Rutger Dahmen, MD1,2, Saskia Buijsmann, MD, PhD1,2, Petra C. Siemonsma, PT, PhD1, Maarten Boers, MD, PhD3, Gustaaf J. Lankhorst, MD, PhD4 and Leo D. Roorda, MD, PT, PhD1
From the 1Amsterdam Rehabilitation Research Center, Reade, 2Department of Rehabilitation, Slotervaart Hospital, 3Department of Epidemiology and Biostatistics, and 4Department of Rehabilitation, VU University Medical Center, Amsterdam, The Netherlands
OBJECTIVES: An estimated 55–90% of patients with rheumatoid arthritis have foot problems. Therapeutic footwear is frequently prescribed as part of usual care, but data on its use and effect is incomplete. This study aimed to investigate the use and effects of therapeutic footwear.
METHODS: Patients with rheumatoid arthritis receiving custom-made therapeutic footwear for the first time formed an inception cohort. Patients reported their therapeutic footwear use on 3 consecutive days in activity diaries 14 and 20 weeks after delivery of the footwear. The Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC) was used as the primary outcome of lower-extremity-related pain and activity limitations, and the Health Assessment Questionnaire (HAQ) as a secondary outcome measure of activity limitations, both at baseline and 26 weeks after therapeutic footwear delivery.
RESULTS: The cohort comprised 114 rheumatoid arthritis patients (median disease duration 10 years). Mean (standard deviation) therapeutic footwear use was 54 (25)% of the time patients were out of bed. The median (interquartile range) WOMAC score improved from 41 (27–59) to 31 (16–45) (p < 0.001). Secondary outcome measures improved significantly.
CONCLUSION: Therapeutic footwear was used with moderate intensity by most rheumatoid arthritis patients and was associated with a substantial decrease in pain and activity limitations. Therapeutic footwear is a relevant treatment option for patients with rheumatoid arthritis and foot problems.
Key words: therapeutic footwear; rheumatoid arthritis; use; compliance; effects; activity limitation; pain; cohort study.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Rutger Dahmen, Amsterdam Rehabilitation Research Center, Reade, P.O. Box 58271, NL-1040 HG Amsterdam, The Netherlands. E-mail: r.dahmen@reade.nl
Accepted Jan 10, 2014; Epub ahead of print Apr 22, 2014
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease frequently characterized by foot problems (1, 2). Between 55% and 90% of patients with RA experience foot problems at some time during the course of their disease (1, 3). The ankle, hindfoot and, especially, the forefoot are usually affected (3). In a cross-sectional study, 81% of 285 consecutive RA patients with a mean disease duration of 10 years reported forefoot pain (2). The prevalence and severity of forefoot joint damage increases during the course of the disease (4). Intra-articular and peri-articular changes, consisting of synovitis, joint damage (erosions) and deformities, all contribute to alteration of plantar pressure, pain and gait disturbance (5, 6). Subsequently, this may lead to limitations in weight-bearing activities, such as standing and walking, and participation restriction (7–10).
Next to pharmacological treatment, usual care for rheumatoid foot problems include the prescription of orthoses, insoles and therapeutic footwear (TF), and orthopaedic surgery (11, 12). Each year, a considerable quantity of TF is prescribed at a considerable financial expense to society. In the Netherlands this is estimated to be 60 million Euros (13).
Prospective research on the effects of TF in RA has not been very promising. A review concluded that there is insufficient evidence to conclusively link footwear interventions with reducing pain and improvement in gait and function in RA (14). However, a systematic review (12) identified randomized controlled trial evidence suggesting that extra-depth shoes are likely significantly to decrease pain on weight-bearing and Health Assessment Questionnaire (HAQ) scores (10), especially if combined with orthoses (1). Limitations of these studies included small sample sizes (1, 10), high numbers of drop-outs (15), exclusive use of factory-made TF (1, 10), and assessment of global effects (1, 10, 15). In addition, studies investigating the association between the use and the effects of TF in patients with RA are lacking.
Although patients with foot problems may benefit from TF, wearing-compliance varies (1, 10, 14), and TF may end up as “just another pair of shoes in the cupboard” (16). A number of studies in RA patients have shown non-use rates of TF of between 3% and 28% (17–20), but these studies are difficult to interpret due to different definitions of use, their cross-sectional design (17, 20) and mixed study population (20–22), and the assessment of TF-use with a single rating scale (17, 20, 21).
The current prospective observational study of a cohort investigated both the use and effects on lower-extremity related pain and activity limitations of custom-made TF, as well as the associations between them.
Patients and Methods
Patient population
This was a prospective observational study in a cohort of patients referred by rheumatologists to the arthritis foot clinic of Reade (formerly the Jan van Breemen Institute), an outpatient clinic for rehabilitation and rheumatology. Inclusion criteria were: definite diagnosis of RA according to the American College of Rheumatology (ACR) revised criteria (1987) (23) and the prescription of custom-made TF for the first time. Exclusion criteria were: presence of relevant comorbidity, such as central or peripheral neurological diseases, and/or an inability to complete questionnaires in the Dutch language. The inclusion of patients occurred between September 2002 and November 2007, and the follow-up period lasted until December 2008. All participants gave written consent, and the study protocol was approved by the medical ethics committee of the Slotervaart Hospital/Reade in Amsterdam.
Intervention
In the Netherlands, TF comprises custom-made shoes (type A) and factory-made shoes (type B). Type A TF is hand-made for the individual patient, whereby a variety of technical adaptations can be incorporated into the shoe and where the patient defines the aesthetic requirements, as stated in our previous article (24). Type B TF is ready-made, with extra depth to accommodate foot and toe abnormalities, and includes a custom-made insole.
The clinical procedure at Reade’s arthritis foot clinic is as follows. During initial consultation, the rehabilitation physician decides whether TF is indicated. If indicated, TF is prescribed by the physician in consultation with the patient and the orthopaedic shoe technician during a second consultation. At this point, the choice between type A and type B shoes is made. This choice is based on the patient’s clinical and personal needs, including technical possibilities and aesthetics. For type A footwear, a model of the foot (a last) is made from a cast based on measurements of the patient’s foot, and a transparent plastic shoe is made based on this last. This shoe is then fitted so that alterations can be made to the last, if necessary, before the final shoe is made. Type A footwear is delivered 10 weeks after prescription. Only type A footwear was included in our study, because they are by far the most commonly prescribed TF in our clinic, probably due to the severity of foot problems seen in the phase that patients are referred to the arthritis foot clinic (25).
Procedure
Baseline variables and effect outcome measures were collected during the patient’s visit to the arthritis foot clinic (baseline assessment [T0]). TF was delivered 10 weeks after T0. Patients were asked to complete diaries on TF use and daily activities on 3 consecutive days, both at 14 and 20 weeks after the delivery of the TF. The first follow-up was at 14 weeks (T1a) and the second follow-up at 20 weeks (T1b). The mean of the measurements of T1a and T1b (a total number of 6 scores) was used as T1. This sampling strategy aimed to average out monthly variation in TF-use. All other outcomes were assessed 26 weeks after delivery of the TF (last follow-up [T2]).
Baseline explanatory variables
Demographic features (gender, age) and data on disease variables (disease duration, presence of rheumatoid factor and erosions, and presence of artificial joints) were collected on standardized clinical forms. The patient’s current disease activity was rated according to erythrocyte sedimentation rate (ESR) in mm/h and measured on a self-reported 3-point Likert scale. The patient’s anti-rheumatic medication was monitored during the study. The localization of inflamed joints was recorded. The standardized forms were also used to collect data on foot characteristics (duration of foot complaints, foot deformity), previous and ongoing therapy for foot problems (podiatry, physiotherapy or surgery) and characteristics of the prescribed TF (shaft height [low, half-length, high]).
Therapeutic footwear-use outcomes
Wearing quotient. Regarding TF use, the primary outcome was a “wearing quotient” calculated from data recorded in the patient diaries on 3 consecutive days, both at 14 (T1a) and 20 (T1b) weeks after the delivery of the TF. Thiele’s optimization strategies to assure and enhance the quality of diary data were applied, which included the usage of simple diaries with straightforward layout, patient instruction and training, limitation of the total number of diary days, depending on the amount of daily records, patient coaching and control of data gathering, and control of the accuracy of the self-registrations (26). Using a diary with a fixed 24-h template, patients first reported TF wearing for every hour, and thereafter completed the type of activities (sitting, lying down, walking indoors and outdoors) undertaken while wearing TF. In addition, each patient recorded the number of hours spent sleeping, being out of bed and resting. The maximum wearing duration was calculated as the number of hours during which the patient was out of bed. The actual wearing duration was defined as the number of hours that the TF was actually worn. Subsequently, the wearing quotient was determined as the ratio: actual/maximum wearing duration. For example, a patient who wakes up at 08.00 h, rests between 13.00 h and 14.00 h and going to bed at 23.00 h, has a maximum wearing duration of 14 h. An actual wearing duration of 7 h for that day implies a wearing quotient of 7/14 = 50%.
Wearing, global. Global wearing was assessed with a Likert-scale after 26 weeks. The patients were instructed to give a global rating of the extent to which they actually wore their shoes on a 3-point scale with response options: (i) (almost) continuously, (ii) only on special occasions, and (iii) rarely or never.
Wear-and-tear. Wear-and-tear was assessed with a wear-and-tear report. A rehabilitation physician and an orthopaedic shoe technician independently rated TF wear-and-tear for both left and right shoes on a 100-mm visual analogue scale (VAS), with 0 and 100 indicating “not at all worn out” and “totally worn out”, respectively.
Therapeutic footwear-effects outcomes
All effect outcome measures were collected at baseline and at 26 weeks after delivery of the TF.
Global. The primary outcome measure regarding TF effect was the total score on the validated Dutch version of the Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC). The WOMAC consists of 24 items divided into 3 subscales: pain (5 items), joint stiffness (2 items) and physical functioning (17 items) (27, 28). The WOMAC is reported to be an appropriate instrument for measuring lower body function in patients with RA (29). The WOMAC was used as a global indicator of lower-extremity related pain and activity limitations.
Pain, global. Global pain was assessed with a 100-mm visual VAS, with 0 and 100 indicating “no pain” and “unbearable pain”, respectively.
Pain, activity-related. Activity-related pain was assessed with the pain subscale of the WOMAC. Furthermore, activity-related pain was assessed with a 100-mm VAS, with 0 and 100 indicating “no pain” and “unbearable pain”, respectively, with separate ratings of pain at rest, while standing and walking.
Pain, joint-related. Joint-related pain of the lower extremity was assessed with Likert scales with response options: (1) none, (2) slight, (3) moderate, and (4) severe. We used the mean score of the left and right joint at issue.
Stiffness, joint-related. Joint-related stiffness was assessed with the joint stiffness subscale of the WOMAC.
Activity limitations, global. Global patient-reported activity limitations were assessed with the validated Dutch version of the Health Assessment Questionnaire (HAQ) (30, 31). The HAQ comprises 20 items in 8 categories (dressing, rising, eating, walking, hygiene, reaching, gripping, and usual activities) (31). When aids or adaptations are indicated the category score was not raised, in order to avoid TF-prescription resulting in an increase in this score.
Activity limitations, lower-extremity related. Lower-extremity related activity limitations were assessed with the physical functioning subscale of the WOMAC.
Activity limitations, specific. Specific activity limitations were assessed with the category scores of the HAQ.
With the exception of the wear-and-tear report, which was based on examination of the TF by the rehabilitation physician and shoe technician, all other outcome measures were patient-reported. The patients, rehabilitation physicians and orthopaedic shoe technicians were blinded with respect to each other’s ratings. Except for the HAQ, all scores were standardized (0–100), with “0” indicating no pain, stiffness or limitations.
Statistical analyses
Categorical variables were described with counts (percentages) and all other variables with the median (interquartile range [IQR]) score.
Use. The maximal and actual wearing duration and the wearing quotient were quantified with the mean (standard deviation [SD]) score. Global wearing was expressed in counts (percentages). Wear-and-tear was quantified by calculating the mean (SD) wear-and-tear scores, as rated by both the rehabilitation physician and the orthopaedic shoe technician.
Effects. Differences in outcomes were investigated with the Wilcoxon signed-rank test for all outcomes. With respect to the primary outcome, p < 0.05 (2-tailed) was considered to be statistically significant. With respect to the secondary outcomes we applied the Dubey/Armitage-Parmar modification of the Bonferroni formula that corrects for multiple correlated outcomes (32). We used an online calculator (33) for 23 outcomes with a mean correlation of 0.24, resulting in a p < 0.0046 (2-tailed), considered to be statistically significant at the family-wise alpha level of 0.05.
Use and effect. A linear regression model was performed to assess the association between use (wearing quotient, global rating and wear-and-tear report) and effect (WOMAC total change score). Change in disease activity as a possible confounding variable was assessed by adding the ESR change score as a covariate to the model. All analyses were performed in SPSS (version 19.0).
Results
Participants
A total of 173 patients were screened; 121 patients fulfilled the selection criteria, 114 of whom agreed to participate in the study; 113 patients received TF; and 96 patients (84%) completed the study (Fig. 1). One patient dropped out before TF delivery. Seventeen patients dropped out after TF delivery, 13 of whom dropped out before T1a. At T1 (T1a plus T1b), 92 patients had completed at least 1 diary for 1 day. At T2, wear-and-tear had been rated for 87 patients, while 94 patients had completed the global rating of wearing.
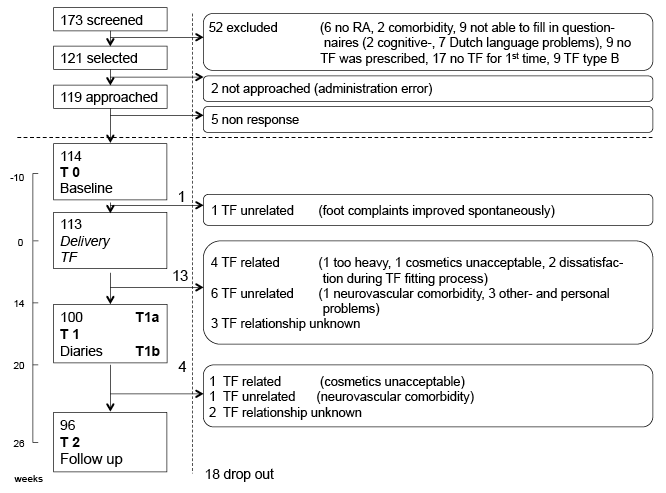
Descriptive data
Most of the patients had advanced RA, and the main indication for TF was forefoot deformity (Table I). At baseline, there were no significant differences between characteristics of patients who dropped out of the study and those who completed the study, except for less joint-related pain in digitus II–V in the patients who dropped out (p < 0.005). On the basis of these data we concluded that there were no relevant differences between the 2 groups. Except for 6 measurements of the ESR, there were no missing data.
|
Table I. Characteristics of the study participants (n = 114) |
|
|
Characteristics |
|
|
Age, years, median (IQR) |
60 (48–67) |
|
Female, n (%) |
95 (83) |
|
Disease duration, years, median (IQR) |
10 (5–16) |
|
Rheumatoid factor positive, n (%) |
86 (75) |
|
Presence of erosions, n (%) |
92 (81) |
|
Patients with joint replacements, n (%) |
20 (18) |
|
Erythrocyte sedimentation rate, mm/h (n = 108), median (IQR) |
16 (9 – 32) |
|
Disease activity, n (%) |
|
|
Low |
56 (49) |
|
Varying |
45 (40) |
|
High |
13 (11) |
|
Localisation of active arthritis, n (%) |
|
|
Feet only or predominantly feet |
33 (29) |
|
Elsewhere |
81 (71) |
|
Duration of foot complaints (years), median (IQR) |
3 (1–7) |
|
Foot deformity, n (%) |
|
|
(Sub)-luxation metatarsal-phalangeal joints |
69 (61) |
|
Hallux valgus |
73 (64) |
|
Claw toes |
57 (50) |
|
Flat foot |
33 (29) |
|
Other (broad forefoot, triple arthrodesis) |
32 (28) |
|
None |
4 (4) |
|
Previous foot therapy, n (%) |
44 (39) |
|
Podiatry |
38 (33) |
|
Physiotherapy |
4 (4) |
|
Surgery |
7 (6) |
|
Other |
7 (6) |
|
Shaft height, n (%) |
|
|
Low |
30 (26) |
|
Half-length |
55 (48) |
|
High |
29 (26) |
|
TF: therapeutic footwear; IQR: interquartile range. |
|
Outcome data
TF-use. TF-use is summarized in Table II. A total of 92 patients completed diaries for a median (IQR) of 6.0 (6.0–6.0) days. Eight had missing data. The mean wearing quotient of 54% (SD 25) indicated that the patients wore their TF a mean of 54% of the time that they were out of bed. Four out of 92 patients had a wearing quotient below 10%, while 10 had a quotient above 90%. The mean actual wearing duration of 7.7 h (SD 3.8) was almost equally divided between the activities of sitting, walking indoors and walking outdoors. Patients lay down for an average of 8 min per day with their shoes on.
|
Table II. Use of therapeutic footwear |
|
|
Activity diaries, h, mean (SD) (n = 92) |
|
|
Wearing quotient (%) |
54 (25) |
|
Potential wearing duration |
14.3 (1.4) |
|
Actual wearing duration |
7.8 (3.8) |
|
Sitting |
2.7 (2.2) |
|
Lying down |
0.1 (0.4) |
|
Walking indoors |
2.3 (1.9) |
|
Walking outdoors |
2.4 (1.3) |
|
Wearing, global, n (%) (n = 94) |
|
|
(Almost) continuously |
65 (69) |
|
Only on special occasions |
13 (14) |
|
Rarely or never |
16 (17) |
|
Wear-and-tear, mean (SD) (n = 87) |
|
|
VAS wear-and-tear |
40 (19) |
|
SD: standard deviation; VAS: visual analogue scale. |
|
The 65 patients who reported “(almost) continuous” TF-use had a mean wearing quotient of 60% (SD 24); the 13 patients who reported TF-use “only on special occasions” had a mean quotient of 38% (SD 22); and the patients who reported TF-use as “seldom or never” had a quotient of 32% (SD 20). Examples of reasons given by patients for not wearing their TF were: “too warm”, “too heavy” and “problems putting the shoes on and taking them off”.
Taking all data into account, and if we assume that the mean wearing quotient was 0% in all patients who dropped out, the mean wearing quotient in all 113 patients having received TF was 38%. A pessimistic estimate of compliance is 57% (95% confidence interval [CI]: 48–66), i.e. 65 (all patients reporting [almost] continuous TF-use) out of 113 (all patients who had TF delivered) TF prescriptions resulting in TF-use. A classification of non-compliance was given when patients reported TF-use as “only on special occasions” or “seldom or never”.
TF-effects. At baseline, most patients reported marked pain during walking and pain in the forefoot (metatarsophalangeal, digitus I and digiti II–V) (Table III). The significantly improved median WOMAC total score was confirmed by significantly improved secondary outcomes, such as the VAS scores for global pain and pain during standing and walking, pain in the forefoot joints and the HAQ total and walking items (Table III).
|
Table III. Effects of therapeutic footwear (n = 96) |
|||
|
Baseline (T0) scorea Median (IQR) |
Follow-up (T2) score Median (IQR) |
p-valueb |
|
|
Global |
|||
|
WOMAC total |
41 (27–57) |
31 (16–45) |
< 0.001 |
|
Pain, global |
|||
|
VAS global |
52 (29–71) |
42 (20–59) |
0.02 |
|
Pain, activity-related |
|||
|
WOMAC pain |
40 (30–60) |
30 (15–45) |
< 0.001 |
|
VAS at rest |
26 (10–51) |
24 (8–49) |
0.18 |
|
VAS during standing |
46 (27–72) |
36 (18–57) |
< 0.001 |
|
VAS during walking |
68 (49–85) |
48 (22–67) |
< 0.001 |
|
Pain, joint-related |
|||
|
Hips |
0 (0–33) |
0 (0–33) |
> 0.30 |
|
Knees |
25 (0–50) |
17 (0–50) |
> 0.30 |
|
Ankles |
33 (0–63) |
17 (0–50) |
0.10 |
|
Midfoot |
50 (17–67) |
33 (0–50) |
0.04 |
|
Metatarsophalangeal |
67 (50–67) |
33 (4–67) |
< 0.001 |
|
Digitus I |
50 (4–67) |
33 (0–50) |
< 0.001 |
|
Digiti II–V |
50 (33–67) |
17 (0–50) |
< 0.001 |
|
Stiffness |
|
||
|
WOMAC stiffness |
50 (25–63) |
38 (25–50) |
< 0.001 |
|
Activity limitations, global |
|||
|
HAQ total |
1.13 (0.75–1.63) |
1 (0.63–1.47) |
0.003 |
|
Activity limitations, lower-extremity related |
|||
|
WOMAC physical functioning |
38 (24–59) |
29 (16–46) |
< 0.001 |
|
Activity limitations, specific |
|||
|
HAQ dressing |
1.00 (0.00–1.00) |
1.00 (0.00–1.00) |
0.19 |
|
HAQ rising |
1.00 (0.00–1.00) |
1.00 (0.00–1.00) |
> 0.30 |
|
HAQ eating |
1.00 (1.00–2.00) |
1.00 (1.00–2.00) |
0.24 |
|
HAQ walking |
1.00 (0.00–2.00) |
1.00 (0.00–1.00) |
< 0.001 |
|
HAQ hygiene |
1.00 (0.00–1.00) |
1.00 (0.00–1.00) |
> 0.30 |
|
HAQ reaching |
2.00 (1.00–3.00) |
2.00 (1.00–3.00) |
> 0.30 |
|
HAQ gripping |
1.00 (1.00–2.00) |
1.00 (0.00–1.00) |
0.22 |
|
HAQ usual activities |
1.00 (1.00–2.00) |
1.00 (1.00–2.00) |
> 0.30 |
|
aExcept for the HAQ, all scores were standardized (0–100), with “0” indicating no pain, stiffness or limitations. bWith respect to the primary outcome (WOMAC total score) p < 0.05 (Wilcoxon signed-ranked test) was considered to be statistically significant, whereas with respect to the secondary outcomes, and after applying Bonferroni correction, p < 0.0046 was considered to be statistically significant. Significant p-values are marked in bold. IQR: interquartile range; HAQ: Health Assessment Questionnaire; VAS: visual analogue scale; WOMAC: Western Ontario and McMasters Osteoarthritis Index; T0: at baseline; T2: follow-up after 26 weeks. |
|||
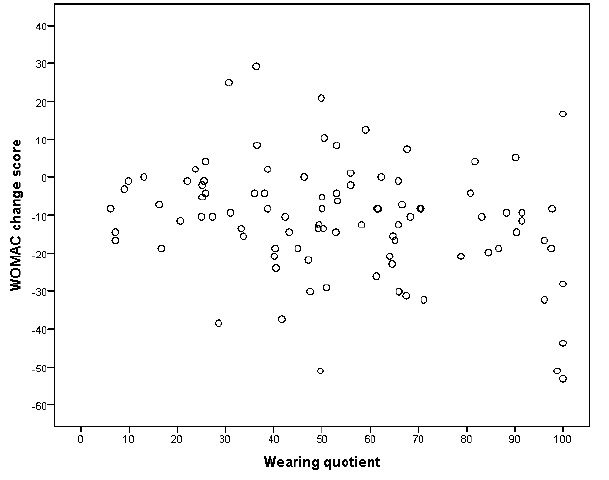
Use and effect. The wearing coefficient was weakly (R2 0.09, p = 0.02) associated with the WOMAC total change score in a model that included ESR (Table IV). These results suggest that use of TF makes an impact on WOMAC total score. For example, a wearing coefficient of 50% rather than 10% implies that the WOMAC score improves with (50–10) × 0.16 = 6.5 points. There was, however, a substantial individual heterogeneity in the mean effect (Fig. 2). Median ESR at T0 and T2 were 17 (9–31) and 19 (8–32), respectively. A decrease in disease activity was documented by the disease activity self-reports in 22 (22%) patients. Furthermore, disease activity remained stable and increased in 49 (48%) and 31 (30%) patients, respectively. Changes in anti-rheumatic medication were documented in 34 (33%) patients. The changes in self-reported disease activity and anti-rheumatic medication were not associated with the WOMAC total change score (data not shown). The other measures of TF-use in the model (global rating of use and wear-and-tear report) showed even weaker associations (not significant; data not shown).
|
Table IV. Regression analysis of effect with use and disease activity |
|||
|
Independent variables |
WOMAC total change scorea |
||
|
B (SE) |
Beta |
p-value |
|
|
Wearing quotient (%) |
–0.16 (0.06) |
–2.57 |
0.02 |
|
Erythrocyte sedimentation rate change score |
0.16 (0.15) |
1.16 |
0.28 |
|
(mm/h) |
R2 = 0.09 (p < 0.02) |
||
|
*Result of the regression of global effect (WOMAC total change score) with use (wearing quotient) and disease activity (erythrocyte sedimentation rate change score). B: unstandardized regression coefficient; Beta: standardized regression coefficient; SE: standard error; WOMAC: Western Ontario and McMasters Osteoarthritis Index. |
|||
Discussion
The results of this study show that patients use prescribed custom-made TF (i.e. type A) with moderate intensity, i.e. slightly more than 50% of the time that they are out of bed. The cohort showed significant improvements in WOMAC total score, and, more specifically, activity-related pain, forefoot joint-related pain, lower-extremity related activity limitations and walking. All of these outcomes could be assumed to show improvement when TF are effective. Somewhat disappointingly, there was only a weak (but significant) association between global use and effect.
These results show that duly designed activity diaries, compared with global wearing rating scales, can provide detailed and relevant information about the use and the purposes of TF. According to our data, patients spent 4.7 h on walking activities during their almost 8 h of (mean) actual wearing duration. This finding is difficult to discuss further, because, to our knowledge, studies using activity diaries to address the activities of adult patients with RA with foot problems (34) or literature addressing the use of shoes in healthy subjects, is lacking. Comparing our results with studies that measure activity using other instruments, our findings may indicate that our patients were rather active in walking during the day. Semanik et al. (35) used the Yale Physical Activity Survey in 185 older women with RA and found a mean physical activity of 3.3 h per day. Piva et al. (36) used activity monitors and found a daily mean number of steps of 7,151 (SD 2,637) in 47 women with RA. Bus et al. (37) introduced an adherence monitor and, in combination with a step activity monitor, found that diabetic patients wore their prescribed TF for a mean of 8.5 h per day and a daily mean number of steps of 8,284 ± 4,794. The self-reported use of TF with the global rating scale is broadly in accordance with the results of other studies (20, 21, 38).
This study provides more detail about the potential effects of TF that, by and large, agree with the results of other studies (10, 15). However, our study is larger and all prescribed TF were custom-made. Furthermore, our study provides a more detailed picture of the effects of TF use, by assessing a substantial number of secondary outcomes.
The weak association between TF-use and effect could simply be due to the availability of TF for specific tasks related to walking being more important for the effect than overall intensity of use. This explanation is supported by the finding that patients reported walking 60% of the time during TF-wearing periods. Patients with RA, just like people without disease, do not need shoes during grooming activities in the morning or while watching television at night. Furthermore, although activity-related pain decreased, it could also be hypothesized that greater TF-use resulted in an increase in activity that could partially offset the beneficial effect. These hypotheses should be addressed in future research.
Limitations of this study include its uncontrolled design, the lack of proper disease activity measurement and other limitations in outcome measurement, and the limited generalizability of the results. At the start of the study, routine standardized measurements of disease activity were uncommon and there was no budget for such measurements within the protocol. The measures of use (diary and wear-and-tear report) are new and have not yet been used elsewhere, let alone validated or compared with monitoring technology. The WOMAC and other measures are validated for RA, but they are not specific for foot pathology, compared, for example, with the Foot Function Index (39). The generalizability of our results is limited, as this study took place in only one secondary-care institution, specialized in rheumatology and rehabilitation. As such, future research is needed to ascertain whether our results can be generalized to non-specialized institutions.
The outcomes of this study emphasize the significance of prescribing TF for patients with RA and foot problems. The results can also be seen as a stepping stone to future controlled studies to unravel the complex, ill-understood and neglected area of TF-use (15) and TF-effects on foot pain and activity limitations. Such a study should also try to address determinants for TF-use and TF-effects, including patient and shoe characteristics and psychological and environmental factors, according to the International Classification of Functioning, Disability and Health model (40), in comparison with waiting-list controls. Furthermore, future studies should investigate shoe characteristics in detail and place a greater emphasis on foot-specific effects.
In conclusion, the majority of patients with RA who are prescribed their first pair of custom-made TF do use them. Although the association between the self-reported use and effect of TF is weak, TF-prescription is associated with relevant effects on forefoot pain and activity limitations. Therefore, prescription of TF should be considered as a realistic and effective treatment option in RA-associated foot problems.
Acknowledgement
The authors thank Jose de Vries for collecting the data.
References
