Otto L. T. Lam, PhD1, Anne S. McMillan, PhD1, Leonard S. W. Li, MBBS2 and Colman McGrath, PhD1*
From the 1Faculty of Dentistry, The University of Hong Kong, Hong Kong SAR, China and 2Department of Rehabilitation Medicine, Tung Wah Hospital, Hong Kong
OBJECTIVES: To explore the influence of socio-demographic and clinical oral health factors on oral health-related quality of life (OHRQoL) in patients after stroke, and to monitor OHRQoL outcomes following the provision of an in-hospital oral health intervention programme.
DESIGN: OHRQoL was measured before and after randomization and provision of oral health promotion interventions in a prospective clinical trial.
SUBJECTS: Eighty-one patients admitted to a stroke rehabilitation ward.
METHODS: OHRQoL was assessed using the Oral Health Impact Profile-14 (OHIP-14) and Oral Health Transition Scale upon admission and 3 weeks later following provision of an oral health promotion programme. Potential factors were examined for their association with OHRQoL outcomes.
RESULTS: Lack of a regular daily brushing habit was significantly associated with 6 of 8 transition scale items (p < 0.01) at baseline, while significant improvements in OHRQoL were observed over the course of the clinical trial for all patients as a whole (p < 0.05).
CONCLUSION: OHRQoL is compromised following stroke and may be influenced by the lack of a regular daily brushing habit during hospitalization. The early re-establishment of an oral hygiene protocol is a priority in stroke rehabilitation wards in order to improve clinical oral health and OHRQoL.
Key words: oral health; stroke; quality of life.
J Rehabil Med 2014; 46: 520–526
Correspondence address: Colman McGrath, Periodontology and Public Health, Faculty of Dentistry, The University of Hong Kong, Pokfulam Road, Hong Kong. E-mail: mcgrathc@hkucc.hku.hk
Accepted Jan 8, 2014; Epub ahead of print XXX ?, 2014
Introduction
The long-term effects that stroke may have on physical, psychological and social functions are well documented (1). Traditionally, rehabilitation outcomes have been based on objective indicators of physical function, such as the Barthel Index (BI) and Rankin scale. Such measures, however, underestimate the full impact of stroke as they are not patient-centred and ignore psychosocial outcomes. Thus, in recent years, the use of general health-related quality of life (HRQoL) measures have been widely advocated and investigated amongst patients following stroke (2).
Few studies, however, have used oral-specific measures to assess oral health-related quality of life (OHRQoL) following stroke. In a longitudinal observational study conducted amongst patients hospitalized in a rehabilitation ward following stroke, a majority of the patients during the acute phase felt that their speech and tooth-brushing abilities, as well as their appearance and overall oral health were worse since the stroke. While mean General Oral Health Assessment Index (GOHAI) (3) scores were markedly improved by the time of hospital discharge and at 6 months following stroke, they were still significantly lower than scores documented in a healthy control group. This suggests that stroke may have a prolonged impact on OHRQoL, which persists until at least 6 months following hospital discharge (4). Hunter et al. (5) additionally reported adverse impacts on OHRQoL in patients 1 year after hospital discharge.
Previous studies have reported a diverse set of factors that may be associated with OHRQoL. These have included objective indicators of oral health status (e.g. number of posterior occluding pairs, carious teeth, prosthetic status), as well as socio-demographic factors (gender, age, education, frequency of dental attendance) (6–8). As the majority of these studies have been conducted amongst community-dwelling or institutionalized subjects, however, the influence of these predictors may not be equally applicable to patients following stroke. As both OHRQoL and clinical oral health are adversely affected following stroke (9), the identification of such predictors may be crucial in informing resource allocation, and the planning and implementation of oral healthcare intervention programmes in stroke rehabilitative wards.
The aims of this study were: (i) to explore socio-demographic and clinical oral health factors that may influence OHRQoL in patients following stroke; and (ii) to monitor OHRQoL outcomes following the provision of an in-hospital oral health intervention programme.
Methods
Patients with stroke were recruited whilst receiving rehabilitative care at Tung Wah Hospital in Hong Kong. Patients had previously received acute care at Queen Mary Hospital, Hong Kong, and were stabilized prior to their transfer to rehabilitative care. Inclusion criteria were: a BI score < 70 (10), age 50 years and over, and transfer to the rehabilitation ward from an acute care hospital within 7 days. Patients were excluded if they had severe cognitive impairment (Mini Mental State Examination (MMSE) score ≤ 9) (11) or nasogastric tube insertion/placement, or if they were unable to follow a 1-step command, or were edentulous. All patients were randomly assigned using a random number table and a block randomization method to receive either: (i) oral hygiene instruction (OHI) only, (ii) OHI and 0.2% chlorhexidine (CHX) mouth-rinse, or (iii) OHI, 0.2% CHX mouth-rinse, and assisted brushing over a 3-week period in the rehabilitation ward, as described previously (12, 13). Random assignment of patients was performed by a research assistant using a colour-coding scheme. The principal investigator was blinded to treatment allocation. OHI was provided by the principal investigator (a registered dentist), while mouth-rinse administration and assisted brushing were performed by nursing-care aides, trained by certified dental hygienists. Patients were monitored for tooth-brushing difficulties by nursing-care aides, who provided hand-over-hand guidance if patients were unable to clean tooth surfaces (buccal, occlusal, lingual) in a systematic manner. Informed consent was obtained directly from patients, and family members, and/or primary caregivers were notified of the study. Ethics approval was obtained from the Institutional Review Board of the University of Hong Kong.
HRQoL and OHRQoL were measured at baseline (prior to intervention initiation), and following completion of the clinical trial at 3 weeks. All assessments were performed at the bedside in the rehabilitation ward. Objective clinical measures were collected as described previously (12, 13).
Questionnaires were conducted by a trained interviewer, and consisted of previously validated Chinese versions of the Short Form Health Survey 12 (SF-12) (14), Oral Health Impact Profile 14 (OHIP-14) (15), and Oral Health Transition Scale (4, 16).
HRQoL was measured with the SF-12 (17), which encompasses 12 items belonging to 8 conceptual health domains: general health (GH), physical functioning (PF), bodily pain (BP), role-physical (RP), mental health (MH), vitality (VT), social functioning (SF), and role-emotional (RE). Pre-specified weights are assigned to each individual item, allowing the derivation of physical component summary (PCS), and mental component summary (MCS) scores, with higher scores indicating better HRQoL.
OHRQoL was assessed with OHIP-14 (18) and Oral Health Transition Scale (16). The OHIP-14 is a 14-item instrument that captures the 7 dimensions of Locker’s theoretical model of oral health: handicap, social disability, psychological disability, physical disability, psychological discomfort, physical pain, and functional limitation. Responses are coded using a 5-point Likert scale (0 = never, 1 = hardly ever, 2 = occasionally, 3 = fairly often, 4 = very often). Overall OHIP scores may be computed by summing scores to the 14 items, in which case higher scores will reflect poorer OHRQoL (5, 19).
The oral health transition scale (16) comprises 8 questions that gauge the patient’s perceptions regarding their general appearance, general oral health, general comfort of their mouth, and ability to chew hard and soft foods, speak, swallow food, and use a toothbrush, before and after the stroke (4, 16). Responses are coded using a 5-point Likert scale (1 = much better, 2 = somewhat better, 3 = no difference, 4 = somewhat worse, 5 = much worse).
Objective clinical oral health indicators (caries status, denture status, number of teeth, number of posterior occluding tooth pairs, unrestored anterior spaces, Community Periodontal Index [CPI] scores, dental plaque and gingival bleeding scores), socio-demographic (age, gender, educational attainment, receipt of financial assistance, employment status) and behavioural variables (dental attendance, tooth-brushing habits, smoking status), stroke-related outcomes (functional disability, previous stroke, swallowing impairment [Royal Brisbane Hospital Outcome Measure for Swallowing] (20), dominant side affected), were documented as described previously (12). These factors, as well as general health-related quality of life, were examined for their association with OHIP-14 scores and Oral Health Transition Scale Item scores.
The study was registered with the Hong Kong Clinical Trial Register (Study identifier: HKCTR-1159) and the US National Institutes of Health (Study identifier: NCT01265043).
Statistical analysis
A complete-case analysis was performed in accordance with the Consolidated Standards of Reporting Trials (www.consort-statement.org) and Altman (21). Within-group comparisons of OHIP-14 and SF-12 scores at baseline and at the end of the clinical trial were made using the Wilcoxon signed-rank test. Change scores were calculated by subtracting post-treatment scores from pre-treatment scores. Comparisons of change scores between groups were made via Kruskal-Wallis one-way analysis of variance (ANOVA), and the Mann-Whitney U test for individual pairwise comparisons. Within-group comparisons of Oral Health Transition Scale Items were made via the McNemar test. χ2 tests were used to examine differences between groups with regards to Oral Health Transition Scale items (worse, not worse).
Univariate analyses (unadjusted) of possible explanatory factors for OHIP-14 scores at baseline and review were performed via a negative binomial regression model, as residuals were not normal following data transformation, and to account for over-dispersion associated with Poisson regression. Variables with a p-value of 0.100 or below were then subjected to negative binomial regression analyses for determination of significant factors (adjusted model). Significant variables were further analysed in an “interaction model”. No pairwise interaction terms were found to be significant in the interaction model. Likewise, univariate analyses of possible explanatory factors for Oral Health Transition Scale items were subjected to logistic regression. Explanatory models were derived by entering variables with p-values of 0.100 or below into multiple logistic regression analyses. Variables significant in multiple logistic regression analyses were examined in an interaction model. No pairwise interaction terms were found to be significant in the interaction model. In all cases variables were examined for multicollinearity (variance inflation factor (VIF) > 10) (22) prior to multivariate analyses.
The statistical level chosen was 0.05. All tests were performed using the statistics software package PASW 18.0 for Windows (SPSS Inc., Chicago, USA).
Results
A total of 102 patients were recruited into the study, with 81 patients being reviewed at the conclusion of the clinical trial (6, 12). The majority (17 subjects) not reviewed at the conclusion of the clinical trial were lost due to early recovery and discharge from the hospital. One patient self-discharged himself from hospital care, while two additional patients were transferred to another hospital for assessment during the study period. One patient was withdrawn from the study due to non-compliance with the mouth-rinse. Demographic variables (gender, age, education level, working status, receipt of social assistance, dental attendance, brushing habits during hospital stay) were not significantly different between patient treatment groups. Time since stroke onset was 13.0 days (standard deviation [SD] 6.8), at the time of recruitment. Sixty-eight patients had had an ischaemic stroke, while 13 patients were diagnosed as having had a haemorrhagic stroke. A total of 16 patients had previously been diagnosed with a prior stroke. The median BI score at baseline was 53.0 (interquartile range 29.0–67.0). Stroke type and BI score were not significantly different between treatment groups.
Significantly higher median SF-12 scores were observed across groups in both the PCS (p < 0.001) and MCS (p < 0.001) component scales on review (median SF-12PCS = 45.7 [34.6–55.4]; median SF-12MCS = 32.9 [25.6–44.0]) compared with baseline (median SF-12PCS = 28.2 [24.8–31.6]; median SF-12MCS = 30.7 [27.1–35.4]), while SF-12 change scores were not significantly different between treatment groups.
No significant differences in total median OHIP-14 scores and median domain scores were present between groups at baseline. Significantly lower OHIP-14 scores were observed across groups on review (median score = 4.0 [1.0–9.0]) compared with baseline (median score = 7.0 [2.0–14.0]; p = 0.014). Changes in OHIP-14 scores between groups, did not reach statistical significance.
In a negative binomial regression model for OHIP-14 scores at baseline, lack of tertiary education (rate ratio: 4.34, p = 0.003, 95% confidence interval (CI): 1.65–11.37), SF-12MCS (rate ratio: 0.98, p = 0.030, 95% CI: 0.96–1.00), and SF-12PCS (rate ratio: 0.94, p = 0.004, 95% CI: 0.90–0.98) were significantly associated with OHRQoL (Table I). Baseline OHIP-14 scores (rate ratio: 1.04, p = 0.004, 95% CI: 1.01–1.07), and SF-12MCS (rate ratio: 0.96, p < 0.001, 95% CI: 0.94–0.98) were associated with scores at 3 weeks.
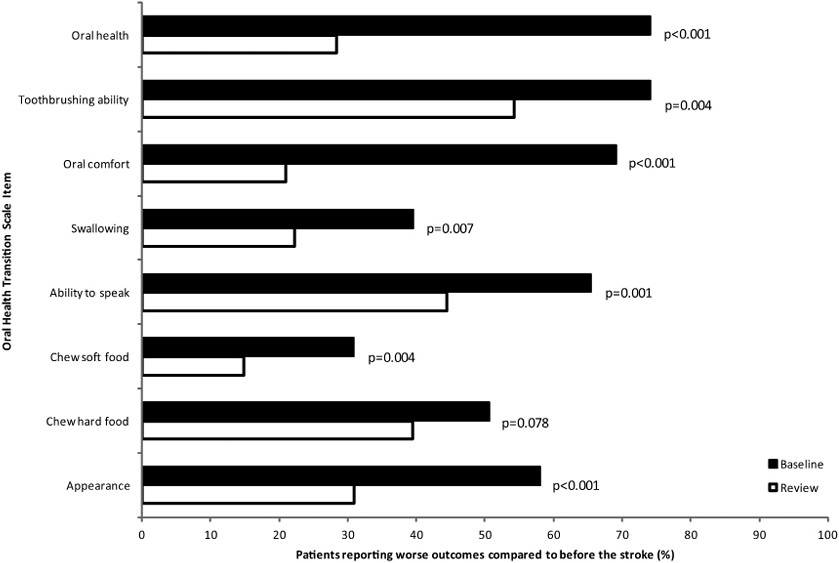
At baseline, approximately three-quarters of patients reported that their tooth-brushing ability and oral health were worse following their stroke, while over half of the patients felt that their appearance, ability to chew hard food, ability to speak, and oral comfort were worse (Fig. 1). No differences were observed between groups at baseline, except for chewing soft (p = 0.043) and hard food (p = 0.024), for which a larger proportion of patients in the OHI group felt that these abilities were worse off following their stroke. Taking all patients into consideration, a significantly smaller proportion of patients felt that their appearance (p < 0.001), oral comfort (p < 0.001), oral health (< 0.001), and ability to chew soft food (p = 0.004), speak (p = 0.001), swallow (p = 0.007), and brush their teeth (p = 0.004), were worse at the end of the clinical trial compared with baseline. Although a trend towards a smaller percentage of patients reporting a decreased ability to chew hard food was observed, this did not achieve statistical significance. No differences between groups in changes in oral health transition scale items over the interventional period were observed.
|
Table I. Clinical and socio-demographic factors associated with Oral Health Impact Profile-14 (OHIP-14) scores in patients with stroke (negative binomial regression model) |
|||||||||||||||
|
Factor |
Baseline |
Review |
|||||||||||||
|
Unadjusted |
Adjusteda |
|
Unadjusted |
Adjustedb |
|||||||||||
|
Rate ratio |
p-value |
95% CI |
Rate ratio |
p-value |
95% CI |
Rate ratio |
p-value |
95% CI |
Rate ratio |
p-value |
95% CI |
||||
|
Age |
1.01 |
0.268 |
0.99–1.03 |
1.00 |
0.749 |
0.97–1.02 |
|||||||||
|
Gender |
0.93 |
0.779 |
0.57–1.53 |
0.841 |
0.534 |
0.49–1.45 |
|||||||||
|
Plaque Index |
1.33 |
0.294 |
0.78–2.25 |
1.06 |
0.798 |
0.70–1.61 |
|||||||||
|
Gingival Bleeding Index |
1.00 |
0.695 |
0.98–1.01 |
1.00 |
0.836 |
0.99–1.02 |
|||||||||
|
Barthel Index |
1.00 |
0.446 |
0.99–1.01 |
1.00 |
0.941 |
0.99–1.02 |
|||||||||
|
Swallowing disability |
1.53 |
0.091 |
0.94–2.50 |
1.33 |
0.237 |
0.83, 2.14 |
0.91 |
0.840 |
0.38–2.22 |
||||||
|
Dominant side affected |
1.00 |
0.993 |
0.62–1.62 |
1.14 |
0.638 |
0.67–1.93 |
|||||||||
|
DMFT |
1.02 |
0.134 |
0.99–1.06 |
1.02 |
0.190 |
0.99–1.06 |
|||||||||
|
Denture |
1.00 |
0.987 |
0.60–1.69 |
0.86 |
0.602 |
0.49–1.52 |
|||||||||
|
Unrestored anterior space |
1.03 |
0.918 |
0.56–1.91 |
0.85 |
0.638 |
0.43–1.67 |
|||||||||
|
Number of teeth |
0.22 |
0.959 |
0.90–1.03 |
0.97 |
0.456 |
0.91–1.04 |
|||||||||
|
Number of posterior |
|||||||||||||||
|
occluding pairs |
0.93 |
0.104 |
0.85–1.02 |
0.91 |
0.190 |
0.86–1.03 |
|||||||||
|
CPI |
0.74 |
0.239 |
0.45–1.22 |
0.72 |
0.211 |
0.42–1.21 |
|||||||||
|
Smoking |
0.94 |
0.849 |
0.59–1.75 |
1.22 |
0.572 |
0.62–2.39 |
|||||||||
|
Last dental visit |
1.19 |
0.567 |
0.66–2.13 |
0.98 |
0.962 |
0.52–1.88 |
|||||||||
|
Receiving financial assistance |
0.61 |
0.128 |
0.32–1.15 |
0.80 |
0.540 |
0.40–1.62 |
|||||||||
|
Lack of tertiary education |
3.89 |
0.007 |
1.46–10.36 |
4.34 |
0.003 |
1.65, 11.37 |
1.92 |
0.219 |
0.68–5.43 |
||||||
|
Lack of regular brushing habit |
1.38 |
0.221 |
0.83–2.29 |
0.57 |
0.248 |
0.22–1.48 |
|||||||||
|
Employment status |
0.90 |
0.707 |
0.53–1.55 |
1.02 |
0.937 |
0.57–1.85 |
|||||||||
|
Previous stroke |
1.61 |
0.109 |
0.90–2.89 |
1.76 |
0.087 |
0.92–3.35 |
0.93 |
0.798 |
0.56, 1.57 |
||||||
|
Number of co-morbidities |
1.11 |
0.324 |
0.90–1.38 |
1.17 |
0.200 |
0.92–1.45 |
|||||||||
|
Treatment group |
|||||||||||||||
|
CHX + assisted brushing vs OHI |
– |
– |
– |
0.788 |
0.455 |
0.42–1.47 |
0.68 |
0.133 |
0.42, 1.12 |
||||||
|
CHX vs OHI |
– |
– |
– |
0.544 |
0.069 |
0.28–1.05 |
0.72 |
0.220 |
0.43, 1.22 |
||||||
|
SF-12 MCS |
0.975 |
0.010 |
0.96–0.99 |
0.98 |
0.030 |
0.96, 1.00 |
0.948 |
< 0.001 |
0.93–0.97 |
0.96 |
< 0.001 |
0.94, 0.98 |
|||
|
SF-12 PCS |
0.949 |
0.034 |
0.91–1.00 |
0.94 |
0.004 |
0.90, 0.98 |
0.952 |
0.011 |
0.92–0.99 |
0.97 |
0.120 |
0.94, 1.01 |
|||
|
OHIP-14 score at baseline |
– |
– |
– |
1.06 |
< 0.001 |
1.03–1.09 |
1.04 |
0.004 |
1.01, 1.07 |
||||||
|
aOmnibus Test: p < 0.001. Goodness of fit: deviance/df = 1.242, Pearson χ2/df = 0.921. bOmnibus Test: p < 0.001. Goodness of fit: deviance/df = 1.298, Pearson χ2/df = 1.068. DMFT: decayed, missing, and filled teeth; CPI: community periodontal index; 95% CI: 95% confidence interval; CHX: chlorhexidine; OHI: oral hygiene instruction; SF-12: Short Form Health Survey 12; PCS: physical component summary; MCS: mental component summary: df: degrees of freedom. |
|||||||||||||||
In multiple logistic regression models (adjusted for independent variables with p ≤ 0.100) for oral health transition scale items, patients who did not have regular daily brushing habits were significantly more likely to report worse appearance (OR = 5.75, p = 0.002), ability to chew hard (OR = 11.35, p < 0.001) or soft (OR = 19.83, p = 0.005) food, oral comfort (OR = 5.54, p = 0.007), ability to brush (OR = 3.83, p = 0.035), and oral health (OR = 4.97, p = 0.004) at baseline compared with before their stroke (Table II). Worse oral comfort was additionally predicted by denture wear during hospital stay (OR = 6.06, p = 0.020), swallowing disability (OR = 5.75, p = 0.014), and a previous history of stroke (OR = 5.84, p = 0.049). Patients in whom the stroke affected their dominant side were 5 times more likely to report a worsened physical ability to brush their teeth at baseline (OR = 5.19, p = 0.017). Generic measures of HRQoL were also significantly associated with OHRQoL at baseline, with worse ability to chew hard food being predicted by lower SF-12MCS scores (OR = 0.94, p = 0.021), and worse ability to speak being predicted by lower SF-12PCS scores (OR = 0.91, p = 0.042).
Likewise, at 3 weeks, lower SF-12MCS scores predicted worse ability to chew hard food (OR = 0.92, p = 0.001) and swallow food (OR = 0.87, p = 0.002), as well as reduced oral comfort (OR = 0.95, p = 0.034), while lower SF-12PCS scores predicted worse physical ability to brush (OR = 0.90, p = 0.015). Patients with a prior stroke were 8 times more likely to report a worsened ability to swallow food at the review assessment (OR = 8.25, p = 0.035). In addition, poorer periodontal status, as indicated by objective clinical measures (e.g. GBI, CPI), predicted a response of “no difference” as opposed to “worse” for transition items investigating appearance (baseline), chewing of hard food (baseline), and ability to swallow (review). Patients reporting worse outcomes at baseline were also more likely to report worse outcomes on review for 6 out of 8 oral health transition scale items.
|
Table II. Factors associated with Oral Health Transition Scale items at baseline and review, in a multiple logistic regression modela |
||
|
Item |
Baseline |
Review |
|
Appearance is worse |
Lack of regular brushing habit (OR = 5.75; p = 0.002; 95% CI [1.91–17.34]) GBI (OR = 0.97; p-value = 0.043; 95% CI [0.94, 1.00]) (Nagelkerke R2 = 0.22) |
Transition item worse at baseline (OR = 15.88; p < 0.001; 95% CI [3.37–74.84]) (Nagelkerke R2 = 0.32) |
|
Ability to chew hard food is worse |
Lack of regular brushing habit (OR = 11.35; p < 0.001; 95% CI [2.91–44.25]) SF-12 MCS (OR = 0.94; p = 0.021; 95% CI [0.90–0.99]) CPI (≥ 6mm) (OR = 0.27; p = 0.029; 95% CI [0.08–0.87]) (Nagelkerke R2 = 0.44) |
SF-12 MCS (OR = 0.92; p = 0.001; 95% CI [0.87–0.97]) Transition item worse at baseline (OR = 16.76; p < 0.001; 95% CI [4.37–64.31]) (Nagelkerke R2 = 0.49) |
|
Ability to chew soft food is worse |
Lack of regular brushing habit (OR = 19.83; p = 0.005; 95% CI [2.50, 157.50]) (Nagelkerke R2 = 0.27) |
Transition item worse at baseline (OR = 9.75; p = 0.002; 95% CI [2.35–40.41]) (Nagelkerke R2 = 0.24) |
|
Ability to speak is worse |
SF-12 PCS (OR = 0.91; p = 0.042; 95% CI [0.84, 1.00]) (Nagelkerke R2 = 0.08) |
Transition item worse at baseline (OR = 10.85; p < 0.001; 95% CI [2.86–41.19]) (Nagelkerke R2 = 0.27) |
|
Ability to swallow food is worse |
Number of teeth (OR = 0.73; p = 0.002; 95% CI [0.59–0.89]) Smoking (OR = 0.10; p = 0.018; 95% CI [0.01–0.67]) Employment status (OR = 0.194; p = 0.020; 95% CI [0.05–0.78]) (Nagelkerke R2 = 0.36) |
CPI (≥ 6mm) (OR = 0.05; p = 0.015; 95% CI [0.01–0.56]) Previous stroke (OR = 8.25; p = 0.035; 95% CI [1.16–58.83]) SF-12 MCS (OR = 0.87; p = 0.002; 95% CI [0.79–0.95]) Transition item worse at baseline (OR = 8.32; p = 0.015; 95% CI [1.52–45.57]) (Nagelkerke R2 = 0.59) |
|
Comfort of mouth is worse |
Lack of regular brushing habit (OR = 5.54; p = 0.007; 95% CI [1.58–19.40]) Swallowing disability (OR = 5.75; p = 0.014; 95% CI [1.43–23.19]) Denture (OR = 6.06; p = 0.020; 95% CI [1.33–27.59]) Previous stroke (OR = 5.84; p = 0.049; 95% CI [1.01–33.81]) (Nagelkerke R2 = 0.34) |
SF-12 MCS (OR = 0.95; p = 0.034; 95% CI [0.90–1.00]) (Nagelkerke R2 = 0.14) |
|
Physical ability to brush is worse |
Lack of regular brushing habit (OR = 3.83; p = 0.035; 95% CI [1.10–13.32]) Dominant side affected (OR = 5.19; p = 0.017; 95% CI [1.34–20.15]) DMFT (OR = 1.10; p = 0.046; 95% CI [1.00–1.20]) (Nagelkerke R2 = 0.35) |
SF-12 PCS (OR = 0.90; p = 0.015; 95% CI [0.82–0.98]) Transition item worse at baseline (OR = 5.27; p = 0.008; 95% CI [1.55–17.92]) (Nagelkerke R2 = 0.27) |
|
Oral health is worse |
Lack of regular brushing habit (OR = 4.97; p = 0.004; 95% CI [1.66–14.91]) (Nagelkerke R2 = 0.15) |
|
|
aAdjusted for clinical and socio-demographic factors with p < 0.100 in unadjusted models. OR: odds ratio; 95% CI: 95% confidence interval; DMFT: decayed, missing, and filled teeth; MCS: mental component summary; GBI: Gingival Bleeding Index. |
||
Discussion
Lack of tertiary education and the absence of a regular daily brushing habit, were found to be significantly associated with worse OHRQoL at baseline. Overall improvements were observed in OHRQoL amongst patients with stroke, regardless of which oral health promotion intervention was provided during the hospitalization period.
Oral health outcomes have traditionally been based on objective indicators such as CPI and decayed, missing, and filled teeth (DMFT) scores, which provide valuable information regarding the presence and extent of existing or past oral pathological processes. Indeed, the provision of an oral health promotion programme significantly improved clinical indices of oral hygiene in the present patient group (12). The exclusive use of such measures, however, fails to provide a holistic assessment of the impact of oral disease, as they ignore effects on the functioning and subjective well-being of the person as a whole (23).
OHRQoL was impaired at baseline, and scores were markedly poorer than those reported from healthy community-dwelling elderly subjects (24). Significant improvements were seen in the total OHIP-14 scores, as well as scores in the functional limitation, psychological discomfort, and social disability subscales by the end of the clinical trial. No differences were observed between groups, indicating that the different OHP interventions were equivocal in terms of the patient’s perceived effectiveness.
In addition to before and after comparisons of group summary means, and raw change scores for measuring change in life quality, global transition judgments have been advocated, as this method takes into account the patients’ subjective view as to how much change is considered meaningful (25). The oral health transition scale provided an additional insight into OHRQoL in the present study, and revealed that approximately three-quarters of patients perceived that their tooth-brushing ability and oral health were worse after, compared with before, their stroke. Over half of the patients felt that their appearance, ability to chew hard food, ability to speak, and oral comfort were worse. These results were consistent with the finding of poor OHRQoL as measured by the OHIP-14. Complaints were probably related to motor impairments affecting the limbs (26), as well as accompanying somatosensory deficits (27), oral stereognosis, weakened orofacial musculature, oral apraxia, and hyposalivation (28–33). By the end of the clinical trial, improvements were seen in all transition scale items except for “ability to chew hard food”. This may have been explained by the fact that the food provided by the hospital was of a fluid or semi-solid consistency.
Both physical and mental components of the SF-12 measure were significantly associated with OHIP-14 scores at baseline and review, confirming prior reports of considerable correlation between OHRQoL and general health-related quality of life (GHRQoL) (34). Clinical oral health indicators, such as plaque and gingival bleeding scores, were not found to have significantly influenced OHIP-14 scores, supporting previous suggestions that such latent diseases are unlikely to affect subjective perceptions of oral health status (35).
Having attained tertiary-level education was associated with lower OHIP-14 scores, confirming a previous report of the positive effects that higher levels of educational attainment may have on OHRQoL (6).
Patients without a regular daily brushing habit since hospitalization were more likely to report worse outcomes for a majority of oral health transition scale items (appearance, ability to chew hard or soft food, oral comfort, ability to brush, overall oral health). The lack of a regular daily brushing habit probably reflected direct effects of the stroke on manual dexterity and brushing ability, as well as the absence of oral hygiene materials within the rehabilitation ward at baseline (12). As the lack of a daily oral hygiene regimen was associated with worsened self-perceived oral health, this also suggests that the re-implementation of such a self-care regimen (e.g. via the provision of oral hygiene materials and tooth-brushing assistance) may be fundamental in improving subjective oral health status amongst patients in the early stages of recovery following stroke. Indeed, subjective measures of oral health were significantly improved following the institution of an oral health promotion programme in the present study. On review, lack of a regular brushing habit was no longer a significant factor, while patients reporting worsened outcomes were also more likely to have done so at baseline, reflecting a regression towards the mean for a majority of the oral health transition scale items. SF-12 scores, however, were not consistently associated with oral health transition scale items, emphasizing the inadequacy of GHRQoL instruments and need for oral specific measures when examining OHRQoL (36).
The lack of significance between OHRQoL and other objective oral health indicators, such as denture wearing, the number of remaining natural teeth, posterior occluding pairs, and unrestored anterior tooth spaces, contrasts from what has been reported in healthy elderly subject groups (37, 38). This may have been due to the possibility that the effects of these clinical indicators were less readily apparent amongst patients in the hospital environment. For example, a decreased number of remaining natural teeth and the presence of unrestored anterior tooth spaces must be considered in the context of the rehabilitation ward, where meals consisted of soft foods only and social visits were few, respectively. Thus, the full impact on OHRQoL may not have been realized until after hospital discharge, and a return to the home environment (16). The influence of clinical oral health indicators may also have been overshadowed by general adverse effects of the stroke, such as hemiplegia. For instance, affliction of the dominant side was demonstrated to have significantly affected the patients’ perceived physical ability to brush their teeth.
Paradoxically, poorer periodontal health (as indicated by CPI), as well as smoking prior to hospital admission, appeared to be inversely associated with 3 of the 8 oral health transition scale items. This may have been due to a generalized neglect and lowered self-evaluations and expectations of oral health prior to the stroke, such that any additional effects on OHRQoL that the stroke and subsequent hospitalization may have had, were minimal.
Although the use of transition scales in the current study may have been affected by recall bias (16), this was unlikely, as patients had sustained the stroke within 2 weeks of the baseline assessment. It was not feasible in this study to consider the full range of stroke-related variables (e.g. visual-spatial difficulties, extra-oral feeding impairments, etc.), which may have had an effect on clinical and self-perceived oral health, and this is recognized as a limitation. While it is acknowledged that the validity of self-reported information, such as OHRQoL and GHRQoL, may be an additional limitation in the present study, prior studies conducted amongst cognitively impaired subjects (MMSE scores above 10) have found such QoL measures to be valid and reliable (39). As no negative control group was utilized, the direct effect of an oral health promotion programme could not be elucidated, as both OHRQoL and GHRQoL are likely to change naturally during the rehabilitative period following stroke (16), with improvements in function, as well as changes in “terms of reference” and the implementation of coping and adaptation patterns being likely factors (40). The use of a negative control group, however, would have been considered unethical, given the frail nature of this patient group.
An additional limitation was that this study did not control for co-interventions (e.g. swallowing, feeding). It is recognized that oral care does not operate in a vacuum, and co-interventions are frequent, especially in rehabilitation settings. Nevertheless, the present study reports the effects of oral care in the context of traditional rehabilitation care. It was not feasible in this study to control for, or make changes to, standard protocols of staff in other disciplines.
While a number of clinical, demographic, and behavioural factors were assessed for their influence on OHRQoL, the lack of a daily tooth-brushing habit appeared to be the factor most consistently associated with poorer life quality outcomes during the initial stages of recovery following stroke. As the early re-establishment of daily oral hygiene regimens is also one of most easily modifiable of the examined factors, implementation of such protocols may be considered a priority in stroke rehabilitation wards for the improvement of clinical oral health, and OHRQoL.
Acknowledgements
The authors would like to thank staff at the rehabilitation ward, as well as the pharmacy and occupational therapy departments of Tung Wah Hospital for their assistance with this project. This work was supported by the Committee of Research and Conference Grants of the University of Hong Kong.
References
