Hannah L. Barden, BappSc(OT)1,2, Ian J. Baguley, MBBS, PhD1,4, Melissa T. Nott, PhD1,3 and Christine Chapparo, PhD2
From the 1Brain Injury Rehabilitation Service, Westmead Hospital, and 2Faculty of Health Sciences, The University of Sydney, Sydney, 3School of Community Health, Charles Sturt University, Albury, NSW and 4Sydney Medical School, The University of Sydney, Sydney, Australia
OBJECTIVES: Evaluate upper limb performance in adults receiving botulinum toxin-A injections for upper limb spasticity using Dynamic Computerised Hand Dynamometry and current clinical measures.
DESIGN: Pre-test/post-test clinical intervention study.
Subjects/Patients: Twenty-eight participants with spasticity following acquired brain injury.
METHODS: Botulinum toxin-A effects were measured 4 weeks post-injection using Dynamic Computerised Dynamometry. Current clinical upper limb performance measures spanning the International Classification of Functioning, Disability and Health domains were also conducted at the Body Function and Structure (Modified Ashworth Scale; Tardieu Scale) and Activity (Action Research Arm Test; Goal Attainment Scaling; patient disability and carer burden scales) domains. Dynamic Computerised Dynamometry hand performance measures were correlated with performance on current clinical measures.
RESULTS: Significant post botulinum toxin-A changes were identified on current clinical measures and the Dynamic Computerised Dynamometry. Dynamic Computerised Dynamometry results correlated with current clinical measures demonstrating functional upper limb change across the Body Function and Structure and Activity domains.
CONCLUSION: Dynamic Computerised Dynamometry sensitively assesses the effects of botulinum toxin-A on upper limb spasticity during a simple, functionally based, grasp and release task. Unlike current measures, the Dynamic Computerised Dynamometry provides information across the Body Function and Structure and Activity domains of the International Classification of Function.
Key words: rehabilitation; muscle spasticity; upper extremity; muscle strength dynamometer; adult.
J Rehabil Med 2014; 46: 00–00
Corresponding address: Hannah L. Barden, Researcher, Brain Injury Rehabilitation Service, Westmead Hospital, PO Box 533, Wentworthville, NSW, Australia, 2145. E-mail: hbar1204@uni.sydney.edu.au
Accepted Nov 11, 2013; Epub ahead of print Feb 14, 2014
INTRODUCTION
The primary action of the upper limb (UL) in the performance of everyday tasks is to position the hand to allow an individual to grasp, release and manipulate objects. Following upper motor neuron (UMN) lesions, UL motor function may be limited by negative features (e.g. weakness, reduced motor control and fatiguability) and/or positive features (e.g., spasticity, muscle overactivity and clonus) (1). This latter group of features/symptoms are amenable to treatment with botulinum toxin A (BTX-A) (2).
There is strong evidence for the efficacy of BTX-A to treat UL spasticity at the Body Function and Structure level of the International Classification of Functioning, Disability and Health (ICF) (3). Commonly used Body Function and Structure level measures used in clinical practice include the Modified Ashworth Scale (MAS) (4) and Tardieu scales (5). In multiple studies, BTX-A has been shown to reduce involuntary muscle overactivity as measured by both the MAS (6–8) and the Tardieu scale (9, 10). However, given the primary purpose of the UL, it is more important to measure the efficacy of BTX-A during the performance of a functionally based UL task.
Despite anecdotal reports, data supporting the role of BTX-A in producing significant active functional change in UL performance is limited and few clinical measures are available to document such change at the Activity or Participation level of the ICF (11). For example, the Action Research Arm Test (ARAT) (12) and the modified Goal Attainment Scale (GAS) (13) both assess at the Activity level, whereas global measures such as the Assessment of Quality of Life (AQoL) (14) assess at the Participation level. Patient/client self-report measures are also used to provide a key insight into perceived outcomes including the Global Assessment of Benefit (GAB) (8). Recent clinical studies have demonstrated that GAS and GAB are sensitive to change when used to assess focal spasticity interventions, while the AQoL is insensitive to improvement (8). Demonstrating the effect of spasticity management on UL performance across all 3 ICF domains has proven difficult, due to the unrelated nature of each domain (11). Evidence of functional improvement (towards active or passive functional goals) (15) following BTX-A injection is necessary to enable access to funding for therapy and to better evaluate the ‘black box’ of spasticity management (16).
Therefore, to address the clinical need for measuring functional changes observed following BTX-A injections, a novel approach was developed to measure motor performance during a simulated functional grasp and release task (17). The psychometric properties of Dynamic Computerised hand Dynamometry (DCD) have been established, with fair/good to excellent levels of test-retest reliability (18) and moderate to good degree of construct, concurrent and predictive validity (19). However, further investigation is required to assess the clinical utility of the measure following spasticity intervention. This study aimed to evaluate UL performance changes in adults with UMN syndrome who received BTX-A injections for UL muscle spasticity in an outpatient clinical setting by: i. Evaluating change in UL performance following BTX-A injections as measured by DCD and current clinical measures. ii. Mapping observed changes to the Body Function and Structure and Activity domains of the ICF.
METHODS
This pre/post-test study was approved by the local Institution Human Research Ethics Committee. Participants provided written informed consent prior to study involvement.
Participants
Participants met the following inclusion criteria: age > 17 years, first onset of ABI, positive UMN features of greater than 3 months duration and sufficient grip strength in the affected UL to hold the dynamometer (minimum = 0.75kg). Exclusion criteria included: bilateral UL neurological disease, other causes of UL weakness and inability to understand instructions.
Instruments
A Biometrics G100 Precision Dynamometer (Jamar configuration) (Biometrics Ltd, Cwmfelinfach, Gwent,UK) was used to collect DCD data. The raw dynamometer signal was sampled at 400 Hz and amplified through an amplifier (model: ML142) (ADInstruments, Bella Vista, Australia) to a PowerLab 26 (model: ML856) (ADInstruments, Bella Vista, Australia). Real-time data was displayed on a computer using LabChart 7.0.2 software (ADInstruments, Bella Vista, Australia).
Procedure
Participants were assessed on the day of and approximately 4 weeks after BTX-A injection. All assessments were conducted by two trained occupational therapists.
Dynamic Computerised Hand Dynamometry
Dynamometry data was collected with participants seated in the standard testing position of the American Society of Hand Therapists (20) with two minor modifications: First, the elbow and forearm were supported on the armrest of the chair or wheelchair (consistent with the Southampton protocol (21)). Second, either the second or third Jamar position was used to assess power grip, with position 3 used for larger hands (22). A static wrist position was self-selected by participants for testing.
Participants performed a series of grasp and release cycles in the power grip position. The dynamometer was re-zeroed prior to each participant assessment. Real-time visual feedback of force and velocity was provided via a laptop. Three pre-test trials were conducted to ensure comprehension of the task and to gather baseline data:
Pre-trial 1: A single maximum force grasp and release cycle. Participants were given the verbal command: “squeeze as hard as you can”.
Pre-trial 2: A single maximum speed grasp and release cycle. Participants were given the verbal command: “squeeze as quick(ly) as you can”.
Pre-trial 3: A single grasp and release cycle integrating maximum force and maximum speed. Participants were given the verbal command: “now do both of those things together – squeeze as hard and as quickly as you can”.
After successful completion of the pre-test trials, participants completed the assessment protocol of 10 continuous grasp and release cycles using the integrated maximum force and maximum speed task from Pre-trial 3. The protocol was completed first with the non-injected hand followed by the injected hand.
Measurement instruments
A battery of measurement instruments were selected to encompass the Body Function and Structure and Activity levels of the ICF. These were selected from published measures in spasticity research with a view to collecting both objective and self-report information. These measures, outlined below, were collected by two trained occupational therapists who were blinded to subsequent injections.
Body Function and Structure level measurements
MAS and Tardieu Scales. The MAS and Tardieu Scales were measured at the elbow, wrist and fingers using standardised protocols (4, 5). The MAS scores for the elbow, wrist and fingers of the affected hand were summed to give a “Composite Spasticity Index” as described by Francis et al. (2, 23). An extension of this concept was applied to calculate a “Tardieu Spasticity Angle Composite Index” by summing the Tardieu spasticity angles at the elbow, wrist and fingers.
Michigan Hand Outcomes Questionnaire (MHQ). The MHQ (24) is a self-reported measure rating hand performance for both the affected and non-affected hand across 6 sub-categories. This study used the pain sub-scale of the MHQ to measure pain frequency and severity.
Activity and Participation level measurement
ARAT. Global UL performance was assessed using the ARAT subscales of grasp, grip, pinch, and gross movements, producing a score range of zero (inability to perform any part of the task) to 57 (full task completion) (25). Due to large within-sample variability on this measure, the sample was stratified into Low and High ARAT score groups based on pre-injection ARAT scores per Shaw et al. (26). Participants scoring ≤ 3 were stratifed as the “Low ARAT group” and those who scored 4–57 were the “High ARAT group”, combining the two categories of patients defined by Shaw et al. as “some retained function”.
GAS. GAS has been adapted for use with UL spasticity populations (13). The GAS measures goal attainment on a 5-point ordinal scale (worse than baseline; baseline performance; anticipated level of achievement; a little more than expected; much more than expected), in relation to a specific goal negotiated between the patient and the therapist. In this study, two collaborative functional task related goals were set with individual participants and/or their carers. BTX-A injection strategies were designed around maximising the likelihood that these goals would be achieved.
Patient Disability (PDS) and Carer Burden Scale (CBS). The PDS and CBS (27) measure perceived UL performance from the participant and the carer perspectives (if the participant required a carer). Both the PDS and the CBS are measured on a 5-point Likert scale (0 = no disability/carer burden; 1 = mild disability/carer burden; 2 = moderate disability/carer burden; 3 = severe disability/carer burden; 4=maximum disability/carer burden) according to performance on UL tasks such as dressing the UL or cutting fingernails.
Perceived treatment benefit
GAB. The overall perceived benefit from the BTX-A injections was reported subjectively by the participants/carers and the clinician. Ratings were made on a 5 point Likert scale (much worse; worse; same; some benefit and great benefit) (8) based on how the person rated the overall benefit received to the arm since the last injection.
Data processing
DCD data were processed off-line using LabChart 7.0.2 and analysed using SPSS Version 20, per Barden et al. 2012 (18, 19). The Force and Force velocity curves produced by participants’ consecutive grasp and release cycles were processed and elements of hand performance extracted from the data. These elements included: Maximum and Minimum Isometric Force measured in kg; Voluntary and Involuntary Isometric Grip Work measured (kg.s); Contraction and Relaxation duration measured in (s); and Maximum and Minimum Force Velocity (kgs-l).
Maximum Isometric Force (Fmax) occurs at the peak of each grasp cycle, while Minimum Isometric Force (Fmin) is the lowest magnitude of force applied to the dynamometer at the end of the release component of each cycle (17). Isometric Grip Work (28), represented by the area under the Force Curve, is composed of Voluntary and Involuntary grip work. Voluntary grip work is the task-specific effort put towards the task of grasp and release, while involuntary grip work is the non-purposeful component of motor effort not directed towards the grasp and release task (18, 19). Contraction and relaxation duration represent the time taken to perform either the grasp or release phase of the cycle. Contraction duration is measured as the time taken between one Minimum Force (Fmin) marker and the next Maximum Force (Fmax). Similarly, Relaxation duration is the time taken from one Maximum Force (Fmax) marker to the next Minimum Force (Fmin) marker. The maximum and minimum rate of force generation in the contraction (Max Fvel) or release phase (Min Fvel) were derived from the Force Velocity (Fvel) Curve (17).
Data analysis
Per protocol, DCD grasp and release data from the central 8 of 10 completed cycles were processed (18, 19) for Fmax, Fmin, Voluntary and Involuntary Isometric Grip Work, Max Fvel, Min Fvel, Contraction and Relaxation duration.
Change in UL performance following BTX-A injections was analysed using Wilcoxon sign-ranks test (z) for ordinal or skewed data. Significance level was set at p < 0.05 for all paired comparisons. Effect sizes (ES) were calculated using the method: ES = z/√n (where n = number of matched pairs) as described by Corder & Foreman (29). ES was interpreted as per Cohen in 1988 (30) where 0.2 represents a small, 0.5 represents a medium and 0.8 represents a large ES (31).
The relationships between DCD measures and existing clinical measures of UL performance were calculated using Spearman’s rank order correlations. Post-injection data was used for all correlations which were considered to have a: good to excellent relationship above 0.75; moderate to good relationship between 0.50 and 0.75; fair relationship between 0.25 and 0.50, and little or no relationship between 0.00 and 0.25 (32).
RESULTS
The demographics of the 28 adults with ABI who participated in this study are displayed in Table I.
|
Table I. Participant demographics |
|
|
Demographic variables |
ABI n = 28 |
|
Age in years: mean (SD) |
51 (17) |
|
Sex: (n) male:female |
15:13 |
|
Hand dominance pre injury/event: (n) right:left |
25:3 |
|
Hand dominance post injury/event: (n) right:left |
17:11 |
|
Change in hand dominance (n) |
11 |
|
Diagnosis: (n) stroke traumatic brain injury |
22 6 |
|
Years post injury/event: median (range) |
2.5 (0.5–39) |
|
BTX-A dosage: Dysport:Botox (n) |
15:13 |
|
Mean dosage: mean (range) Dysport (u) Botox (u) |
740 (500–1200) 200 (25–400) |
|
ABI: acquired brain injury; SD; standard deviation. |
|
Botulinum toxin-A related change
Participants demonstrated statistically significant change in UL performance following BTX-A injection on assessment using the DCD and clinical measures. These are outlined below according to the ICF domains in Table II.
Body Function and Structure domain. At the Body Function and Structure level, significant reductions in spasticity were measured by the MAS and the Tardieu spasticity angle composite indices with medium to large ES (see Table II; ES = 0.75 and 0.74 respectively). Pain severity reduced significantly following BTX-A injection with a medium ES (ES = 0.69), however, pain frequency did not show significant change post injection.
Activity domain. In the Activity domain, significant changes were found on level of disability (PDS) and goal attainment (GAS; see Table II). The greatest post BTX-A change in the ICF Activity domain was measured by the GAS, with a 33% improvement and a medium to large ES (ES = 0.78). Self-reported disability with the PDS demonstrated significant reduction following BTX-A with a medium ES, however, a corresponding reduction in carer burden was not found. Participants in neither the “Low” nor “High” ARAT group demonstrated change in UL performance. The pre and post injection median scores of the “Low” group remained at zero. The “High” ARAT group achieved a non-significant 4-point median improvement following BTX-A injection (refer to Table II).
|
Table II. Pre/Post botulinum toxin A injection upper limb changes |
|||||
|
Variable |
Pre injection n = 28 Median (IQR) |
Post injection n = 28 Median (IQR) |
z |
p |
Effect size |
|
Body Structure & Function |
|||||
|
Modified Ashworth Scale, composite |
4 (3.4) |
2.5 (2.9) |
–3.97 |
0.001 |
0.75 |
|
Tardieu Spasticity Angle, composite |
118 (39) |
60 (90) |
–3.53 |
0.001 |
0.74 |
|
Pain frequency |
4 (2) |
5 (1) |
–0.61 |
0.54 |
– |
|
Pain severity |
3 (1) |
2 (2) |
–2.07 |
0.04 |
0.69 |
|
Activity |
|||||
|
Goal Attainment Scale |
37.6 (0.1) |
50.0 (13.2) |
–4.12 |
< 0.001 |
0.78 |
|
Patient Disability Scale |
12 (9) |
9 (15) |
–2.33 |
0.02 |
0.45 |
|
Carer Burden Scale |
2 (6) |
1 (5) |
–1.51 |
0.13 |
– |
|
Low ARAT group (n = 11) |
0 (0) |
0 (0) |
–0.82 |
0.41 |
– |
|
High ARAT group (n = 16) |
19 (22) |
23 (23) |
–0.43 |
0.67 |
– |
|
Self-reported benefit |
|||||
|
GAB: clinician |
3 (0) |
4 (1) |
–4.62 |
< 0.001 |
0.89 |
|
GAB: participant |
3 (0) |
4 (1) |
–4.69 |
< 0.001 |
0.94 |
|
DCD |
|||||
|
Maximum Force, kg |
8.1 (4.1) |
4.9 (6.0) |
–2.28 |
0.02 |
0.44 |
|
Minimum Force, kg |
3.1 (2.4) |
2.4 (1.5) |
–2.52 |
0.01 |
0.48 |
|
Maximum Force Velocity, kgs–1 |
19.2 (28.2) |
10.1 (22.2) |
–1.63 |
0.10 |
– |
|
Minimum Force Velocity, kgs–1 |
–14.1 (26.6) |
–7.5 (24.7) |
–2.38 |
0.02 |
0.46 |
|
Voluntary Work, % |
61.8 (19.9) |
75.2 (20.9) |
–2.38 |
0.02 |
0.46 |
|
Involuntary Work, % |
38.2 (19.9) |
24.8 (20.9) |
–2.38 |
0.02 |
0.46 |
|
Contraction duration, s |
0.46 (0.37) |
0.48 (0.24) |
–1.99 |
0.05 |
0.38 |
|
Relaxation duration, s |
0.65 (0.49) |
0.52 (0.25) |
–3.39 |
0.001 |
0.65 |
|
p < 0.05. Wilcoxon signed ranks tests. GAB: Global Assessment of Benefit; ARAT: Action Research Arm Test; kg: kilograms; Fvel: Force Velocity; kgs–1: kg per second; effect size: 0.2 = small; 0.5 = medium and 0.8 = large; IQR: interquartile range. |
|||||
Perceived treatment benefit. Benefit to the UL was reported by both the participant and the clinician using the GAB. A median one point improvement was achieved approximately 4 weeks following UL BTX-A injections, representing a change from “Same” to “Some benefit”. Perceived treatment benefit was associated with a large ES (ES = 0.89–0.94).
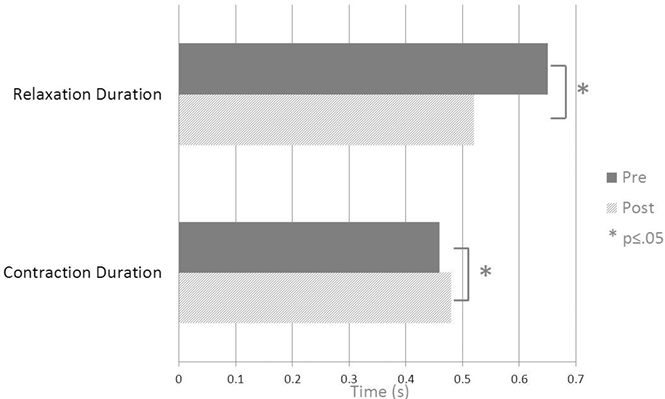
Dynamometry. Seven of the 8 DCD components demonstrated significant change following BTX-A injections (Voluntary and Involuntary Isometric Grip work; Fmax and Fmin; Min Fvel; Contraction and Relaxation duration). Participants demonstrated improved motor speed, being able to release the dynamometer 20% faster following BTX-A injection (relaxation duration: median 0.65 s down to 0.52 s with a medium effect size of 0.65; see Table II and Fig. 1). This effect was accompanied by a 0.72 kg median improvement in between-contraction relaxation of the hand. Participant’s ability to generate force (Fmax) decreased by a median of 3.2 kg following BTX-A injections. Despite this reduction in strength, Voluntary Isometric Grip work improved by 14%; that is, participants were able to direct a greater proportion of their voluntary effort towards the grasp and release task.
Relationship between Dynamometry and clinical measures
Relationships between DCD and current clinical measures were statistically significant across the Body Function and Structure and the Activity domains of the ICF for all measures excluding GAS (Table III). At the Body Function and Structure level of the ICF, a lower score on the Modified Ashworth and Tardieu scales (indicating reduced spasticity) correlated with greater Voluntary Grip Work. Higher MAS and Tardieu scores correlated with greater residual spasticity as measured by higher Fmin values (i.e. reduced ability to relax grasp). In the Activity domain of the ICF, higher ARAT scores correlated with higher Fmax, increased speed of force generation (Max Fvel), and shorter Contraction and Relaxation duration. Improved carer burden and disability scales scores also correlated with shorter Contraction and Relaxation duration.
|
Table III. Correlation between dynamic computerised hand Dynamometry (DCD) and existing clinical measures |
|||||
|
DCD |
MAS |
Tardieu |
ARAT |
CBS |
PDS |
|
Voluntary Grip Work, % |
–0.42* |
–0.49* |
0.07 |
–0.27 |
–0.14 |
|
Maximum Force, kg |
0.09 |
0.16 |
0.50* |
–0.27 |
–0.10 |
|
Minimum force, kg |
0.45* |
0.47* |
0.10 |
–0.01 |
0.08 |
|
Contraction duration, s |
0.13 |
0.14 |
–0.54* |
0.62* |
0.48* |
|
Relaxation duration, s |
0.04 |
0.22 |
–0.56* |
0.59* |
0.48* |
|
Max Fvel, kgs–1 |
–0.07 |
0.09 |
0.63* |
–0.38 |
–0.23 |
|
Min Fvel, kgs–1 |
–0.01 |
–0.19 |
–0.56* |
0.36 |
0.20 |
|
*p < 0.05. Spearman’s rank order correlation presented. Fvel: Force Velocity; MAS: Modified Ashworth Scale (composite value); Tardieu: Tardieu Spasticity Angle (composite value) ARAT: Action Research Arm Test; CBS: Carer Burden Scale; PDS: Patient Disability Scale. |
|||||
Discussion
BTX-A injections are a well-established, safe and effective intervention for reducing involuntary muscle activity after UMN injury (3, 8). This study aimed to investigate the efficacy of BTX-A injections at the ICF Body Function and Structure, and the Activity domains, and to correlate these measures with DCD, a novel task based procedure for measuring grasp and release (17).
The observed hand performance changes achieved in this study at the Body Function and Structure domain are supported by the existing literature. Within this domain, resistance to passive movement at slow (MAS) and fast velocities (Tardieu Scale) reduced with BTX-A, a finding that has been extensively demonstrated in the literature (8–10). In addition, the changes measured by the MAS and the Tardieu spasticity angle composite indices were similar in their ES. For functional tasks involving multiple segments of the upper-limb, composite scores assessing spasticity of the arm as a multi-segmental functional unit may relate better to Activity level tasks that typically rely on integrated movement of multiple UL segments, e.g. reach and grasp.
In addition to the observed reduction in muscle spasticity, self-reported pain severity reduced significantly. Other studies have demonstrated mixed results for UL pain-related changes following BTX-A injections, with similar numbers of studies demonstrating beneficial effects (33, 34) compared to no reduction in spasticity-related pain (8, 27, 35). Participants who reported pain did not always relate this pain to muscle spasticity, however pain typically impacted on performance of daily activities and on ability to sleep.
In contrast to the well reported changes in Body Function and Structure, it has proven much more challenging to scientifically demonstrate BTX-A associated changes at the ICF Activity and Participation levels (3, 26). This study contributes to the emerging evidence supporting functional change following UL BTX-A injections, as significant change occurred on nearly half of the Activity domain measures. Significant improvements in self-selected goals (measured by the GAS) were found, with similar or greater goal attainment compared with previous literature (8, 36). In this study, injection strategies were chosen with direct reference to the patient’s goals, rather than purely on the injecting physician’s standard practice, and the improved GAS outcomes could be associated with this individualised goal focused injection strategy (16). A similar argument may be made for the observed improvements in pain. Such an individualised goal focused injector strategy is in accordance with recommendations of the International Consensus Statement (3) to optimise functional outcomes post UL BTX-A injections.
Participants reported lower disability for UL tasks and activities following BTX-A injection as measured by the PDS and also reported benefits on the GAB. A client centred approach to UL spasticity management is important to facilitate optimal UL outcomes from the participant’s perspective, making it advantageous for self-reported measures such as the GAB to be included in an UL spasticity management outcome battery (3). In addition, carers reported a mild reduction in carer burden following injection, however, this change did not reach statistical significance.
In the ICF Activity domain, ARAT scores did not change for the Low ARAT groups and only marginally improved (a non-significant 4-point improvement) in the High ARAT group following BTX-A. This finding contrasts with previous literature, where the majority of studies have demonstrated positive change on the ARAT following BTX-A (37, 38). However, Suputtitada & Suwanwela (39) demonstrated in a dose ranging study both an improvement and a reduction in ARAT scores that were dependent on BTX-A dosage. A number of reasons present themselves to explain the current negative finding. Group ARAT scores in this study varied widely (total ARAT pre-injection scores ranged from zero to 57), with significant floor effects in keeping with previous research (11, 19, 40). Finally, the movements required of different ARAT subtests (e.g. pinch) may have been irrelevant to the person’s self-selected goals, and were therefore not addressed by the BTX-A injector strategy.
The pattern of spasticity change using current clinical measures was paralleled by changes in 7 of the 8 DCD elements of hand performance following BTX-A injection. Of note, BTX-A significantly reduced the time taken to release the dynamometer (20% faster relaxation) and also reduced residual inter-cycle spasticity (Fmin) by 23%. Despite a reduction in maximum force generation (Fmax) of around 40%, the combined changes produced a statistically significant 14% improvement in the amount of voluntary effort that participants could direct towards the task.
To address the hypothesis that DCD is able to measure across the Body Function and Structure and Activity domains of the ICF, correlational analysis demonstrated a number of key associations between DCD and UL spasticity measures. Force components of the DCD (residual inter-cycle spasticity and Voluntary Isometric Grip Work) showed moderate correlations with measures at the Body Function and Structure level (MAS and Tardieu composite indices). In contrast, time based elements of the DCD correlated with the ICF Activity level (ARAT, CBS and PDS). The moderate relationship between the timed DCD elements and the CBS and PDS support the idea that the efficiency of UL performance is an important consideration in self or carer-reported reduction in disability and carer burden. In “real-world” situations, the efficient completion of a task requires the correct amount of force to be applied, in the correct sequence and with appropriate timing (41). Such timing issues are not addressed with impairment measures (such as the MAS and Tardieu), however, are captured by the DCD. In this study, higher force generation (Fmax) and longer contraction and shorter relaxation durations were moderately correlated with higher ARAT scores, suggesting that greater motor control and speed of muscle recruitment were associated with greater hand performance.
This paper highlights a number of advantages of the DCD approach to measuring the effects of spasticity on the UL. DCD data provides objective, ratio level measurement of force, velocity and time based elements observable during a simulated functional grasp and release task. Further, DCD simultaneously measures negative and positive features of the UMN syndrome (19) (in keeping with recommendations of the International Consensus Statement (3)). This is an important consideration, given the central role of negative UMN features as a prognostic sign in those with UMN lesions (42). Finally, the correlational analysis undertaken in this study suggests that DCD sub-components validly assess the effect of UMN syndromes across the separate ICF constructs of the Body Function and Structure, and Activity domains (11).
DCD is a physiological measure which is able to demonstrate UL change following BTX-A injection using repeated grasp and release of a dynamometer to represent a common functional task. Unlike other Activity domain measures, the DCD has very small floor effects with participants requiring a minimum of 0.75 kg of force to hold and grasp the dynamometer. An advantage of DCD is that it provides a non-invasive, quantitative measure of the underlying physiology of hand performance during a simulated functional grasp and release task. This advantage over current clinical measures, has application for both clinical and research purposes.
The limitations of this study include the small number of participants and the heterogeneity of the sample (containing both stroke and TBI participants). While this latter feature may be a research design limiting factor, it is consistent with clinical outpatient services. This research primarily focused on motor elements of the UMN syndrome. Future research may consider investigating other components that adversely impact on UL performance, such as motor planning and sensory processing that may also correlate with DCD measures, or other novel outcome measures such as the Tardieu Spasticity Composite score. Finally, research is required to look at the impact of BTX-A at the Participation domain of the ICF.
In conclusion, DCD was shown to demonstrate improvement following BTX-A injections consistent with current clinical measures, and correlated with current clinical measures demonstrating functional UL change across the Body Function and Structure, and the Activity ICF domains. In particular, force components of the DCD correlated with Body Function and Structure domain and timed components correlated with measures of the Activity domain. DCD provides potential advantages over current clinical measures in being able to simultaneously measure ratio level data for both the positive and negative features of UMN syndrome and bridges the gap between the Body Function and Structure, and the Activity domains of the ICF.
Acknowledgement
This paper is derived from data collected as part of an investigator initiated study supported by Ipsen Australia. H. L. Barden’s PhD candidature is supported by an Australian Postgraduate Award, The George Burniston Cumberland Foundation Fellowship 2011 and the Helga Pettitt Faculty of Health Sciences Postgraduate Study Award 2011. The funding bodies had no influence on the interpretation of data nor the conclusions drawn.
Conflicts of interest: Barden: Honoraria from Ipsen and Allergan – Modest; Baguley: Research Grant from Ipsen – Significant, Advisory Board for Ipsen and Allergan – Modest, Honoraria from Ipsen and Allergan – Modest; Nott: Advisory Board for Allergan, Honoraria from Ipsen and Allergan – Modest; Chapparo: None.
REFERENCES
