Matagne Heutink, MSc1, Marcel W. M. Post, PhD1, Peter Luthart, PT2, Marijke Schuitemaker, PT3, Sandra Slangen, PT4, Jolante Sweers, PT5, Lonneke Vlemmix, PT5 and Eline Lindeman, MD, PhD1†
From the 1Brain Center Rudolf Magnus and Center of Excellence for Rehabilitation Medicine, University Medical Center Utrecht and De Hoogstraat Rehabilitation, 2De Hoogstraat Rehabilitation, Utrecht, 3Rehabilitation Center Het Roessingh, Enschede, 4Adelante Zorggroep, Hoensbroek and 5Rijndam Rehabilitation Center, Rotterdam, The Netherlands. †This author is deceased (September 27th 2012)
OBJECTIVE: To explore the long-term outcomes of CONECSI (COping with NEuropathiC Spinal cord Injury pain), a multidisciplinary cognitive behavioural treatment programme in persons with spinal cord injury.
DESIGN: Long-term follow-up pre-post-intervention design.
SUBJECTS: A total of 29 subjects with a spinal cord injury and chronic neuropathic pain from 4 Dutch rehabilitation centres.
METHODS: Primary outcomes were pain intensity and pain-related disability (Chronic Pain Grade questionnaire). Secondary outcomes were mood (Hospital Anxiety and Depression Scale), participation in activities (Utrecht Activities List), and life satisfaction (Life Satisfaction Questionnaire). Random coefficient analysis was used for the analyses of measurements before (t1), immediate post-intervention (t2), and 6 (t3), 9 (t4), and 12 (t5) months follow-up.
RESULTS: The analyses showed significant improvements on pain intensity (t1–t2 and t1–t5) and pain-related disability (t1–t2, t1–t4, and t1–t5), anxiety and participation in activities (t1–t2, t1–t3, and t1–t5).
CONCLUSION: This exploratory study suggests that a multidisciplinary cognitive behavioural programme might have lasting improvements on pain intensity, pain-related disability, anxiety, and participation in activities in people with chronic neuropathic spinal cord injury pain and highlights the potential of such programmes.
Key words: spinal cord injuries; neuralgia; chronic pain; longitudinal studies; intervention studies; cognitive therapy.
J Rehabil Med 2014; 46: 540–545
Correspondence address: M.W.M. Post, PhD, De Hoogstraat Rehabilitation, P.O. Box 85238, 3508 AE Utrecht, The Netherlands. E-mail: M.Post@dehoogstraat.nl
Accepted Jan 14, 2014; Epub ahead of print May 8, 2014
INTRODUCTION
Spinal cord injury (SCI) is a serious condition and adjusting to the physical and psychosocial consequences of SCI is a great challenge for the person involved (1). The prevalence of depression and anxiety is elevated in people with SCI (2), and their average life satisfaction is substantially below that of the general population (3). Chronic pain is one of the major consequences of SCI and affects about 70% of this population (4). One type of pain many people with SCI have to cope with is chronic neuropathic spinal cord injury pain (CNSCIP), which strongly affects daily functioning and is associated with depression, anxiety and overall quality of life (5–7). A review showed that no less than 40% of persons with SCI reported intense neuropathic pain (4). Neuropathic pain is initiated by a primary injury to the nervous system and involves abnormal sensations, such as burning, electrical and shooting sensations, and often reduced touch sensation and allodynia (8). The mechanisms underlying CNSCIP are only partly understood, and it is still unclear why some SCI patients develop neuropathic pain and others with apparently similar injuries do not (9).
CNSCIP is difficult to treat (10). Approaches that have been used to treat CNSCIP include pharmacological treatments (e.g., anticonvulsants, antidepressants, opioids, or non-steriodal anti-inflammatory drugs) and non-pharmacological treatments (e.g., physical methods, massage, psychological treatments, acupuncture, physiotherapy and exercise). However, to date none of these provide sufficient relief in the majority of the SCI population (11–14). Therefore, there is a need for effective treatments for CNSCIP.
In recent years more attention has been given to psychological treatments because research showed relationships between psychological factors and maintenance and aggravation of CNSCIP (7, 15). Psychological treatment is targeted on pain cognitions, e.g., catastrophising, pain-related beliefs and coping, and social factors, to improve psychological and physical functioning in persons with chronic pain (16). Intervention studies examining the potential for such comprehensive, cognitive behavioural therapy (CBT)-based treatment programmes to benefit persons with SCI and pain showed promising results (17–20), in terms of changes in anxiety (17, 19, 20), and depression (17, 20).
In response to these findings, the CONECSI (COping with NEuropathiC Spinal cord Injury pain) trial was conducted to evaluate the effectiveness of a comprehensive, multidisciplinary cognitive behavioural programme for coping with CNSCIP (21). This randomised controlled trial demonstrated a short-term decrease in both primary outcome measures (pain intensity and pain-related disability) and 2 out of 4 secondary outcome measures (anxiety and participation in activities), although compared to the control group no short-term treatment effect was found for pain intensity and only a trend was found for pain-related disability (p = 0.059) (22). However, the duration of follow-up was restricted to 3 months post-intervention. A long-term follow-up might have shown stronger favourable effects of cognitive behavioural treatment if people get more experience in applying principles learned in the programme in their daily life. To our knowledge, except from one study (17), data on the long-term outcomes of cognitive behavioural interventions for CNSCIP is lacking to date.
The objective of this study was therefore to explore the long-term outcomes of the CONECSI trial. The hypothesis was that the intervention would result in a long-term decrease of pain intensity and pain-related disability, and in improvement of mood, participation in activities, and life satisfaction.
METHODS
Study design
The CONECSI trial is an unblinded multicentre randomised controlled trial. Participants were randomly allocated to an immediate intervention group or to a waiting list control group within each participating rehabilitation centre. The control group was invited for the programme after a waiting period of 6 months. Measurements were performed in both groups before starting the programme (t1), immediately after intervention (t2), and at 6 months (follow-up, t3). These results have been published earlier (22). Since a waiting-list control group was used in this trial, it was possible to perform additional follow-up measurements in the intervention group at 9 (t4) and 12 (t5) months after the start of the intervention. But these long-term measurements were not possible in the control group within the time frame of the study. Therefore only the participants in the intervention group are included in the current long-term follow-up study.
Ethical considerations
The Medical Ethics Committees of the University Medical Centre Utrecht and the participating rehabilitation centres have approved the study protocol. Written informed consent was obtained from each participant. The trial is registered in the Dutch Trial Register (NTR1580).
Study population
Participants were recruited from 4 Dutch rehabilitation centres with a specialisation in SCI rehabilitation: De Hoogstraat Rehabilitation, Utrecht, Adelante Zorggroep, Hoensbroek, Rehabilitation Center Het Roessingh, Enschede, and Rijndam Rehabilitation Center, Rotterdam. Eligible persons met the following inclusion criteria: (1) SCI (determined by the physiatrists of the 4 SCI departments of the rehabilitation centres); (2) at least 18 years old; (3) at least 1 year after discharge from first inpatient SCI rehabilitation; (4) main pain type is neuropathic pain; (5) duration of neuropathic pain at least 6 months; and (6) pain intensity score in the previous week of at least 40 on the 0–100 numerical rating scale of the Chronic Pain Grade (23). Exclusion criteria were: (1) SCI caused by metastatic tumour; (2) previous CBT for coping with pain after SCI (determined by a psychologist); (3) inability to function in a group due to psychopathology; and (4) insufficient mastery of the Dutch language.
Procedure
Physiatrists from the 4 rehabilitation centres selected former patients from their centre meeting inclusion criteria 1, 2, and 3. The selected patients were sent a questionnaire to determine if they met the inclusion criteria 4, 5, and 6 and exclusion criterion 1. A trainer of the intervention (psychologist or nurse practitioner) checked in an interview for the other exclusion criteria before final inclusion in the CONECSI trial.
Intervention
This multidisciplinary programme consists of 10 3-h sessions over a 10-week period and a comeback session 3 weeks after the tenth session. Each meeting was supervised by a psychologist and a physiotherapist (the trainers) from the local centre in 3 centres, and by a nurse practitioner and a physiotherapist (the trainers) from the local centre in 1 centre. The programme comprises educational, cognitive, and behavioural elements targeted at coping with CNSCIP. Two theoretical models were used in the programme, the BioPsychoSocial (BPS) model (24) and the Activating event-Belief-Consequence (ABC) model (25). These two models were explained in educational sessions and in guided group discussions using fictitious cases. These models were applied in sports workshops and homework assignments. Further elements of the programme were: information on SCI and CNSCIP; goal setting; information by a physiatrist specialised in SCI rehabilitation and a physiatrist specialised in chronic pain rehabilitation; information on movement and pain; information on assertiveness and communication about pain; introduction to relaxation exercises; information on pain, mood, and stress; and information on social aspects and partner, family, and friends.
A detailed description of the study protocol and the CONECSI trial has been reported elsewhere (21).
Instruments
Pain intensity and pain-related disability were measured with the Chronic Pain Grade questionnaire (CPG) (23). Participants rated their pain intensity on a Numeric Rating Scale for mean pain, worst pain, and current pain (pain intensity score 0–100), and the degree of pain interference with daily activities, work/household activities, and recreational/social activities (pain-related disability score 0–100). The internal consistency for the pain intensity score and the pain disability score in an SCI population was excellent (Cronbach’s α 0.95 and 0.94, respectively) (7). In the present study, the CPG has been adapted to ask for neuropathic pain (“The following questions relate to neuropathic pain due to spinal cord injury”) in the past week instead of the past 6 months. The mean of the CPG scores at inclusion and at t1 score was used as the baseline (t1) score (22).
Anxiety and depression were measured with the Hospital Anxiety and Depression Scale (HADS) (26). The HADS is a 14-item self-report measure. It contains two 7-item scales: one for anxiety and one for depression, both with a score range of 0–21. It is a valid and reliable measure and responsive to change (26). Woolrich et al. (27) reported a good internal consistency in an outpatient population with SCI, with a Cronbach’s α of 0.85 for the anxiety and 0.79 for the depression scale.
Participation in activities was measured with Utrecht Activities List (UAL) (28, 29). The UAL is a Dutch adaptation of the Craig Handicap Assessment and Rating Technique (CHART) (30). Participation in activities is assessed by the time spent on activities such as paid work, study, housekeeping, voluntary work, hobbies, and sports in hours per week.
Life satisfaction was measured with the Life Satisfaction Questionnaire (LiSat-9) (31, 32). The LiSat-9 consists of a global item ‘life as a whole’ and 8 domain-specific items: ‘activities of daily living’, ‘leisure’, ‘vocational situation’, ‘financial situation’, ‘sexual life’, ‘partnership relationship’, ‘family life’, and ‘contacts with friends’. These 9 variables are rated on a 6 point scale (very dissatisfying to very satisfying), with higher scores reflecting greater satisfaction. The internal consistency of the total score (mean of all item scores) was good (Cronbach’s α of 0.80) in a Dutch SCI population (33).
Demographic characteristics assessed at baseline were age, gender, educational level, and marital status. Functional independence was assessed with the Barthel Index (BI) (34). The Dutch translation showed good validity and reliability (Cronbach’s alpha 0.87) in people with SCI (35). Type of pain (musculoskeletal pain; visceral pain; spasm pain; neuropathic pain below, above, or at injury level; pain from syringomyelia; and non-SCI related pain) was assessed by self-report, as well as time post injury, cause of injury, and level and completeness of the lesion. The questions of the DN4 (36) were used to check for the presence of neuropathic pain. The DN4 questionnaire is a validated instrument with a specificity for detecting neuropathic pain of 82.9% and a sensitivity of 89.9% (36).
Neurological lesion level was defined as the highest motor level. Completeness was distincted in motor complete (AIS grades A and B) versus motor incomplete (AIS grades C and D). Neurological levels below T1 were defined as paraplegia, neurological levels at or above T1 were defined as tetraplegia. The physiatrist was asked if there was any doubt about the patient’s answer about the type of pain or the neurological lesion level, or if the answer was missing. Cause of injury was differentiated into traumatic (traffic, work, and sports accident; fall from height; surgery; and other) and non-traumatic SCI (inflammation; tumour; and other).
Statistical analyses
Participant’s characteristics were calculated at t1 (baseline). The pain intensity score and the pain-related disability score of the CPG, the anxiety and depression score of the HADS, total participation in activities of the UAL, and the life satisfaction sum score of the LiSat-9 were calculated for the measurements t1 to t5. Descriptive statistics were computed using means (standard deviations (SDs)). The courses of the outcomes of the intervention over time were analysed using random coefficient analysis (multilevel analysis) (37). The hierarchy in the data of this study is the repeated measurement “time” (t1–t5) (level 1), which is grouped within the individual subjects (level 2), who are grouped in the rehabilitation centres (level 3). Six models were calculated, each with one of the outcome measures as dependent variable (pain intensity, pain-related disability score, anxiety, depression, total participation in activities, and life satisfaction) in a multilevel regression analysis and time modelled with 4 dummy variables (t1–t2, t1–t3, t1–t4, t1–t5) as the determinant. The intercept or slope is fixed, unless the –2 log likelihood of the model with a random intercept or slope is significantly lower (the model is better) then the –2 log likelihood of the fixed intercept or slope model.
Statistical analyses were performed using SPSS statistical program for Windows (version 19.0) and MLwiN program of the Centre for Multilevel Modelling, Institute of Education, University of London (version 2.25). Significance was set at a p-value less than 0.05.
RESULTS
Participants characteristics
A total of 31 persons were randomised in the intervention group of the CONECSI trial. Two persons were excluded from the current analyses because they could not participate in the programme due to health problems before the start of the programme. The mean age of the participants at t1 was 56.5 years (SD 12.1). The median time between the onset of SCI and inclusion was 5.4 years (range 1.9–23.7) and the median duration of CNSCIP at inclusion was 4.5 years (range 1.6–23.7). The mean Barthel Index score was 12.7 (SD 5.8) on a 0–20 scale. The mean number of self-reported pain types was 2.5 (SD 1.2). More men than women participated in this study, and the majority had a traumatic SCI, paraplegia, and lived with a spouse (Table I).
|
Table I. Baseline characteristics of study sample (n = 29) |
|
|
Sample characteristics |
n (%) |
|
Gender (male) |
21 (72.4) |
|
Married/living with a spouse |
23 (79.3) |
|
Level of education (lowa) |
14 (48.3) |
|
Traumatic SCI |
25 (86.2) |
|
Paraplegic level of SCI |
18 (62.1) |
|
Motor incomplete SCI |
14 (48.3) |
|
Pain type |
|
|
Musculoskeletal |
15 (51.7) |
|
Visceral |
6 (20.7) |
|
Neuropathic above |
4 (13.8) |
|
Neuropathic at level |
15 (51.7) |
|
Neuropathic below |
24 (82.8) |
|
Spasm |
7 (24.1) |
|
Syringomyelia |
1 (3.4) |
|
Other |
1 (3.4) |
|
aIncomplete primary education, primary school, junior secondary technical education, general secondary education (lower level). SCI: spinal cord injury. |
|
Course of primary and secondary outcome measures
Table II shows descriptive data of pain intensity, pain-related disability, participation in activities, anxiety, depression, and life satisfaction at each measurement time-point.
|
Table II. Descriptives of pain intensity, pain-related disability, participation in activities, anxiety, depression, and life satisfaction scores at each measurement (mean and standard deviation) |
|||||||
|
Outcome |
Maximum range |
Actual range |
t1 (n = 29) |
t2 (n = 29) |
t3 (n = 29) |
t4 (n = 28) |
t5 (n = 24) |
|
Pain intensity |
0–100 |
20–90 |
69.0 (9.8) |
65.3 (12.9) |
66.1 (13.2) |
66.3 (12.3) |
65.7 (16.6) |
|
Pain-related disability |
0–100 |
0–83.33 |
49.0 (21.2) |
38.8 (25.1) |
39.0 (24.7) |
40.1 (23.7) |
42.4 (23.9) |
|
Participation in activities |
0–no max |
0–91 |
33.1 (22.7) |
41.8 (18.8) |
43.1 (16.7) |
35.5 (22.5) |
42.7 (17.3) |
|
Anxiety |
0–21 |
0–19 |
7.2 (4.1) |
5.9 (3.6) |
6.1 (3.6) |
6.7 (3.4) |
5.4 (3.1) |
|
Depression |
0–21 |
1–18 |
7.2 (3.6) |
6.7 (4.0) |
6.8 (3.1) |
6.4 (3.2) |
6.0 (3.8) |
|
Life satisfaction |
1–6 |
1–6 |
4.1 (1.0) |
4.0 (1.1) |
4.2 (0.9) |
4.0 (1.1) |
4.4 (0.8) |
|
The mean scores differ slightly from the scores in the figures with MlwiN-estimated values. |
|||||||
Multilevel analysis showed that pain intensity significantly decreased between the time periods t1–t2 and t1–t5, and that pain-related disability decreased between the time periods t1-t2, t1–t4 and t1–t5. The decrease in pain-related disability between t1 and t3 was just outside significance (Table III, Fig. 1).
|
Table III. Multilevel linear regression models for pain intensity and pain-related disability pre- and post-intervention, and 6, 9, and 12 months follow-up (n = 29) |
||||||||
|
Variables |
Model for pain intensity |
Model for pain-related disability |
||||||
|
β |
SE |
p |
β |
SE |
p |
|||
|
Constant |
69.023 |
2.141 |
48.965 |
4.298 |
||||
|
Time (t1–t2) |
–3.712 |
1.850 |
0.044* |
–10.155 |
3.913 |
0.009* |
||
|
Time (t1–t3) |
–2.931 |
2.329 |
0.208 |
–9.999 |
5.104 |
0.050 |
||
|
Time (t1–t4) |
–3.077 |
1.872 |
0.101 |
–8.697 |
3.960 |
0.028* |
||
|
Time (t1–t5) |
–5.520 |
2.004 |
0.006* |
–8.544 |
4.165 |
0.040* |
||
|
All models had random intercepts. All models had fixed slopes, except for the Time (t1–t3) and (t1–t5) covariates of pain intensity and for the Time (t1–t3) covariate of pain-related disability. *p < 0.05. t1: measurement 1, pre-intervention; t2: measurement 2, immediate post-intervention; t3: measurement 3, 6 months follow-up; t4: measurement 4, 9 months follow-up; t5: measurement 5, 12 months follow-up; SE: standard error. Beta (β) stands for a non-standardised regression coefficient in multilevel analyses. |
||||||||
No changes over time were found for the secondary outcome measures HADS depression and life satisfaction (Table IV). HADS anxiety scores significantly decreased and UAL scores significantly increased across t1–t2, t1–t3, and t1–t5 (Table IV and Fig. 2).
|
Table IV. Multilevel linear regression models for the secondary outcome measures pre- and post-intervention, and 6, 9, and 12 months follow-up (n = 29) |
|||||||
|
Variables |
Model for participation in activities |
Model for anxiety |
|||||
|
β |
SE |
p |
β |
SE |
p |
||
|
Constant |
33.172 |
3.628 |
7.207 |
0.662 |
|||
|
Time (t1–t2) |
8.635 |
2.984 |
0.004* |
–1.413 |
0.477 |
0.003* |
|
|
Time (t1–t3) |
9.395 |
2.949 |
0.001* |
–1.256 |
0.471 |
0.008* |
|
|
Time (t1–t4) |
0.985 |
2.986 |
0.741 |
–0.357 |
0.477 |
0.453 |
|
|
Time (t1–t5) |
8.668 |
3.107 |
0.005* |
–1.427 |
0.497 |
0.004* |
|
|
Variables |
Model for depression |
Model for life satisfaction |
|||||
|
β |
SE |
p |
|
β |
SE |
p |
|
|
Constant |
7.241 |
0.650 |
4.102 |
0.186 |
|||
|
Time (t1–t2) |
–0.558 |
0.468 |
0.234 |
–0.202 |
0.126 |
0.110 |
|
|
Time (t1–t3) |
–0.374 |
0.462 |
0.418 |
0.063 |
0.125 |
0.617 |
|
|
Time (t1–t4) |
–0.644 |
0.468 |
0.168 |
–0.178 |
0.126 |
0.159 |
|
|
Time (t1–t5) |
–0.821 |
0.487 |
0.091 |
0.196 |
0.132 |
0.139 |
|
|
All models had random intercepts. All models had fixed slopes. *p < 0.05. t1: measurement 1, pre-intervention; t2: measurement 2, immediate post-intervention; t3: measurement 3, 6 months follow-up; t4: measurement 4, 9 months follow-up; t5: measurement 5, 12 months follow-up; SE: standard error. Beta (β) stands for a non-standardised regression coefficient in multilevel analyses. |
|||||||
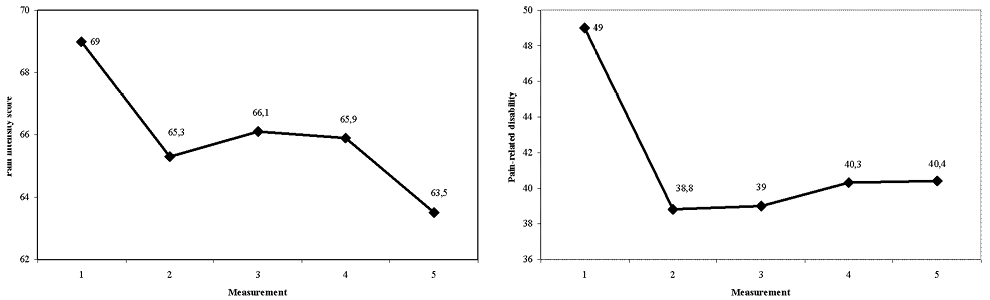
Fig. 1. Pain intensity score and pain-related disability score of the Chronic Pain Grade questionnaire.
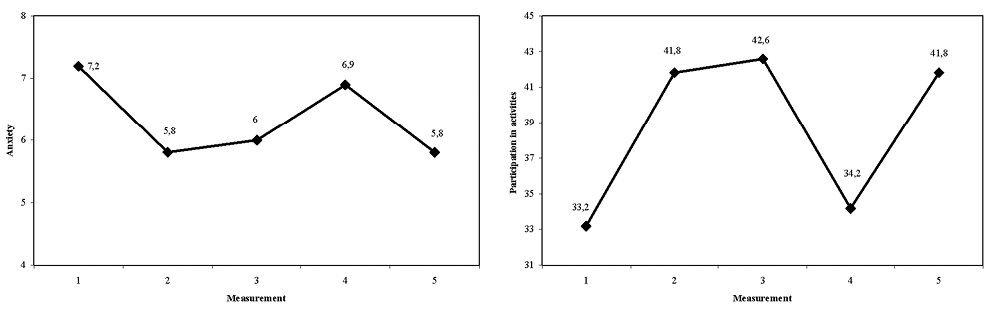
Fig. 2. Anxiety score of the Hospital Anxiety and Depression Scale and participation in activities score of the Utrecht Activities List.
DISCUSSION
The present study shows that the CONECSI intervention, a multidisciplinary cognitive behavioural programme for coping with CNSCIP, had favourable long-term outcomes on the primary outcomes pain intensity and pain-related disability, and the secondary outcomes anxiety and participation in activities. This study adds long-term outcomes (at 9 and 12 months) to the earlier reported short-term results of the CONECSI trial (22).
Overall, there was no significant difference between the scores at 12 months and the scores immediately after intervention (t2; 3 months), confirming the hypothesis that improvements during the intervention would be maintained at follow-up, although the patterns of scores were variable over time for 3 of the 6 outcome variables. Only pain intensity showed a further decrease after the end of the intervention at 12 months. Although the scores changed in the right direction, no significant change over time was found for depression and life satisfaction. This is in line with the previously reported short-term results (22).
Long-term outcomes
A decrease of pain intensity is usually not a focus of cognitive behavioural programmes, but it is a common “side effect” of improvements in physical and psychological functioning (16). In this study we indeed found a decrease in pain intensity, together with improvements in pain-related disability, anxiety, and participation in activities. This study confirms the conclusions of earlier studies (17, 19) that comprehensive psychosocial pain treatment programmes are promising treatment options for persons with disabilities and pain.
Norrbrink Budh et al. (17) found also change of anxiety and no change in life satisfaction after the intervention, but in contrast to our study they found change of depression, and no change in pain intensity at 12 months follow-up. Nicholson Perry et al. (19) found a reduction in anxiety in their treatment group, and a trend towards improvement on pain intensity and depression at 1 month post-treatment, but the depression scores returned to pre-treatment levels at 9 month follow-up. Both anxiety and depressed mood were addressed in our CBT programme, but maybe it is easier to modify feelings of anxiety than depressed mood by CBT. Life satisfaction was measured using a questionnaire on satisfaction with various life domains, including satisfaction with vocational situation and sexual life, which might explain the lack of change of life satisfaction scores in this study. Dorstyn et al. (38) found in a meta-analysis that most treatment effects of CBT for the management of psychological outcomes following SCI were minimal or not sustained at follow-up. The results of the current study showed little relapse, maybe because of the booster session 3 weeks after the final group session. Nevertheless, further attention for relapse prevention is required (19). Booster session(s) or comeback session(s) in the third to ninth month after finishing the intervention might be helpful.
Strengths and limitations of this study
This is one of the few longitudinal studies reporting long-term effect of CBT for coping with CNSCIP. The loss to follow-up was minimal and the use of random coefficient analysis allowed the inclusion of all participants in the statistical analyses. However, the sample size was small. There were only 29 persons analysed in this study, although the repeated measurements increased the statistical power of the analyses. Another limitation is that, because of the use of a waiting-list control group, it was not possible to perform the current analyses in a controlled design. The results of this study should therefore be considered exploratory and in need for further confirmation. There is a need for further research utilising larger samples and longer term measurements to study the effectiveness of CBT-based interventions for CNSCIP.
Implications
Our findings highlight the potential of cognitive behavioural programmes to learn people with SCI cope with neuropathic pain. Our finding of a long-term decrease of pain intensity needs confirmation, but is encouraging and might make the CBT approach more attractive to patients. The programme focussed on CNSCIP, but it can easily be adapted to include other types of pain. CBT is easy to implement in clinical practice, since it is an accepted treatment in other diagnostic groups. More research is needed on treatment modalities, i.e. individual or group, or internet-based, and on timing, i.e. soon after persons with SCI start experiencing neuropathic pain, or only after pharmaceutical and non-pharmaceutical treatments show insufficient pain relief.
Acknowledgements
The authors would like to thank the participants in this study, the participating Dutch rehabilitation centres, and the co-trainers in these centres: Lilian Pfennings (De Hoogstraat Rehabilitation, Utrecht); Conny Overdulve (Adelante Zorggroep, Hoensbroek); Wim van de Vis (Rehabilitation Center Het Roessingh, Enschede); Hilde Schors and Nicole Vrijens (Rijndam Rehabilitation Center, Rotterdam).
This study was performed within DALI for PAIN, a national programme that focuses on neuropathic pain care optimisation. DALI for PAIN is an initiative of Pfizer. This project was supported by an unrestricted grant from Pfizer, reference number 007-04.
References
