montreal General Hospital, Montreal, Canada and 13Allergan Ltd, Marlow International, The Parkway, Marlow, Buckinghamshire, UK
OBJECTIVE: Evaluate changes in active and passive function with onabotulinumtoxinA + standard of care within goal-oriented rehabilitation programmes in adults with focal post-stroke spasticity.
METHODS: Prospective, 24-week double-blind study with an open-label extension. Subjects were randomized to onabotulinumtoxinA + standard of care or placebo + standard of care, at baseline and at 12 weeks, if judged appropriate, with follow-up to 52 weeks. The primary endpoint was the number of patients achieving their principal active functional goal at 24 weeks (or 10 weeks after an optional second injection). Secondary endpoints included achievement of a different active or a passive goal at this timepoint.
RESULTS: The intent-to-treat population comprised 273 patients. The proportion of patients achieving their principal active functional goal and secondary active functional goal with onabotulinumtoxinA + standard of care was not statistically different from placebo + standard of care. Significantly more patients achieved their secondary passive goal with onabotulinumtoxinA + standard of care (60.0%) vs. placebo + standard of care (38.6%) (odds ratio, 2.46; 95% confidence interval, 1.18–5.14) as well as higher Goal Attainment Scaling levels for upper limb and ankle flexor subgroups.
CONCLUSIONS: Addition of onabotulinumtoxinA to standard of care as part of goal-oriented rehabilitation in post-stroke spasticity patients significantly increased passive goal achievement and was associated with higher levels of active function.
Key words: botulinum neurotoxin A; functional change; goal attainment scaling; onabotulinumtoxinA; post-stroke spasticity; rehabilitation.
J Rehabil Med 2014; 46: 504–513
Correspondence address: Professor Anthony B. Ward, North Staffordshire Rehabilitation Centre, Haywood Hospital, Stoke on Trent, ST6 7AG, UK. E-mail: Anthony.Ward@uhns.nhs.uk
Accepted Feb 18, 2014; Epub ahead of print Apr 8, 2014
INTRODUCTION
Numerous clinical studies have shown the benefits of treatment with botulinum neurotoxin type A (BoNT-A), in improving passive function and in reducing disability (as assessed by the modified Ashworth Scale (MAS) and/or the Disability Assessment Scale) in the upper limbs (UL) of patients with post-stroke spasticity (PSS) (1–6). However, demonstrating improvements in active function in clinical trials has proved more difficult. Several studies have indicated that non-pharmacological interventions, e.g. electrostimulation, splinting/orthosis and strength or task-oriented training programmes (7–9) may improve motor function in general populations of stroke survivors with a range of motor impairments. However, patients with PSS demonstrate a range of different impairments relative to the global stroke survivor population. A number of randomised controlled studies published to date have specifically and prospectively evaluated improvements in activity and participation with BoNT-A in patients with PSS (10–16), with differing results.
Unlike many previous studies, the BOTOX® Economic Spasticity Trial (BEST) (17) aimed to reflect usual clinical practice as far as possible and across different healthcare systems. This has been difficult to achieve up to now within the restrictions of a clinical trial, which typically do not provide for goals and outcomes to be tailored to the individual needs of the patients with PSS. Previous studies also included patients whose potential for functional improvement was small and in which functional goal achievement was not a principal objective (10, 18, 19). BEST evaluated changes in active functional goals in a selected group of PSS patients with evidence of some preserved agonist and antagonist function (e.g. in UL – grasping a fork; for lower limb (LL) – standing and ambulation). Only two other published studies have undertaken an evaluation of BoNT-A on active function in patients with PSS with some preserved UL function (14, 16). However, participants were also required to demonstrate at least one item of the REsistance to PAssive movement Scale (REPAS) (20) with a spasticity score of ≥1 across the relevant joints for which the primary outcome was defined. Additionally, a subgroup of patients was also identified with ankle plantarflexor spasticity in the gastrocnemius, soleus and tibialis posterior muscles, who received specific localized treatment, as it has been established that spasticity in these muscles limits walking endurance (21, 22).
BEST assessed whether goal-oriented rehabilitation in patients with focal or multifocal PSS is an effective approach, and evaluated whether the effects of treatment were comparable for patients with UL or LL PSS. It was the first study in adult patients with PSS to target individualized, patient-defined functional goals specifically as a primary endpoint, using Goal Attainment Scaling (GAS) although some studies have utilized this as a secondary outcome measure (1, 11). GAS is a validated methodology that has been used to measure changes in function over time in patients with acquired brain injury, and the effects of BoNT-A in individuals with PSS (1, 2). A key feature of GAS is that, rather than simply identifying whether a pre-defined goal has been achieved, it allows quantification of the level of goal attainment, due to the intervals defined on the scale (23).
Using GAS is more reflective of usual clinical practice than other outcome measures but has not been described before in large-scale prospective clinical trials. Smaller studies, however, have indicated that this approach, by engaging patients in goal setting, can lead to a beneficial sense of empowerment (2, 5, 24).
METHODS
BEST is a prospective, multicentre, randomized, double-blind, placebo-controlled Phase IIIb study with an open-label extension, conducted in Germany, Sweden, the United Kingdom, and Canada (Phase IV). The study design and protocol have been published previously (17), but are briefly summarized below. BEST was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice and the study protocol was approved by an independent ethics committee at each participating site.
Study population
Consecutive patients at each centre were considered for the study. Participation in the study was limited to men and women aged 18–85 years who: had experienced a stroke due to a primary cerebral haemorrhage/infarction or subarachnoid haemorrhage, leading to a hemiplegia/hemiparesis, ≥ 3 months before the screening visit, were considered as suitable and had the potential for functional gains following treatment with onabotulinumtoxinA for upper or lower limb spasticity. Patients with a fixed contracture as a result of spasticity and with causes of spasticity other than stroke were excluded.
Study design and setting
Between October 2007 and July 2009 a screening visit was followed by the baseline visit (with administration of the first injection of study medication), first assessment (after 12 weeks), optional second injection of study medication (at 12–24 weeks), second assessment (10 weeks after the second injection, or 24 weeks if the patient had received only one injection) and follow-up after 52 weeks.
Patients were randomized to onabotulinumtoxinA (BOTOX®, Allergan, Inc., Irvine, CA, USA) + standard of care (SC) or placebo + SC (in a 1:1 ratio) (Fig. 1) and the treatment arms were stratified according to location of spasticity (UL or LL). The study period lasted for a total of 52 weeks; 22–34 weeks of double-blind treatment (at which time the primary endpoint of the study was evaluated), followed by an open-label phase. During the double-blind period, patients received either a single injection of onabotulinumtoxinA or placebo, with a second dose at a minimum of 12 weeks, if the treating physician thought they would benefit from a second treatment. During the open-label phase, all patients were eligible to receive onabotulinumtoxinA injections, with a minimum inter-injection interval of 12 weeks. A maximum of 800 U of study medication was available to the investigator for any single treatment session. While minimum doses for each muscle were recommended in the study protocol, the principal investigators agreed that, in order to reflect clinical practice, individual patients’ dosing was to be at each investigator’s discretion based upon their clinical experience. This may not have reflected the manufacturer’s label.
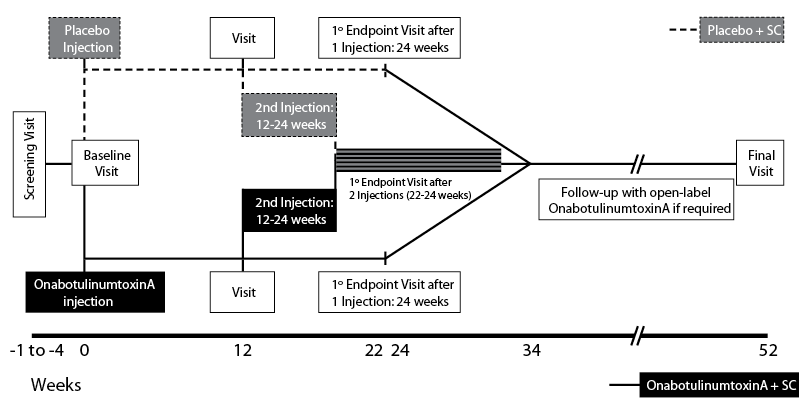
Each participating centre individually determined SC in terms of available resources and usual practice in that centre. Therefore SC was anticipated to differ between individual patients and centres across the study but for some, this may well have been a more intensive programme of care than prior to study entry, e.g., physical therapy, occupational therapy and SC focussed on their active functional goal achievement.
Study endpoints
The primary endpoint of BEST was the number of patients in each arm achieving their principal active functional goal at 24 weeks (or 10 weeks after the optional second injection of study drug) as determined by the investigator.
Secondary endpoints included (17): the number of patients achieving their principal active functional goal at 12 and 52 weeks, as determined by the investigator; secondary active or passive goal attainment at 12, 24 weeks (or 10 weeks after the optional second injection of study drug) and 52 weeks, as determined by the investigator; the median level of functional goal attainment (principal and secondary) as determined by the investigator (at 12, 24, and 52 weeks); REPAS-26 score at baseline and each study visit; and documentation of the occurrence of treatment-emergent adverse events (TEAEs) during the study.
Outcome measures
Goal Attainment Scaling. The study investigators underwent training in the use of GAS prior to the study and during the initial recruitment into the study to ensure an appropriate level of skill in setting realistic and achievable goals that were meaningful to the patients (25). The patient designated a primary limb as that in which they wanted to achieve improved active function during the study. The patient and investigator together then defined the principal active functional treatment goal, based on an objective treatment measure, to be achieved by the study intervention for the primary assessment limb. The patient and investigator also defined a secondary active or passive functional treatment goal. If a secondary active functional goal was chosen, this was set either for a different active function in the primary assessment limb or for an active function in another affected limb.
A 6-point modified version of GAS was chosen to capture deterioration in function (Table I) (25).
|
Table I. The 6-point modified version of Goal Attainment Scaling used in BOTOX® Economic Spasticity Trial (25) |
|
|
Level |
Description |
|
–3 |
Goal not achieved – deterioration from baseline level |
|
–2 |
Goal not achieved – much less than expected level of outcome |
|
–1 |
Goal not achieved – less than expected level of outcome |
|
0 |
Goal achieved – expected level of outcome |
|
+1 |
Goal achieved – better than expected level of outcome |
|
+2 |
Goal achieved – much better than expected level of outcome |
Resistance to passive movement. Changes in REPAS scores were assessed in order to demonstrate changes in the severity of spasticity (20). The REPAS is a validated, standardised composite measure that consists of 26 items across different joints, each of which are rated according to the MAS (0 = no increase in muscle tone to 4 = limb rigid in flexion or extension (26)), and includes assessments of the shoulder, elbow, forearm, wrist, finger, hip, knee, and foot (20). A higher score indicates more severe impairment, with a maximum total body score of 104 (upper limbs, 64; lower limbs, 40). The REPAS has previously been used to evaluate the effects of BoNT-A treatment on upper limb function in a small study involving stroke patients (27).
Statistical analyses
Populations used for the statistical analyses presented in this paper are: (1) All available-patients (AAP) population, which included all randomized patients regardless of study medication administration and was used to present demographic and baseline characteristics; (2) Intent-to-treat (ITT) population, which included all patients who were randomized and received a baseline injection of study drug, regardless of when they withdrew, and was used to present all efficacy and safety data.
Sample size calculations were performed for the primary efficacy endpoint of principal active functional goal achievement and also the secondary efficacy endpoint of the median level of principal active functional goal achievement, both at 24 weeks/10 weeks after the optional second injection of study drug. For the primary efficacy endpoint, it was expected that at least 80% of the patients in the onabotulinumtoxinA + SC treatment group would achieve or exceed their principal active functional goal, whereas less than 50% of the patients in the placebo + SC treatment group would achieve this outcome. Using these assumptions and with α = 0.05 and power equal to 80%, the estimated sample size required to detect a statistically significant difference using a two-sample continuity corrected two χ2 test of equal proportions ranged from 28 to 91 per treatment group. Assuming placebo and onabotulinumtoxinA proportions of patients achieving functional goal at 0.6 and 0.8, respectively, and a dropout rate of 15%, the maximum number of patients to be enrolled was 210.
For the secondary efficacy endpoint, the median level of the principal active goal achieved by the patients in the onabotulinumtoxinA + SC treatment group, based on 80% of patients achieving this goal, was assumed to range from 0 to 1 (ie, the score to increase by 2 or 3, respectively, from baseline). The median level achieved by the patients in the placebo + SC treatment group, based on 40% or 50% of patients achieving goal, was assumed to range from –2 to –0.5. For a comparison achieved using a Wilcoxon-Mann-Whitney rank sum test, assuming a probability of 0.6 that an observation in the placebo + SC group would be less than an observation in the onabotulinumtoxinA + SC group, a sample size of 131 in each treatment group (total of 262) would have 80% power at a 2-sided α = 0.05. The number of patients to be enrolled was therefore 300, to adjust for a dropout rate of 15%.
A final sample size was determined with 300 enrolled patients allowing for a 15% dropout rate, a power of 80% when testing the primary hypothesis, and allowed sufficient sample for secondary analysis. All hypothesis testing was carried out at the 5% (two-sided) significance level unless otherwise specified. Descriptive summary statistics were presented for continuous variables: median, minimum and maximum, arithmetic mean, 95% confidence intervals (CI), standard deviation (SD) and standard error. In non-parametric summaries and analyses, the minimum, maximum, median, 5th, 25th, 75th, and 95th percentiles were calculated. The median difference between treatment groups and the corresponding 95% CI were presented in statistical analysis outputs. For categorical variables, counts and percentages were presented, and comparison of treatment groups performed using generalized linear models to provide odds ratios (ORs), corresponding 95% CI and p-values.
For the primary endpoint, the number and percentage of patients who achieved their principal active functional goal were summarized. The OR for onabotulinumtoxinA + SC vs. placebo + SC was reported, along with the 95% CI and two-sided p-value.
For goal achievement, the OR for onabotulinumtoxinA + SC vs. placebo + SC for each secondary efficacy outcome measure was reported, along with the 95% CI and two-sided p-value. For the level of goal achievement, the median difference between onabotulinumtoxinA + SC and placebo + SC was calculated using the Cochran-Mantel-Haenszel raw mean score, using non-parametric scores. This was reported, along with the corresponding 95% CI.
Pre-specified subgroup analyses were performed according to the location of spasticity. These were chiefly: upper limb spasticity, lower limb spasticity, and also lower limb ankle plantar flexor spasticity (the gastrocnemius, soleus and tibialis posterior). Achievement of the principal active functional goal, secondary goals, and level of goal achievement were calculated as above for each of these subgroups.
Randomised spasticity location was a stratification factor in all the analyses. Country, treatment by country interaction, age, gender and time since stroke were included in the primary efficacy endpoint analysis for the ITT population using logistic regression. Significant covariates were identified at a significance level of α = 0.1 using a backward selection method.
Trial registration: EudraCT 2007-000735-26 (https://www.clinicaltrialsregister.eu/ctr-search/search?query=2007-000735-26).
RESULTS
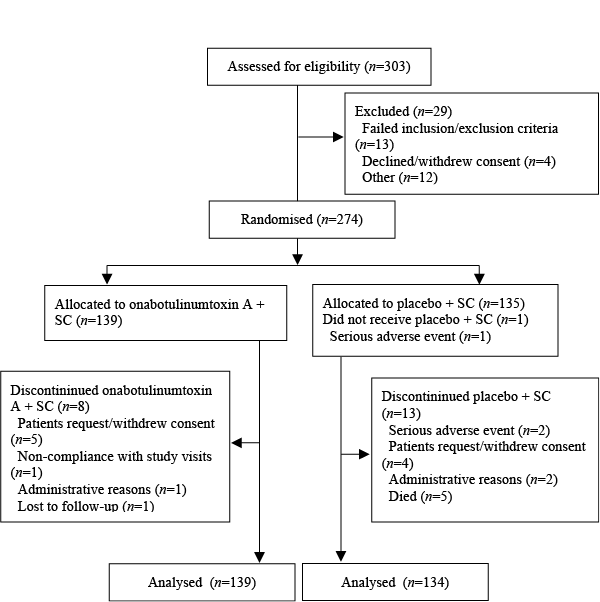
Patient disposition is shown in Fig. 2. A total of 303 patients were screened for the study, of whom of 274 were randomized to study treatment and formed the AAP population. One patient withdrew from the study prior to receiving the first injection of study medication (ITT population = 273 patients). For the primary endpoint of principal active functional goal attainment (at 24 weeks, or 10 weeks after the second injection), the number of assessable patients was 137 in the onabotulinumtoxinA + SC group and 132 in the placebo + SC group. A total of 21 patients withdrew from the study (onabotulinumtoxinA + SC, n = 8; placebo + SC, n = 13). The reasons are presented in Fig. 2.
In terms of baseline clinical and demographic characteristics, the treatment groups were comparable (Table II). Notably, a substantial proportion of patients were aged < 65 years, and for many, their last stroke had occurred > 12 months prior to study entry. More than 90% of patients demonstrated moderate or severe spasticity at baseline (Table I). Seventy-three percent of patients had received physiotherapy in the 3 months prior to baseline.
|
Table II. Baseline characteristics (all available patients population) |
||
|
OnabotulinumtoxinA + SC (n = 139) |
Placebo + SC (n = 135) |
|
|
Gender, n (%) |
||
|
Male |
85 (61.2) |
76 (56.3) |
|
Female |
54 (38.8) |
59 (43.7) |
|
Race, n (%) |
||
|
Caucasian |
136 (97.8) |
130 (96.3) |
|
Other |
3 (2.2) |
5 (3.7) |
|
Age, years |
||
|
Median (range) |
64.11 (22.6–81.2) |
61.86 (26.8–82.4) |
|
< 65 years, n (%) |
73 (52.5) |
54 (40.0) |
|
Type of stroke, n (%) |
||
|
Cerebral ischaemic |
102 (73.4) |
102 (75.6) |
|
Cerebral haemorrhagic |
30 (21.6) |
27 (20.0) |
|
Subarachnoid haemorrhage |
6 (4.3) |
6 (4.4) |
|
Not known |
1 (0.7) |
0 |
|
Severity of strokea, n (%) |
||
|
Mild |
9 (6.5) |
5 (3.7) |
|
Moderate |
99 (71.2) |
98 (72.6) |
|
Severe |
31 (22.3) |
32 (23.7) |
|
Affected limbs, n (%) |
||
|
Right arm |
56 (40.3) |
46 (34.1) |
|
Right leg |
57 (41.0) |
46 (34.1) |
|
Left arm |
82 (59.0) |
89 (65.9) |
|
Left leg |
82 (59.0) |
88 (65.2) |
|
Severity of spasticityb, n (%) |
||
|
Mild |
7 (5.0) |
8 (5.9) |
|
Moderate |
103 (74.1) |
101 (74.8) |
|
Severe |
29 (20.9) |
25 (18.5) |
|
Time since stroke, months |
||
|
Median (range) |
24.05 (2.9–252.3) |
21.29 (3.0–402.6) |
|
> 12 months, n (%) |
94 (67.6) |
95 (70.4) |
|
aAs assessed by the treating physician. Mild: minor deficit, functionally non-impairing odds ratio mild functional deficit with some restriction of lifestyle; moderate: moderate deficit significantly interfering with activities of daily living; severe, dependent, requiring chronic care. bMild: all joints with a REPAS Ashworth score of < 2 in the limb associated with the primary goal; moderate: REsistance to PAssive movement Scale Ashworth score of 2 or 3 in any joint in the limb associated with the primary goal; severe: REPAS Ashworth score of 4 in any joint in the limb associated with the primary goal. SC: standard of care. |
||
Treatment
A summary of study medication administration is presented in Table III. During the double-blind and open-label periods, the median doses administered were similar for both treatment groups. However, the range of cumulative doses during the open-label period were broad, as a small number of individual patients received relatively high doses of onabotulinumtoxinA. A total of 225 patients received open-label onabotulinumtoxinA (113 initially randomised to onabotulinumtoxinA and 112 initially randomised to placebo).
|
Table III. Study medication doses administered throughout the study (intention-to-treat population) |
||
|
Study medication injection |
OnabotulinumtoxinA + SC |
Placebo + SC |
|
First injection, n |
139 |
134 |
|
Total patient population, median units (25th percentile, 75th percentile) |
340 (250, 400) |
300 (220, 400) |
|
Upper limb only, median (range) |
275 (100 to 450) |
260 (100 to 600) |
|
Lower limb only, median (range) |
300 (100 to 740) |
282.5 (100 to 400) |
|
Both upper and lower limbs, median (range) |
400 (200 to 800) |
400 (160 to 725) |
|
Optional second injection, n |
102 |
94 |
|
Total patient population, median units (25th percentile, 75th percentile) |
365 (230, 400) |
350 (230, 400) |
|
Upper limb only, median (range) |
212.5 (100 to 700) |
250 (100 to 600) |
|
Lower limb only, median (range) |
300 (100 to 570) |
275 (150 to 400) |
|
Both upper and lower limbs, median (range) |
400 (150 to 750) |
400 (200 to 650) |
|
Cumulative dosing during open-label phasea, n |
113 |
112 |
|
Total patient population, median units (25th percentile, 75th percentile) |
600 (320, 800) |
525 (300, 800) |
|
Upper limb only, median (range) |
400 (100 to 1000) |
300 (100 to 1900b) |
|
Lower limb only, median (range) |
360 (100 to 1300) |
470 (180 to 860) |
|
Both upper and lower limbs, median (range) |
725 (130 to 1600) |
697.5 (150 to 1500) |
|
aCumulative doses from all injections during the open-label phase of the study. bOne patient received a cumulative dose of 1900 U during the open-label treatment phase. SD: standard deviation. |
||
Primary endpoint
Despite trends in favour of onabotulinumtoxinA + SC vs. placebo + SC the difference for principal active functional goal achievement between groups at the primary endpoint was not statistically significant (Table IV). Principal active functional goal achievement at 12 weeks was comparable for patients receiving onabotulinumtoxinA + SC or placebo + SC. Similar results were observed at 52 weeks (i.e. following the open-label period when subjects randomised to placebo injections could receive optional onabotulinumtoxinA injections). Median level of principal active functional goal achievement was similar for onabotulinumtoxinA + SC and placebo + SC at 24 weeks, or 10 weeks after the second injection.
|
Table IV. Goal achievement, assessed using the goal attainment scale at (intention-to-treat population) |
||||||||
|
Week 12 |
Week 24/10 weeks after second injection |
Week 52 |
||||||
|
OnabotulinumtoxinA + SC (n = 139) |
Placebo + SC (n = 134) |
OnabotulinumtoxinA + SC (n = 139) |
Placebo + SC (n = 134) |
OnabotulinumtoxinA + SC (n = 139) |
Placebo + SCa (n = 134) |
|||
|
Principal active functional goal achievement, % |
33.1 |
28.9 |
40.9 |
33.3 |
45.0 |
52.4 |
||
|
OR (95% CI); p-value |
1.20 (0.69 to 2.10); p = 0.512 |
1.36 (0.81 to 2.29); p = 0.247 |
0.74 (0.44 to 1.23); p = 0.239 |
|||||
|
Secondary functional (active or passive) goal achievement, % |
40.5 |
34.7 |
51.6 |
40.7 |
53.8 |
55.6 |
||
|
OR (95% CI); p-value |
1.31 (0.77 to 2.22); p = 0.324 |
1.62 (0.95 to 2.76); p = 0.079 |
0.94 (0.57 to 1.55); p = 0.803 |
|||||
|
Secondary active functional goal achievement, % |
38.5 |
33.3 |
39.2 |
43.8 |
46.3 |
47.3 |
||
|
OR (95% CI); p-value |
1.44 (0.63 to 3.31); p = 0.386 |
0.94 (0.40 to 2.23); p = 0.896 |
1.21 (0.54 to 2.71); p = 0.646 |
|||||
|
Secondary passive functional goal achievement, % |
41.9 |
35.8 |
60.6 |
38.6 |
59.2 |
62.3 |
||
|
OR (95% CI); p-value |
1.22 (0.60 to 2.49); p = 0.580 |
2.46 (1.18 to 5.14); p = 0.016 |
0.82 (0.41 to 1.62); p = 0.562 |
|||||
|
aPatients randomised to placebo could received optional injections of onabotulinumtoxinA after 24 weeks (a total of 112 patients randomised to placebo received open-label onabotulinumtoxinA). CI: confidence interval; OR: odds ratio; SC: standard of care. |
||||||||
Secondary endpoints
There were no statistically significant differences in goal achievement between groups for all secondary goals (active or passive combined), and secondary active goals alone, at 12, 24 or 52 weeks (Table IV). However, the proportion of patients demonstrating secondary passive goal achievement at the primary endpoint was significantly greater with onabotulinumtoxinA + SC (60.6%) than with placebo + SC (38.6%) (OR 2.46; 95% CI 1.18 to 5.14; p = 0.016) (Table IV). The median level of goal achievement was not significantly different between groups for secondary goals (active and passive) or secondary active goals. However, the median level of secondary passive goal achievement was significantly greater with onabotulinumtoxinA + SC than with placebo + SC (0 vs. –1; treatment difference, 1.0 (95% CI 0.0 to 1.0); p = 0.01).
Mean (standard deviation (SD)) REPAS summated total scores were similar for onabotulinumtoxinA + SC and placebo + SC groups at baseline (20.9 (SD 9.1) vs. 21.7 (SD 9.2)), and were significantly reduced at 24 weeks, or 10 weeks after the second injection (mean –3.9 (95% CI –5.0 to –2.9) vs. –2.8 (95% CI –3.9 to –1.7)). Similar changes were observed in patients with UL or LL spasticity (Table V).
|
Table V. Change from baseline in REsistance to PAssive movement Scale Summated total score (intention-to-treat population) |
||
|
Spasticity location |
OnabotulinumtoxinA + SC |
Placebo + SC |
|
Upper limb spasticity, n |
62 |
62 |
|
Baseline, mean (SD) |
20.1 (8.2) |
21.2 (8.4) |
|
Change at 24 weeks/10 weeks after the second injection, mean (95% CI) |
–4.3 (–5.7 to –2.8) |
–1.7 (–2.9 to –0.4) |
|
Lower limb spasticity, n |
77 |
72 |
|
Baseline, mean (SD) |
21.5 (9.8) |
22.1 (9.8) |
|
Change at 24 weeks/10 weeks after the second injection, mean (95% CI) |
–3.7 (–5.2 to –2.2) |
–3.7 (–5.4 to –1.9) |
|
CI: confidence interval; SC: standard of care; SD: standard deviation. |
||
For UL principal active functional goals, statistically significant higher GAS scores were attained by patients receiving onabotulinumtoxinA + SC compared with placebo + SC (Table VI). Of 116 patients who had an UL principal active functional goal, the most common goals were eating/drinking (n = 30), dressing (n = 18), and washing (n = 8). For principal active functional goals pertaining to LL function, there was no significant difference between treatments for GAS scores. Of 157 patients who had a LL principal active functional goal, 140 (89%) had a goal related to ambulation. There were no significant differences between onabotulinumtoxinA + SC and placebo + SC, in terms of GAS scores for either UL or LL secondary active functional goals (Table VII). Secondary UL functional goals were focussed on dressing (n = 9), feeding (n = 9) and washing (n = 3), and for lower limb, ambulation (n = 38), climbing stairs (n = 16) and independent transfers (n = 2).
|
Table VI. Level of principal active functional goal attainment, assessed at week 24, or 10 weeks after the second injection |
||
|
OnabotulinumtoxinA + SC |
Placebo + SC |
|
|
Upper limb principal active functional goalsa, ITT population (assessable patients), n |
n = 62 (n = 54) |
n = 62 (n = 52) |
|
+2 |
3 (5.6) |
1 (1.9) |
|
+1 |
7 (13.0) |
3 (5.8) |
|
0 |
11 (20.4) |
9 (17.3) |
|
–1 |
19 (35.2) |
16 (30.8) |
|
–2 |
12 (22.2) |
20 (38.5) |
|
–3 |
2 (3.7) |
3 (5.8) |
|
Median |
–1 |
–1 |
|
Median difference (95% CI); p-value |
0.0 (0.0 to 1.0); p = 0.034 |
|
|
Lower limb principal active functional goalsb, ITT population (assessable patients), n |
n = 77 (n = 69) |
n = 72 (n = 66) |
|
+2 |
3 (4.3) |
11 (16.7) |
|
+1 |
9 (13.0) |
5 (7.6) |
|
0 |
18 (26.1) |
14 (21.2) |
|
–1 |
17 (24.6) |
12 (18.2) |
|
–2 |
19 (27.5) |
21 (31.8) |
|
–3 |
3 (4.3) |
3 (4.5) |
|
Median |
–1 |
–1 |
|
Median difference (95% CI); p-value |
0.0 (–1.0 to 0.0); p = 0.724 |
|
|
aITT: intention-to-treat; b SC: standard of care. |
||
|
Table VII. Level of secondary active functional goal attainment, assessed at week 24, or 10 weeks after the second injection |
||
|
OnabotulinumtoxinA + SC, n |
Placebo + SC, n |
|
|
Upper limb secondary active functional goalsa, ITT population (assessable patients), n (%) |
n = 62 (n = 27) |
n = 62 (n = 23) |
|
+2 |
2 (7.4) |
1 (4.3) |
|
+1 |
5 (18.5) |
3 (13.0) |
|
0 |
4 (14.8) |
6 (26.1) |
|
–1 |
3 (11.1) |
2 (8.7) |
|
–2 |
11 (40.7) |
10 (43.5) |
|
–3 |
2 (7.4) |
1 (4.3) |
|
Median |
–1 |
–1 |
|
Median difference (95% CI) |
0.0 (–1.0 to 1.0); p = 0.935 |
|
|
Lower limb secondary active functional goalsb, ITT population (assessable patients), n (%) |
n = 77 (n = 24) |
n = 72 (n = 25) |
|
+2 |
1 (4.2) |
5 (20.0) |
|
+1 |
2 (8.3) |
2 (8.0) |
|
0 |
6 (25.0) |
4 (16.0) |
|
–1 |
10 (41.7) |
3 (12.0) |
|
–2 |
5 (20.8) |
10 (40.0) |
|
–3 |
0 |
1 (4.0) |
|
Median |
–1 |
–1 |
|
Median difference |
0.0 (–1.0 to 1.0); p = 0.813 |
|
|
aITT: intention-to-treat; bSC: standard of care. |
||
Subgroup analyses
A separate analysis was performed for the subgroup of patients with ankle plantarflexor spasticity (n = 78) who had received injections in the 3 key plantarflexor muscles (gastrocnemius, soleus and tibialis posterior), during the double-blind period of the study. There was no statistically significant difference between onabotulinumtoxinA + SC and placebo + SC for principal active functional goal achievement at the primary endpoint. However, the level of principal active functional goal attainment for this subgroup was significantly higher (p = 0.030) in the onabotulinumtoxinA + SC treatment group as compared to the placebo + SC group, at the primary endpoint. For the same subgroup of patients, a significantly higher percentage of patients randomized to onabotulinumtoxinA + SC achieved their secondary goal (active or passive) (62.9%) compared with patients randomized to placebo + SC (36.4%) (p = 0.029). The level of secondary goal attainment as assessed by the investigator, was also significantly higher (p = 0.003) in the onabotulinumtoxinA + SC group as compared to the placebo + SC group at the primary endpoint. The mean change from baseline in Ashworth ankle score (as part of REPAS) for this subgroup at 10 weeks post-second injection was –0.8 with onabotulinumtoxinA + SC vs. 0 with placebo + SC (p = 0.003).
When considering patient age as a covariate, there was a significant correlation with achievement of the principal active functional goal at the primary endpoint (parameter estimate, –0.047; p = 0.006), i.e. younger patients were more likely to demonstrate goal achievement than older patients. There was also a significant correlation between age and achievement of secondary goals at the primary endpoint (parameter estimate, –0.081; p < 0.001). Time since stroke was also significantly correlated with principal active functional goal achievement (parameter estimate, –0.754; p = 0.022); a shorter duration since stroke was associated with an increased likelihood of goal achievement.
Safety
For the entire study duration, double-blind and open-label phases, the incidence of TEAEs were slightly higher for onabotulinumtoxinA + SC than placebo + SC (Table VIII). The most frequently-reported treatment-related TEAEs were local muscle weakness (onabotulinumtoxinA + SC, 5.0%; placebo + SC, 0.7%), falls (onabotulinumtoxinA + SC, 1.4%; placebo + SC, 0%), and musculoskeletal pain (onabotulinumtoxinA + SC, 1.4%; placebo + SC, 0%). All other TEAEs occurred in less than 1% of patients in either group. The study was not powered to detect statistically significant differences between groups for TEAEs.
|
VIII. Treatment-emergent adverse events (intention-to-treat population) |
||
|
OnabotulinumtoxinA + SC (n = 139) n (%) |
Placebo + SC (n = 134) n (%) |
|
|
TEAEs reported DB phase |
74 (53.2) |
66 (49.3) |
|
Treatment-related TEAEs reported DB phase |
14 (10.1) |
5 (3.7) |
|
TEAEs reported during OL phase |
96 (42.7) |
|
|
Treatment-related TEAEs reported OL phase |
8 (3.6) |
|
|
Serious TEAEs reported during DB phase |
19 (13.7) |
16 (11.9) |
|
Treatment-related serious TEAEs DB phase |
1 (0.7) |
0 |
|
Serious TEAEs reported OL phase |
29 (12.9) |
|
|
Treatment-related serious TEAEs reported OL phase |
0 |
|
|
TEAE: treatment-emergent adverse event; DB: double blind; OL: open label; SC: standard of care. |
||
One patient experienced a treatment-related SAE (Serious Adverse Event; increased muscle spasticity), which occurred during the double-blind period in the onabotulinumtoxinA + SC group. Two patients in the placebo group experienced SAEs leading to withdrawal: pneumonia (n = 1) and polymyalgia rheumatica (n = 1). There were 5 deaths reported during the entire study, and all occurred in patients randomized to placebo + SC. Three occurred during the double-blind period (cerebrovascular accident; myocardial ischaemia; pneumonia and cardiac failure); and two occurred during the open-label phase (pneumonia; cerebral infarction and circulatory collapse). None were considered to be related to study treatment.
DISCUSSION
In this study, the primary endpoint of principal active functional goal achievement was not significantly better with onabotulinumtoxinA + SC vs. placebo + SC at Week 24/10 weeks after the second injection, and similar findings were obtained for all secondary goals and secondary active goals. However, the results presented here demonstrate a significant improvement in passive function with onabotulinumtoxinA + SC vs. placebo + SC, as measured by the proportions of patients achieving their secondary passive goals. In addition, the median level of passive secondary goal achievement was significantly higher in the onabotulinumtoxinA + SC group than in the placebo + SC group at the primary endpoint. This was seen in previous studies (1, 5, 11).
When looking at the subgroup analyses, the median level of principal active functional goal achievement in patients with UL spasticity was significantly greater for onabotulinumtoxinA + SC vs. placebo + SC. There was no difference between treatment groups in terms of GAS scores for lower limb goals, and the mean change from baseline in REPAS summated total score was also similar.
The range of goals encompassing both upper and lower limbs may disguise the benefits seen in the more specific UL and ankle plantarflexor sub groups. Indeed it is possible that the hurdle of functional disability was greater in patients with a primary goal associated with their LL. As such, 90% of principal active functional goals in the LL concerned ambulation. To be able to stand or transfer is different from UL function with regard to restoring or learning new skills. Ambulation is a multilevel problem and several factors other than LL spasticity may affect LL functioning, thus presenting unique challenges. There are fundamental differences in the neural control of hand and leg movement, considering the contributing role of spinal reflexes in central pattern generation for gait (28, 29) vs. the almost exclusively higher control of fine hand movements (30). The potential for neural and muscle plasticity responsiveness may therefore differ for upper vs. lower limbs, translating into different capacities for functional achievements after stroke. Notably, those patients who received injections in the key ankle plantarflexors, demonstrated a significant benefit of onabotulinumtoxinA + SC relative to placebo + SC for median level of principal active functional goal achievement, and also for median level of all secondary goals. It has been established that in PSS patients, this group of muscles contributes to walking endurance (21, 22), and that specific localized treatment with BoNT-A may enhance mobility and gait quality (31, 32). It may therefore be that focussing on specific areas of spasticity such as the ankle plantarflexors leads to more focussed rehabilitation and a greater likelihood of goal attainment.
BEST has provided additional insights during the open-label phase. In patients initially randomized to placebo + SC, but subsequently treated with onabotulinumtoxinA after week 24, the proportions of patients achieving their principal and secondary goals at week 52 increased consistently, and was therefore comparable to that for patients who had received onabotulinumtoxinA + SC throughout the study. This may indicate that even delayed onabotulinumtoxinA treatment can augment SC. With a longer duration of the motor disorder, PSS patients may benefit from a preparatory reconditioning of neural and muscle function, as well as structural characteristics, such as is provided by SC, in order to optimise the additional effect of onabotulinumtoxinA. In reducing spasticity, BoNT-A may facilitate the reversal of maladaptive muscle changes (33, 34).
As a methodology, GAS has been validated in numerous studies, across disease areas and disciplines (1, 5, 35, 36). In the small, prospective observational cohort study (n = 16) published by Ashford & Turner-Stokes (1), patients with shoulder girdle and upper proximal limb spasticity (regardless of pathology) set goals analyzed after 16 weeks of BoNT-A treatment. GAS scores improved in 15 of 16 patients over the study period, with goals achieved or over-achieved in these 15 individuals. In a separate, larger study, patients with UL spasticity demonstrated a significant benefit of BoNT-A treatment vs. placebo for GAS score (11).
BEST showed that goal-oriented spasticity care is an effective approach, and the authors recommend that it should be adopted as standard practice. Active patient participation in goal-setting is valuable and it may empower patients and lead to greater motivation to achieve their goals. However, it is important that clear and specific goals be set; they should be easily measured and achievable. This study also demonstrated that improvements in activity related to goals can be achieved, even with a mean duration of 4 years post-stroke.
Many patients included in BEST had a significantly longer post-stroke duration (approximately two-thirds were > 1 year), and this may have had an impact on the results they were able to achieve. Indeed, the exploratory analysis indicated that there was a negative relationship between longer time since stroke and principal active functional goal achievement. This would suggest that early intervention in patients with PSS is potentially clinically advantageous (15). A subgroup analysis of data from patients less than 2 years following stroke could provide an insight into this issue. However, studies involving patients with PSS in post-acute rehabilitation (≤ 3 months after the event) (37–39) have not prospectively evaluated the effects of early treatment initiation; such studies would provide additional information.
No new safety concerns were raised during the study, which included doses up to 800 U per visit. Notably, the numbers of treatment-related TEAEs reported decreased over time (double-blind period vs. open-label period), even though the doses of study treatment remained of a comparable magnitude. These findings are interesting when considering the similar incidences of muscle weakness reported in BEST and an earlier study evaluating lower doses of onabotulinumtoxinA in patients with hand and wrist spasticity, over a much shorter period (12 weeks) (3). Several previous studies have comprehensively assessed the safety of onabotulinumtoxinA in patients with PSS, reporting findings in line with those observed in BEST (1, 3, 18).
The results of this study may have been confounded by the degree of improvement observed with SC alone; this was not predicted in the statistical planning (as described by Borg et al., 2011 (17)). During the study, SC was possibly of a higher quality or more intensive than that usually received by the participants. However, in this respect BEST supports the hypothesis that a high standard of SC is an important and effective intervention in patients with PSS. There were more marked differences between groups for goal attainment in UL spasticity, which is a well-established target for BoNT-A therapy, albeit using other assessment tools, such as the MAS (1-4, 11).
Study limitations
BEST reflected usual clinical practice, and inherent in this type of study design are important limitations and intrinsic variability. The broad variety of goals defined in the study’s ‘real-world’ design, although tailored to individuals needs, makes comparison of outcomes inherently more difficult than using pre-defined clinical outcomes. Additionally, although investigators were directed to ensure that goals were realistic and considered to be challenging but achievable, the difficulty in reaching the predefined goal achievement may have been greater than anticipated in some cases.
Furthermore, GAS had not been widely used in the participating centres prior to the study and a significant element of learning in goal-setting and scaling was therefore apparent during the conduct of the study (4). Indeed, the goals set at the beginning of BEST and those set towards the end may have differed due to increasing experience (11). Also, the degree of functional disability will have varied from patient to patient dependent upon their pattern and severity of stroke and spasticity.
Although standardized within each centre, each centre determined what SC meant for them in order to reflect its clinical practice as far as possible. Additionally, patients would have received different levels of physical therapy prior to entering the study. These factors may have increased variability of the potential benefits provided by this support. While general guidance was provided concerning the administration of study medication (minimum suggested doses for individual muscles) the selection of target muscles and doses injected was decided by the individual investigators on the basis of a patient’s requirements, and their own experience, in order to facilitate the achievement of their personalized functional goals.
Conclusions
OnabotulinumtoxinA provides additional benefits helping patients with focal and multifocal spasticity to improve passive function for enhanced activity and participation. The data presented here support some recommendations for clinical practice, namely, the development of goal attainment as standard rehabilitation practice, together with adequate training in how to use goal attainment (40). Additionally, in this study, a shorter duration since stroke was associated with an increased likelihood of goal achievement. While adequate physical training is a good method to re-establish function in patients with longterm PSS using GAS to identify and achieve goal-oriented outcomes, patients need to be actively engaged in it and in the goal setting process.
Although the proportion of patients achieving their principal active functional goal with onabotulinumtoxinA + SC did not differ significantly compared to that for patients with placebo + SC, a trend was seen in improving active function GAS score in pre-defined subgroups of PSS patients with UL goals and for goals where ankle plantar flexor spasticity was the target of onabotulinumtoxinA therapy.
ACKNOWLEDGEMENTS
Full details of the acknowledgements and members of the BEST Study Group can be found in Appendix S11.
Conflicts of interest: Professor Anthony B. Ward has participated in research studies, for which unrestricted grants have been provided by Allergan. He has been the recipient of honoraria and fees for presentations at meetings and congresses and for participating in Advisory Boards. He has also received, in the past, honoraria and fees from Ipsen, Medtronic and Merz for presentations at meetings and congresses.
Professor Jörg Wissel has participated in research studies, for which unrestricted grants have been provided by Allergan, Elan, Merz and Ipsen. He has been the recipient of honoraria and fees for presentations at meetings and congresses and for participating in Advisory Boards from Allergan, Eisai, Ipsen, Merz and Medtronic.
Professor Jörgen Borg has been the recipient of honoraria and fees for presentations at meetings and congresses and for participating in Advisory Boards.
Dr Christoph Herrmann has participated in research studies, for which unrestricted grants have been provided by Allergan. He has been the recipient of honoraria and fees from Allergan, Ipsen, Medtronic and Merz for presentations at meetings and congresses.
Professor Jai Kulkarni has been the recipient of honoraria and fees from Allergan and Ipsen for presentations at meetings and congresses.
Dr Kristina Lindgren has no conflicts of commercial interest in this study.
Dr Mohamed Sakel has been the recipients of honoraria and fees from Allergan and Ipsen for presentations at meetings and congresses.
Dr Per Ertzgaard has been the recipient of honoraria and fees from Allergan and Medtronic for presentations at meetings and congresses and for participating in Advisory Boards.
Dr Iris Reuter has received honoraria and fees from Allergan, Ipsen, Merz and Medtronic for presentations at meetings and congresses and for participating in Advisory Boards.
Dr Patrik Säterö has received honoraria and fees from Allergan for presentations at meetings and congresses.
Dr Satyendra Sharma has no conflicts of commercial interest in this study. He has received honoraria and fees from Allergan and Merz for presentations at meetings and congresses, and for serving as a faculty member in an educational programme sponsored by Allergan.
Dr Theodore Wein has participated in research studies for which unrestricted grants have been provided by Allergan, Sanofi, Bristol Myers Squibb, Pfizer and the National Institutes of Health. He has received honoraria for participating in congresses, Advisory Boards and accredited CME activities from Allergan, Bristol Myers Squibb, Sanofi, Pfizer and Servier. In addition, he has received consultancy fees from Allergan.

1http://www.medicaljournals.se/jrm/content/?doi=10.2340/16501977-1817
REFERENCES
