Annelie Gutke, PhD1,2, Christina B. Olsson, PhD3,4, Nina Völlestad, PhD5, Birgitta Öberg, PhD1, Lena Nilsson Wikmar, PhD4 and Hilde Stendal Robinson PhD5
From the 1Department of Medical and Health Sciences, Div Physiotherapy, Linköping University, Linköping, 2Institute of Neuroscience and Physiology, Department of Neuroscience and Rehabilitation/Physiotherapy, Sahlgrenska Academy, University of Gothenborg, Gothenburg, 3Centre for Family Medicine, Stockholm County Council, 4Department of Neurobiology, Care Sciences and Society, Div of Physiotherapy, Karolinska Institutet, Stockholm, Sweden and 5Department of Health Sciences, Institute of Health and Society, The Medical Faculty, University of Oslo, Norway
OBJECTIVE: To explore the association between disability and sick leave due to lumbopelvic pain in pregnant women in 3 cohorts in Sweden and Norway and to explore possible factors of importance to sick leave. A further aim was to compare the prevalence of sick leave due to lumbopelvic pain.
Design/subjects: Pregnant women (n = 898) from two cohorts in Sweden and one in Norway answered to questionnaires in gestational weeks 10–24; two of the cohorts additionally in weeks 28–38.
METHODS: Logistic regression models were performed with sick leave due to lumbopelvic pain as dependent factor. Disability, pain, age, parity, cohort, civilian status, and occupational classification were independents factors.
RESULTS: In gestational weeks 10–24 the regression model included 895 cases; 38 on sick leave due to lumbopelvic pain. Disability, pain and cohort affiliation were associated with sick leave. In weeks 28–38, disability, pain and occupation classification were the significant factors. The prevalence of lumbopelvic pain was higher in Norway than in Sweden (65%, vs 58% and 44%; p < 0.001).
CONCLUSION: Disability, pain intensity and occupation were associated to sick leave due to lumbopelvic pain. Yet, there were significant variations between associated factors among the cohorts, suggesting that other factors than workability and the social security system are also of importance.
Key word: low back pain; pelvic girdle pain; pregnancy; sick leave; disability.
J Rehabil Med 2014; 46: 468–474
Correspondence address: Annelie Gutke, Institute of Neuroscience and Physiology, Department of Neuroscience and Rehabilitation/Physiotherapy, Sahlgrenska Academy, University of Gothenborg, Gothenburg, Sweden. E-mail: annelie.gutke@gu.se
Accepted Jan 14, 2014; Epub ahead of print Apr 10, 2014
Introduction
Low back pain and pelvic girdle pain (PGP), commonly referred to as lumbopelvic pain (LPP) is reported by more than 50% of pregnant women (1). This is higher than the 26% of women in the general population of the same age (2). The pregnant women with LPP report difficulties with most daily weight bearing activities. Decreased endurance capacity during walking, sitting and standing are common (3). However, large variations in severity of LPP are seen (4–6).
Today it is common for women to work during pregnancy. LPP can affect women’s work ability resulting in the necessity for sick leave. Besides the personal distress, LPP has therefore great economical consequences for both the individual and the society. The estimated sick leave costs for pregnancy-related LPP in Sweden was in 2003 reported to be approximately 24.6 million Euros and on the rise (7).
Ideally, sick leave should be based on the person’s workability. However, the rate of sick leave has been shown to correlate with the social benefit system in a country, i.e. the better the benefits the more sick leave (8). LPP was the most common cause for sick leave among pregnant women in a previous study comparing Sweden and Norway (9). Furthermore, the rate of sick leave in general during pregnancy was twice as high in Sweden, while the benefits in Sweden were more generous than in Norway. When the previous comparison was made, the social benefits in Sweden included pregnancy benefits while the latter was not offered for PGP in Norway (9). However, this has now changed. Social benefits in pregnancy seem thus now to be similar in the two countries.
The prognosis is good for the majority of women with LPP in pregnancy. However, women that do not recover in the first weeks after delivery, have higher risk for persistent pain (10). As many as every 5th woman are reported to have persistent LPP 2–3 years after pregnancy (11) with difficulties in returning to work (9, 12, 13). Among women with LPP requiring sick leave in pregnancy, the majority reported to have had LPP during a subsequent pregnancy (92%) and also while not pregnant (85%) (14). The women also had higher frequency of sick leave periods after pregnancy. Pregnancy can be seen as a risk for persistent LPP and might increase the need for sick leave due to LPP for many years.
Although one third of the pregnant women report LPP as a severe problem that interferes with activities of daily life and influences their ability to work (9, 15), it has been questioned whether LPP in pregnancy should be looked upon as a normal consequence that the women must endure (16) or a reason for sick leave during pregnancy. Norwegian women are granted paid sick leave if they develop problems ‘‘above normal’’ in pregnancy. This raises questions about what can be labelled as ‘‘normal complaints’’ when it comes to LPP. Back related functional disability and its association to workability should be evaluated when sick leave is considered.
Sweden and Norway are neighbouring countries, comparable in many socio-economical aspects. Since there have been changes over time in the social security system and based on the ongoing debate on prescribing sick leave during pregnancy in both countries, it is interesting to study if there is an association between country and sick leave due to LPP.
Aim
The aim of this study was to explore the association between disability and sick leave due to LPP in pregnant women in 3 cohorts in Sweden and Norway and to explore possible factors of importance to sick leave. A further aim was to compare the prevalence of sick leave due to LPP.
Methods
Study design
The study is cross-sectional and includes participants from two Swedish (Sweden East and Sweden West) and one Norwegian cohort of pregnant women. The data presented is collected at two different times during pregnancy, once between gestational weeks 10 to 24, mean 17 weeks (standard deviation; SD 3) and a second time between 28 to 38 gestational weeks, mean 33 weeks (SD 2). The Swedish and Norwegian public health systems serve nearly 100% of the country’s pregnant women, providing regular free check-ups at the maternity care units (MCUs) during pregnancy and puerperium.
Subjects
The cohort in western Sweden comprised all pregnant women consecutively registered at two MCUs in a community of 26,000 people between August 2001 and September 2003. Swedish-speaking women who were expected to have a normal pregnancy were recruited. Participants received written and oral information about the study from their midwives before giving oral consent. Women were excluded if they had a locomotor system disease; verified diagnosis of spinal problems in the previous 2 months; or a history of fracture, neoplasm, or spinal, pelvic, or femur surgery. All women responded to a questionnaire in gestational week 12 to 18. The Regional Research Ethics Committee approved the study in western Sweden (Dnr Ö 414-00).
The cohort in eastern Sweden comprised of pregnant women recruited from 5 MCUs, 3 in the capital area and 2 in a medium-sized town (approximately 130,000 inhabitants), between March 2005 and September 2006. In the capital MCUs, midwives distributed questionnaires to women between gestational weeks 19 to 21. At the other MCUs, women at the same gestational weeks were sent questionnaires by one of the authors (CO). The women received oral information about the study from the midwives, and written information together with the questionnaire. The Regional Research Ethics Committee approved the study in Eastern Sweden (Dnr 03-503).
The cohort in Norway comprised of Norwegian-speaking women, who registered at 4 MCUs in the capital area between January 2006 and June 2007, in gestational weeks 10 to 24. Women not expected to have a normal pregnancy were excluded. The Regional Committee for Medical Research Ethics and the Norwegian Social Science Data Services gave formal approval for the study (Dnr S-05284).
Social security benefits
A national sickness insurance program covers every Swedish resident over 16 years of age with a regular income. The first 7 days of sickness may be self-certified but thereafter, a certificate by a physician is required. The woman’s employer financially covers the first 14 days of the sick-leave period. Pregnancy benefit can be given 60 days before delivery to a woman with a physically heavy work at the end of pregnancy. Throughout pregnancy there is also pregnancy benefit for women with dangerous work with risk for the pregnancy but not the last 10 days before estimated birth.
The Norwegian national sickness insurance program covers every Norwegian resident. Sick leave for more than 3 days usually requires a doctor’s certificate. The first 16 days of the sick leave period are financially covered by the woman’s employer, but in pregnancy the employer can apply and get these costs refunded by the national health insurance system. Pregnancy benefit can be given during pregnancy to secure the women with work situations that might be of risk for the foetus (physical demanding job, distress) even though the woman is healthy, if no adjustment in work situation is possible.
Assessment
Participants completed questionnaires in gestational weeks 10 to 24. The questionnaires included questions about age, body mass index (BMI), civilian status (single/cohabitant), gestational week, employment (fulltime/part time), number of previous pregnancies, LPP, LPP in previous pregnancy, LPP before first pregnancy, and sick leave (yes/no). Pain intensity was estimated on a visual analogue pain scale (100 mm VAS; 0 indicating no pain and 100 worst imaginable). Mean pain intensity in Sweden was evaluated at the time of filling in the questionnaire, whereas in Norway the mean pain intensity was calculated from worst pain intensity in the morning and in the evening. Evaluation of disability was done with disability rating index (DRI) in Sweden East and in Norway, and by the Oswestry Disability Index (ODI) in Sweden West. Based on data from a previous study where women (n = 80) 3 months postpartum (17) had responded to both DRI and ODI, (correlation coefficient R = 0.9) we created a formula from a regression analysis (disability: ODI = 2.5 + 0.84 × DRI) to recount all disability measures into comparable units.
One, 8, and 11 women were without work in Sweden West, Sweden East and in Norway, respectively. Work data were missing on 2 women from Sweden East.
Occupation was asked for in the questionnaire in Sweden East and in Norway and classified into sedentary or non-sedentary according to the Swedish Standard Classification of Occupations 1996 (SSYK96) (18), an adaptation of the International Standard Classification of Occupations (ISCO-88) published in Geneva in 1990 by the International Labor Office.
Participants from Sweden East and Norway filled in a second questionnaire (variables as above) in gestational weeks 28 to 38 (Sweden East gestational weeks 31 to 38, and Norway 28 to 36).
Statistics
Statistical analyses were performed using the SPSS program, 19.0 (SPSS Inc., Chicago, IL). The Mann-Whitney U test was used for two-group comparisons and the Kruskal-Wallis test was used for multi-group comparisons of data on ordinal and scale level. For nominal data, the χ2 test was performed; Fischer’s exact test as appropriate. The data of sick leave and pregnancy benefit due to LPP (in Sweden East) collected in gestational weeks 28 to 38 was pooled into sick leave.
Backward stepwise logistic regression analysis was performed with sick leave due to LPP as a dependent variable. The initial choice of independent variables (measured in gestational weeks 10–24) was based on our questions regarding the association between disability and sick leave due to LPP and factors that could influence this association. Univariate analyses were carried out to compute crude estimates for independent variables (disability, pain intensity, age, parity, gestational weeks, civilian status, occupational classification and cohort affiliation). The significant independent variables were entered into a backward stepwise logistic regression analysis. The final multivariable models included significant variables. Statistical significance was set at p < 0.05.
Results
The study comprised of 898 women. From western Sweden, out of 457 eligible women 87 were excluded and 62 did not give consent; leaving 308 women answering a questionnaire (Table I). From eastern Sweden, out of 498 eligible women 28 did not give consent. Out of the 470 women receiving questionnaires 311 women answered and returned the questionnaires (Table I) and 272 women answered the second questionnaire (in mean gestational weeks 35). The women who did not answer the second questionnaire reported significantly higher disability in the first questionnaire (median 22.0 vs 14.6, p = 0.03). Out of 385 eligible women in Norway, 59 did not give consent. Out of the 326 women, 279 had answered first questionnaire when being between gestational weeks 10 and 24 (Table I). All 279 women answered the second questionnaire (in mean gestational weeks 30).
At baseline there were some significant differences between the cohorts (Table I). The Sweden West women were younger with higher BMI, had given birth to more children and worked full time to a lower degree. The women from the Swedish East cohort answered both questionnaires when being in a later gestational week.
|
Table I. Characteristics of included women in the cohorts in gestational weeks 10–24 and in gestational weeks 28–38 |
|||||||||
|
Variable in gestational weeks 10–24 |
Women without LPP pain |
Women with LPP pain |
|||||||
|
Sweden West (n = 131) |
Sweden East (n = 175) |
Norway (n = 97) |
Group comparisons |
Sweden West (n = 177) |
Sweden East (n = 136) |
Norway (n = 182) |
Group comparisons |
||
|
Age, years, mean (SD) |
29 (4) |
32 (4) |
32 (4) |
< 0.001 |
29 (5) |
30 (5) |
31 (4) |
< 0.001 |
|
|
Gestational week, mean (SD) |
15 (2) |
20 (2) |
15 (3) |
< 0.001 |
15 (2) |
20 (1) |
16 (4) |
< 0.001 |
|
|
Parity, ≥ 1 parity, n (%) |
66 (50) |
69 (39) |
34 (35) |
0.046 |
111 (63) |
67 (49) |
73 (40) |
< 0.001 |
|
|
Full time work, n (%) |
76 (58) |
N/A |
73 (75) |
0.007 |
79 (45) |
N/A |
113 (62) |
0.002 |
|
|
LPP pain before first pregnancy, n (%) |
39 (30) |
N/A |
33 (37) |
0.273 |
85 (48) |
N/A |
100 (57) |
0.087 |
|
|
LPP pain in previous pregnancy, n (%) |
22/65 (34) |
N/A |
15/36 (42) |
0.435 |
88/108 (82) |
N/A |
51/77 (66) |
0.018 |
|
|
LPP now, n (%) |
N/A |
N/A |
N/A |
N/A |
177 (58) |
136 (44) |
182 (65) |
< 0.001 |
|
|
Sick leave due to LPP, n (%) |
N/A |
N/A |
N/A |
N/A |
19 (11) |
7 (5) |
12 (7) |
0.135 |
|
|
BMI, mean (SD) |
25.3 (4.4) |
N/A |
23.0 (3.7) |
< 0.001 |
25.3 (4.2) |
N/A |
23.6 (3.4) |
< 0.001 |
|
|
Civilian, single, n (%) |
4 (3) |
3 (2) |
0 (0) |
0.218 |
7 (4) |
8 (6) |
9 (5) |
0.714 |
|
|
Pain intensity VAS, median (Q1–Q3) |
N/A |
N/A |
N/A |
N/A |
30 (16–48) |
20 (7–36) |
5 (0–28) |
< 0.001 |
|
|
Disability, median (Q1–Q3) |
N/A |
11 (5–18) |
11 (6–19) |
0.483 |
16 (10–28) |
25 (14–41) |
20 (11–33) |
< 0.001 |
|
|
Disability – no sick leave due to LPP pain, median (Q1–Q3) |
N/A |
N/A |
N/A |
N/A |
14 (8–22) |
24 (14–39) |
18 (11–28) |
< 0.001 |
|
|
Disability – sick leave due to LPP pain, median (Q1–Q3) |
N/A |
N/A |
N/A |
N/A |
34 (28–40) |
55 (45–66) |
38 (34–50) |
0.002 |
|
|
Variable in gestational weeks 28–38 |
Sweden East n = 173 |
Norway n = 215 |
Group comparisons |
||||||
|
Gestational week, mean (SD) |
N/A |
N/A |
N/A |
N/A |
N/A |
34 (1) |
30 (1) |
< 0.001 |
|
|
Occupation classification, n (%) |
|||||||||
|
Sedentary |
N/A |
N/A |
N/A |
N/A |
N/A |
106 (69) |
148 (73) |
0.443 |
|
|
Non-sedentary |
N/A |
N/A |
N/A |
N/A |
N/A |
48 (31) |
56 (27) |
||
|
LPP now, n (%) |
173 (63) |
215 (81) |
< 0.001 |
||||||
|
Sick leave due to LPP, n (%) |
73 (43) |
71 (33) |
0.048 |
||||||
|
Pain intensity VAS, median (Q1–Q3) |
N/A |
N/A |
N/A |
N/A |
N/A |
23 (13–46) |
27 (0–50) |
0.435 |
|
|
Disability, median (Q1–Q3) |
N/A |
N/A |
N/A |
N/A |
N/A |
48 (34–60) |
37 (24–48) |
< 0.001 |
|
|
Disability – no sick leave due to LPP pain, median (Q1–Q3) |
N/A |
N/A |
N/A |
N/A |
N/A |
44 (28–54) |
29 (19–41) |
< 0.001 |
|
|
Disability – sick leave due to LPP pain, median (Q1–Q3) |
N/A |
N/A |
N/A |
N/A |
N/A |
56 (41–64) |
48 (38–56) |
0.005 |
|
|
BMI: body mass index; LPP: lumbopelvic; SD: standard deviation; Q1: quartile 1 (25%); Q3: quartile 3 (75%); N/A: not applicable. |
|||||||||
Gestational weeks 10 to 24
The logistic regression model included 895 cases out of which 38 were on sick leave due to LPP. The 3 factors that were significantly associated with sick leave due to LPP were disability, pain intensity and cohort affiliation (Table II). These variables accounted for 39% of the variance (Nagelkerke r-square 0.394). An interpretation is for example that a woman with a 10 unit increase in disability will have an odds of 2.52 to be on sick leave. Likewise, an increase in pain intensity of 10 mm will have an odds of 1.24 to be on sick leave. We also re-entered gestational week into the final model, first together with cohort and afterwards without cohort in the model. Neither changed the results, and gestational week were not associated to sick leave in the model. Hence, independent factors not significantly associated with sick leave due to LPP were age, parity, gestational weeks, and civilian status.
The prevalence of self-reported LPP differed between the 3 cohorts (p < 0.001) (Table I) as well as between countries (65% vs 44–58% in Norway and Sweden, respectively p < 0.001). The prevalence of self-reported sick leave due to LPP was similar (5%–11%; p = 0.135) (Table I). There was no difference between countries (p = 0.48).
Women on sick leave due to LPP in gestational weeks 10–24 reported higher disability (median 34, 38 and 55 for Sweden West, Norway, Sweden East, respectively) than the women not on sick leave (median 14, 18 and 24, respectively) p < 0.001 (Fig. 1). Disability levels differed significantly between cohorts both in women without and with sick leave due to LPP (p ≤ 0.002).
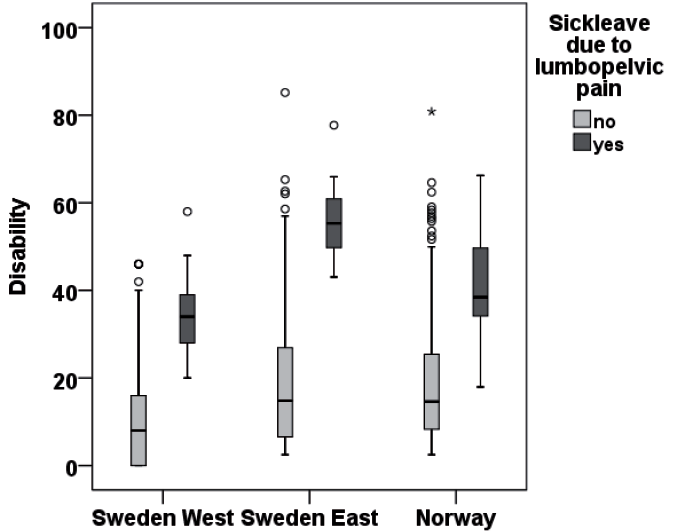
|
Table II. Univariate and multivariable backward logistic regression analyses of factors associated with sick leave due to lumbopelvic pain (LPP) in gestational weeks 10–24 |
|||||
|
Independent variables, n = 895 of which 38 on sick leave |
Sick leave due to LPP in gestational week 10–24 |
||||
|
Univariate crude analyses |
Multivariable adjusted analyses |
||||
|
Crude OR (95% CI) |
p-value |
Adjusted OR (95% CI) |
p-value |
||
|
Age |
0.93 (0.87–1.00) |
0.059 |
|||
|
Parity, Ref: zero parities |
1 |
||||
|
≥ 1 parity |
1.43 (0.75–2.76) |
0.279 |
|||
|
Gestational weeks |
1.01 (0.92–1.11) |
0.814 |
|||
|
Civilian, ref: co-habitant |
|||||
|
Single |
2.53 (0.74–8.74) |
0.141 |
|||
|
Disability |
1.08 (1.06–1.11) |
< 0.001 |
1.10 (1.07–1.13) |
< 0.001 |
|
|
Pain intensity, VAS |
1.05 (1.04–1.07) |
< 0.001 |
1.02 (1.00–1.04) |
0.017 |
|
|
Cohort, ref: Sweden East |
1 |
1 |
|||
|
West |
2.88 (1.91–6.94) |
0.019 |
10.48 (3.15–34.87) |
< 0.001 |
|
|
Norway |
1.95 (0.76–5.03) |
0.166 |
2.62 (0.87–7.94) |
0.088 |
|
|
OR: odds ratio; 95% CI: 95% confidence interval; VAS: visual analogue scale. |
|||||
Gestational weeks 28 to 38
The logistic regression model in gestational weeks 28–38 included 539 cases out of which 146 were on sick leave due to LPP (Table III). The three factors that were significantly associated with sick leave due to LPP were occupational classification, disability, and pain intensity and accounted for 46% (Nagelkerke R2 0.462) of the variance in sick leave due to LPP. In addition, we re-entered both cohort affiliation and gestation week in the final model, however none of the variables changed the result. Independent factors not significantly associated with sick leave due to LPP in gestational weeks 28–38 were age, parity, gestational weeks, civilian status, and cohort affiliation.
In gestational weeks 28 to 38 of pregnancy, more of the Norwegian women reported LPP than the Swedish women (81% and 63% respectively, p < 0.001) (Table I). The proportion of Norwegian and Swedish women receiving sick leave due to LPP differed somewhat (p = 0.048) (Table I). Women on sick leave due to LPP reported a higher disability level than women not on sick leave (p < 0.001). Swedish women were more disabled than their Norwegian counterpart (p ≤ 0.005) (Table I).
|
Table III. Univariate and multivariable backward logistic regression analyses of factors associated with sick leave due to lumbopelvic pain (LPP) in gestational weeks 28–38 |
|||||
|
Independent variables, n = 539 of which 146 on sick leave |
Sick leave due to LPP in gestational week 28–38 |
||||
|
Univariate crude analyses |
Multivariable adjusted analyses |
||||
|
Crude OR (95% CI) |
p-value |
Adjusted OR (95% CI) |
p-value |
||
|
Age |
0.96 (0.92–1.00) |
0.057 |
|||
|
Parity, ref: xero parities |
|||||
|
≥1 parity |
1.57 (1.07–2.30) |
0.022 |
|||
|
Gestational weeks |
0.96 (0.88–1.04) |
0.316 |
|||
|
Civilian, ref: co-habitant |
|||||
|
Single |
1.48 (0.54–4.09) |
0.445 |
|||
|
Occupation, ref: sedentary |
|||||
|
Non-sedentary |
2.40 (1.59–3.63) |
< 0.001 |
3.77 (2.18–6.51) |
< 0.001 |
|
|
Disability |
1.07 (1.05–1.09) |
< 0.001 |
1.04 (1.02–1.06) |
< 0.001 |
|
|
Pain intensity, VAS |
1.05 (1.04–1.06) |
< 0.001 |
1.05 (1.03–1.06) |
< 0.001 |
|
|
Cohort, ref: Sweden East |
|||||
|
Norway |
0.99 (0.68–1.44) |
0.950 |
|||
|
OR: odds ratio; 95% CI: 95% confidence interval; VAS: visual analogue scale. |
|||||
Discussion
The main results of this study were that disability and pain intensity were associated to sick leave due to LPP both in gestational weeks 10–24 and 28–38. In addition, cohort affiliation in early pregnancy and occupational classification in later pregnancy contributed to the explanation of variance in sick leave due to LPP.
The person’s work ability in relation to their job demands should be the basis for sick leave hence self-reported disability is a factor of importance when sick leave is considered. Women on sick leave due to LPP reported higher disability than the women not on sick leave supporting the adequacy of sick leave. The odds for being on sick leave due to LPP in Sweden West were 10 times higher than in Sweden East despite the fact that the Sweden East women reported the highest disability level. This suggests that factors other than disability level influence the decision to sick leave within the same country.
Occupational classification may be a factor to consider when judging workability and need of sick leave. Reported occupational factors decreasing workability in pregnant women with LPP are twisting or bending many times per hour (19), uncomfortable posture (20), and strenuous work (1). One difficulty using occupational classification is that the same occupation may vary regarding these and other factors and thereby the self-reported disability in relation to job demands may be a better choice.
The sick leave due to LPP ranged between 5 and 11% of women with reported LPP. The greatest difference in sick leave rate was seen between the two Swedish cohorts, suggesting that the social security system is not the most important factor. Cultural differences between public health workers in different areas of the same country could also influence the results.
The similar rate of sick leave in Sweden and Norway in the present study is in contrast to the comparison made 25 years ago where the Swedish sick leave rate was double the Norwegian (9). The difference might be explained by methodological differences or also by changes in the social security system concerning pregnancy in both countries over twenty years.
The prevalence of LPP was highest in late pregnancy which was also to be expected (10). The prevalence of LPP was higher in Norway than in the two Swedish cohorts, early as well as later in pregnancy. However, the prevalence was within the previously reported range from all over the world (1, 3) although the prevalence in late pregnancy in Norway was among the highest reported (80%). This might be interpreted as a lower threshold to report LPP although it did not result in sick leave.
The frequency of self-reported sick leave due to LPP in gestational weeks 28–38, was similar in the two countries despite the differences in prevalence of LPP. This could be explained by the generally higher disability reported by the Swedish East women.
Due to the restricted attitude toward sick leave in general in Sweden lately, there seem to be an overall reported decrease in sick leave. In 1986 when the previous comparison was made, PGP was not accepted for sick-leave during pregnancy in Norway. This was changed in 1992, and might contribute to the different results. Further comparison with previous studies is difficult since they are longitudinal and report sick leave during the total pregnancy period (21–25). Sick leave is said to be influenced by the degree of employment outside the home (9). This was similar in the countries in the present study. In 1986 however, more Swedish than Norwegian women were employed outside the home (9). On the other hand, more Norwegian women worked full-time in both studies suggesting that full time work does not explain sick leave due to LPP.
The proportion of women on sick leave due to LPP in pregnancy is the double of that among all women on sick leave in Sweden (26). Since back pain has been reported to be the most common cause for sick leave during pregnancy both in studies (9, 21) and the Swedish national register (7), intervention directed towards LPP during pregnancy is warranted. Since our results suggest that also other factors than disability are associated to sick leave, further studies need to focus on factors important for work ability in pregnant women.
Method discussion
The strength of the present study is the low drop-out rates and thereby good generalizability. Further strength is that cohorts of pregnant women are studied and not only women who seek health care, although 898 women can be seen as a low number from an epidemiological perspective.
The exclusion criteria of women with locomotor system or spinal problems (10) in Sweden West, were not utilised in the two other cohorts. However, in the Norwegian cohort, only 4 of the included women reported to have rheumatoid arthritis (3) and pelvospondylitis (1). We do not believe that this small numbers have importantly influenced our result.
The present study is based on self-reported data from questionnaires. Self-reported sick leave data has shown to be valid compared to data registered by the insurance office (27–29) except for duration of sick leave (29).
The question of sick leave for pregnancy-related LPP is of high interest at present both in Sweden and Norway. We therefore believed that the advantages of having comparable data from 3 cohorts from the two countries exceeded the limitations of the study. Presently in Sweden, the employer pays the first two weeks of sick leave. Sweden East data is collected about 4 years later than data from Sweden West. The only change in the Swedish social security system during the study period was that from mid 2003 to end 2004, the employer paid the first 3 weeks of sick leave. The periods of comparison in Sweden (gestational weeks 12–21 and 31–38) and Norway (gestational weeks 10–24 and 28–36) are similar. Since there is evidence to support that the debut of symptoms is between gestational week 12 and 24 (30), the first comparison should be representative for LPP in pregnancy. The women in Sweden East were in mean in a later gestational week at baseline than the two other cohorts. However, conducting analyses with and without the cohort affiliation did not change either the results or the final regression models. Furthermore, gestational week was not associated with sick leave due to LPP in either of the regression models.
Reported mean pain intensity from Sweden is somewhat different from the calculated mean pain intensity from morning and evening assessments in Norway. The women with pregnancy-related LPP often report higher pain intensity in the evenings as a consequence of physical activity during the day. It is likely that when women report mean pain intensity during a day, they estimate a mean of the morning and the evening pain. Thus we do not believe the difference in methods make an important influence on our results.
The different disability measurements used in the 3 cohorts were recalculated using a formula created by data from a study where the highly correlated (R 0.9) ODI and DRI were used. We also performed an analysis of the regression model in early pregnancy based on DRI (without the Sweden West cohort) and this resulted in the same model. Hence, we evaluated the recalculation formula and the model to be appropriate. It is common in the sick leave debate to focus on proportion of sick leave in populations. However, we believe that the disability associated with the condition is also important to evaluate and therefore the possibility to study disability outweighs the limitation of having different measures.
The factor occupation classification is based on a predefined classification of work being sedentary or non-sedentary (18). It is important though to consider that a work position within the same occupation may differ regarding physical and mental demands and thereby workability. The classification into sedentary/non-sedentary can thus be somewhat uncertain and this should be consider when interpret the result from the regression model in gestational weeks 28–38.
The baseline differences in age, parities and BMI among cohorts at inclusion are small and thereby probably of little clinical importance. A possible interpretation of the differences could be that the higher parity in Sweden is associated with higher BMI and less fulltime work. The fact that the Swedish women had higher parity than the Norwegian women should rather imply higher prevalence of LPP (31).
Acknowledgement
This study was performed after support from The Swedish Council for Working life and Social Research, The University of Oslo, EXTRA funds from the Norwegian Foundation for Health and Rehabilitation, and The Norwegian Fund for Post-Graduate Training in Physiotherapy. Financial support was provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
References
