Muhammed Kılınç, PT, PhD1, Ayşe Livanelioğlu, PT, PhD1, Sibel Aksu Yıldırım, PT, PhD1 and Ersin Tan, MD2
From the 1Department of Physiotherapy and Rehabilitation and 2Department of Neurology, Hacettepe University, Faculty of Health Sciences, Ankara, Turkey
OBJECTIVE: To evaluate and compare the effects of transcutaneous electrical nerve stimulation (TENS) therapy on pain intensity and functional capacity in patients with either peripheral neuropathic pain or central neuropathic pain.
METHODS: A total of 40 patients (20 with peripheral neuropathic pain and 20 with central neuropathic pain) were included in this study. Pain severity, pain quality, and functional capacity were assessed with a visual analogue scale, a neuropathic pain scale, and the Brief Pain Inventory, respectively. A pre–post-treatment design was used. Semmes Weinstein monofilaments were used to evaluate touch sensation. Mild pressure was applied to provoke static mechanical allodynia. The presence of any severe and sharp pains upon pricking was considered a positive sign for hyperalgesia. The 2 groups of patients received 20/30-min sessions of TENS therapy over 4 weeks.
RESULTS: No significant differences were found between the 2 groups regarding the pre-treatment values for visual analogue scale, neuropathic pain scale, and Brief Pain Inventory. The pain parameters in both groups were significantly decreased by TENS therapy for 4 weeks (p < 0.05). The group with peripheral neuropathic pain presented more overall improvements than the group with central neuropathic pain (p < 0.05).
CONCLUSION: TENS therapy can be used successfully in clinical practice as an alternative or supportive treatment.
Key words: peripheral neuropathic pain; central neuropathic pain; transcutaneous electrical nerve stimulation (TENS); physiotherapy.
J Rehabil Med 2014: 46: 00–00
Correspondence address: Muhammed Kılınç, Hacettepe University Faculty of Health Sciences Department of Physiotherapy and Rehabilitation Samanpazari, Ankara, Turkey. E-mail: fzt.muhammedkilinc@gmail.com, muhammedkilinc@yahoo.com
Accepted Nov 21, 2013; Epub ahead of print Feb 18, 2014
Introduction
Neuropathic pain is described as “pain arising as direct consequence of a lesion or disease affecting the somatosensory system” (1). Cases of neuropathic pain are categorized into two main groups based on the primary aetiology of the lesions and the underlying neurobiological mechanisms. These groups are:
• conditions of peripheral neuropathic pain (PNP), which originate from peripheral nervous system lesions, such as traumatic injuries to major peripheral nerves;
• conditions of central neuropathic pain (CNP), which originate from lesions affecting the central nervous system, such as spinal cord injuries (2).
PNP and CNP syndromes are characterized by similar clinical features. In both syndromes, comprehensive neurological examinations reveal motor, sensory and autonomic neural dysfunction (3). Patients usually experience sensations of chill, tingling, itching, pricking and numbness in addition to pain. Moreover, they experience abnormal sensations, such as sensations that feels like electrical shock or burns, which worsen when the numb areas are touched (4, 5).
In its guidelines for the management of neuropathic pain, the European Federation of Neurological Societies states that “despite an increasing number of studies, therapy for neuropathic pain is not yet satisfactory’’. No therapeutic drugs or drug groups have proven effective for treatment of patients with neuropathic pain. Current treatment modalities provide only 30–50% pain relief, at best. Importantly, total abolition of pain is not the ultimate aim of treatment. The goal of treatment is for pain to be reduced to a level that can be handled by the patient (6); thus, novel alternative treatments are required for patients with neuropathic pain.
Transcutaneous electrical nerve stimulation (TENS) is a non-invasive technique that delivers pulsed electrical currents through the intact surface of the skin to activate peripheral nerves. TENS-induced afferent activity inhibits transmission of nociceptive information throughout the central nervous system and leads to hypoalgesia (7). TENS can be implemented in various combinations of frequencies and intensities to alleviate pain (8, 9).
The gate-control theory of pain, originally developed by Melzack & Wall (10), describes the basis for utilization of TENS as a therapeutic tool. TENS-mediated neural stimulation causes release of pain-suppressing opioids that then alter pain perception (7, 9–11).
There are only a limited number of studies that use TENS for treatment of neuropathic pain. Dubinsky (12) reported 2 class II studies that compared TENS with sham-TENS and 1 class III study that compared high-frequency muscle stimulation with TENS for relief of pain associated with mild diabetic peripheral neuropathy. The studies concluded that TENS is effective for reducing pain that arises from diabetic peripheral neuropathy. Luk (13) performed a single-blind, randomized controlled trial, and found that TENS was effective in reducing pain and improving tactile tolerance in patients with neuropathic pain. Furthermore, Cuypers et al. (14) found that long-term TENS treatment improved tactile sensitivity in patients with multiple sclerosis (MS) . Taken together, these results suggest that TENS is a reasonable method to manage neuropathic pain (7).
However, there is an insufficient number of controlled clinical trials investigating the effects of TENS in patients with PNP or CNP. As described above, most of the published subject-related trials focus on diabetic neuropathic pain. Therefore, controlled clinical studies are necessary to investigate the effects of TENS therapies in patients with CNP (patients with stroke, MS, and Parkinson’s disease). Furthermore, these studies should compare the effects of TENS in patients with CNP with those in patients with PNP. The study presented here evaluated and compared the effects of TENS therapy on pain intensity and functional capacity in patients with either PNP or CNP.
Material and Methods
Patients
This study was conducted in the Department of Physiotherapy and Rehabilitation at Hacettepe University, Ankara, Turkey. The study was approved by the university ethics committee and all participants provided written informed consent.
In the period January 2010 to December 2011, all the patients who were diagnosed as having either PNP or CNP syndrome by a neurologist were invited to the study. The diagnoses were made based on patients’ history and signs and the results of neurological examinations. Demographic characteristics (age and pain duration), diagnoses, and medical histories of the patients were recorded. The dates of pain onset and the accompanying complaints (numbness, burning, etc.) were also recorded. All patients included in the study were above 18 years of age, had a Leeds Assessment of Neurological Symptoms and Signs (LANSS) pain score ≥ 12, had had neuropathic pain for at least 6 months, and were resistant to a variety of medical treatments. Any patient who reported having pain other than neuropathic pain, had unstable medical conditions, was already receiving medical treatment for neuropathic pain, had a severe systemic disorder, had a mental illness or communication problem or experienced excessive spasticity (≥ 3 according to the modified Ashworth scale) was excluded from the study.
Evaluation of pain
Pain location (pain drawing). Patients were asked to mark on a diagram of the body the areas where they perceived pain. If there was more than one painful area, each area was marked using different coloured pens to indicate the pain intensities in those areas. Only the most painful area of each patient was considered for this study (15).
Pain intensity (visual analogue scale). Pain intensities were assessed with a visual analogue scale (VAS). Patients were asked to score the most intense (maximal) pain, the least intense (minimal) pain, and the mean pain over the preceding 2 weeks. Patients also scored the pain they were experiencing at the time of evaluation (current pain). These pain measurements were scored from 0 to 10 (where 0 = no pain, and 10 = unbearable pain) (16).
Pain quality (neuropathic pain scale). Pain qualities were evaluated with the Neuropathic Pain Scale (NPS). The NPS is an instrument that assesses specific qualities of neuropathic pain, such as “intense”, “sharp”, “hot”, “dull”, “cold”, “sensitive”, “itchy”, “deep pain”, and “surface pain”. All of these items are rated on a 0–10 scale (where 0 is “no ___” or “not___”, and 10 is “the most ____ sensation imaginable”). In addition, there is an item in the NPS measuring the overall “unpleasantness” of the pain (17).
Pain interference (Brief Pain Inventory). The Brief Pain Inventory (BPI) was used to assess the effects of pain on the lives of the patients. In this questionnaire, patients were asked to rate on a scale of 0–10 the presence, intensity, and characteristics of their pain, the treatments they received, the response to treatments, and the sociocultural effects of the pain based on their experiences over the preceding week (18). Patients are also asked to rate the extent to which their pain interferes with 7 quality-of-life domains that include general activity, walking, mood, sleep, work, relationships with other persons, and enjoyment of life. These scales are bounded by the words “does not interfere” and “interferes completely” (18).
Evaluation of sensations
Light touch (Semmes Weinstein monofilaments). Semmes Weinstein monofilaments were used to evaluate touch sensation. The filaments were pressed perpendicular to the painful skin areas and their local vicinities for 1 s. The test was performed 7 times in each painful area, and the patient was asked each time whether he/she felt the filaments (19).
Warm and cold sensation (hot and cold water tubes). In order to evaluate heat sensations, tubes containing cold (5–10°C) and hot (40–45°C) water were used. Results recorded whether there was sensory loss (20).
Mechanical static allodynia. Mild pressure was applied by touching around the painful area in an attempt to provoke mechanical-static allodynia. Mild pain was recorded as a positive result. The presence of any severe and sharp pains upon pricking the areas with a pin was considered a positive sign for hyperalgesia (20).
One physiotherapist (MK) performed all of the evaluations. The evaluations were performed both at the beginning and end of treatment.
Treatment procedure. TENS therapies were applied for a total of 20 sessions for each patient. The sessions lasted for 4 weeks (5 days per week, 30 min per session). All sessions occurred at the hospital and were performed by one physiotherapist. This physiotherapist was not the evaluator. A Cefar Active XT TENS device (Cefar Medical, Malmö, Sweden) with 2 channels and 4 outlets that produced asymmetrical, biphasic square waves was used for the treatments. The square waves were modified so that they had the following characteristics: a frequency of 80 pulses per second (pps), a pulse width (duration) of 350 µs, and currents up to 60 milliamperes. The intensity of the current was increased throughout the sessions until the patients felt it was at “strong but not painful and not unpleasant” levels (7). The TENS electrodes (Dura-Stick plus 5 × 5 cm, cabled, self-adhesive) were placed diagonally around the target painful areas so that the current crossed these target areas.
Statistical analysis
Statistical analyses of the data were performed with SPSS 15.00 software. Quantitative data were expressed as means and standard deviations (SDs), and qualitative data as percentages. Wilcoxon signed-rank tests were used for within-group analyses. Mann-Whitney U tests were used for analyses that compared different groups. The level of significance was set at p < 0.05.
Results
A total of 51 patients were referred for TENS treatment by the neurologist (28 PNP, 23 CNP). After the first evaluation 11 patients (8 PNP, 3 CNP) declined to participate in the study. The main reasons were: travel problems (n = 5), non-benign pain (n = 3), more severe pain during the attachment and removal of the electrodes (n = 2) and unable to complete questionnaires (n = 1). The study design is shown in Fig. 1. There were no drop-outs from the study, and no major discomfort due to TENS treatment was reported. Only one patient (entrapment neuropathy) reported discomfort, at the time TENS was applied at the first 3 treatment sessions. A total of 40 patients (20 PNP, 20 CNP) were included in this study. The mean age and the duration of the pain were similar between the groups (Table I). The aetiologies of neuropathic pain for the groups are shown in Table I.
The patients’ painful areas were indicated using body diagrams. The distribution of pain locations in both groups are shown in Fig. 2. The two most common painful areas were feet (55%) and hands (20%) in the CNP group, and ankles (30%) and arms (20%) in the PNP group (Fig. 2). Twelve patients in the PNP group and 17 patients in the CNP group reported paraesthesia before and after the treatments. There was no important adverse effect in the treatment group; one patient described paroxysmal numbness at the first 3 treatment sessions.
At the beginning of the trial, the minimal, maximal, mean, and current pain intensities were similar between the CNP and PNP groups (Table II). Post-treatment pain intensity values were significantly lower than pre-treatment values in both groups (p < 0.05). However, the post-treatment values of the mean and current pain intensities were significantly lower in the PNP group than in the CNP group (p < 0.05) (Table II).
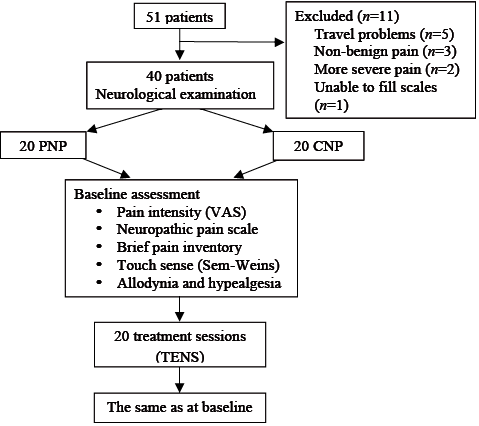
Fig. 1. Study design. VAS: visual analogue scale; PNP: peripheral neuropathic pain; CNP: central neuropathic pain; TENS: transcutaneous electrical nerve stimulation; Sem-weins: Semmes Weinstein monofilaments.
|
Table I. Demographic and sensory characteristics of patients |
|||
|
Characteristics |
PNP group (n = 20) |
CNP group (n = 20) |
p-value |
|
Age, years, mean (SD) |
51.75 (18.23) |
48.5 (18.74) |
0.645 |
|
Duration of pain, months (SD) |
24.15 (24.57) |
33.95 (27.47) |
0.135 |
|
Cause of pain, n (%) Entrapment neuropathy Hereditary neuropathy Diabetes mellitus Lumbar disc hernia Thoracic outlet syndrome Humerus fracture Cerebrovascular accident Multiple sclerosis Spinal cord injury Parkinson’s disease |
6 (30) 5 (25) 4 (20) 2 (10) 2 (10) 1 (5) |
8 (40) 6 (30) 5 (25) 1 (5) |
|
|
Light touch (Semmes Weinstein), mean (SD) |
|||
|
Pre-treatment Post-treatment |
2.6 (1.46) 2.4 (1.04) |
4.4 (1.04) 4.15 (1.26) |
< 0.05 < 0.05 |
|
Patients with allodynia and hyperalgesia, n % |
|||
|
Allodynia |
|||
|
Pre-treatment Post-treatment |
10 (50) 7 (35) |
10 (50) 10 (50) |
|
|
Hyperalgesia |
|||
|
Pre-treatment Post-treatment |
7 (35) 6 (30) |
9 (45) 9 (45) |
|
|
p-values were determined by unpaired t-tests. PNP: peripheral neuropathic pain; CNP: central neuropathic pain. SD: standard deviation. |
|||
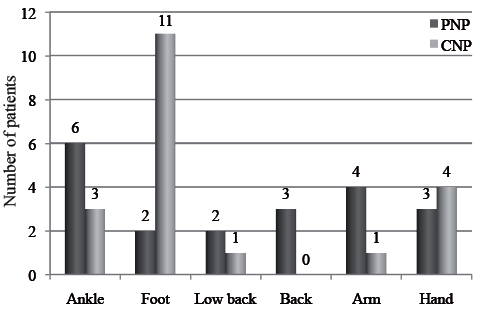
Fig. 2. Location of pain in patients with peripheral neuropathic pain (PNP) and central neuropathic pain (CNP).
|
Table II. Pre- and post-transcutaneous electrical nerve stimulation (TENS) treatment visual analogue scale values of patients with peripheral (PNP) or central neuropathic pain (CNP) |
||||||||||
|
PNP |
CNP |
Between-groups |
||||||||
|
Pre-treatment Mean (SD) [95% CI] |
Post-treatment Mean (SD) [95% CI] |
p-value |
Pre-treatment Mean (SD) [95% CI] |
Post-treatment Mean (SD) [95% CI] |
p-value |
pa |
pb |
|||
|
Mean |
7.32 (1.48) [6.3–7.8] |
4.55 (2.06) [3.7–5.7] |
0.000* |
6.90 (1.62) [5.9–7.2] |
5.9 (1.88) [5.1–6.9] |
0.006* |
0.407 |
0.034* |
||
|
Maximal |
9.05 (0.31) [8.4–9.6] |
6.75 (2.12) [5.7–7.7] |
0.001* |
8.50 (1.53) [7.7–9.2] |
7.2 (1.82) [6.3–8.0] |
0.001* |
0.235 |
0.511 |
||
|
Minimal |
3.15 (2.05) [2.1–4.1] |
1.2 (1.88) [0.3–2.0] |
0.002* |
4.10 (2.46) [2.9–5.2] |
2.95 (3.06) [1.5–4.3] |
0.038* |
0.194 |
0.087 |
||
|
Current pain |
5.50 (2.06) [4.5–6.4] |
3.00 (2.42) [1.8–4.1] |
0.003* |
6.17 (1.96) [5.2–7.0] |
4.9 (2.67) [3.6–6.1] |
0.033* |
0.359 |
0.026* |
||
|
*Wilcoxon signed-rank tests were used for within-group analyses. Mann-Whitney U tests were used for between-group analyses. The level of significance was set at p < 0.05. ap-values of pre-treatment comparisons between PNP and CNP groups; bp-values of post-treatment comparisons between PNP and CNP groups. 95% CI: 95% confidence interval; SD: standard deviation. |
||||||||||
The perceived pain qualities were assessed with the NPS. The pre-treatment pain quality values of the two groups were similar. However, the values of the “intense”, “hot”, “dull”, “cold”, and “itchy” descriptions were lower in the PNP than the CNP group after treatments (p < 0.05). According to the within-group comparisons, the “intense”, “hot”, “sensitive”, and “unpleasant” descriptions improved after TENS therapy in both groups (p < 0.05). Eight qualities (intense, sharp, hot, dull, sensitive, unpleasant, superficial, and deep) improved in the PNP group and 4 (intense, hot, sensitive, and unpleasant) in the CNP group (Table III).
|
Table III. Pre- and post-transcutaneous electrical nerve stimulation (TENS) treatment neuropathic pain scale values of patients with peripheral (PNP)or central neuropathic pain (CNP) |
||||||||||
|
PNP |
CNP |
Between-groups |
||||||||
|
Pre-treatment Mean (SD) [95% CI] |
Post-treatment Mean (SD) [95% CI] |
p-value |
Pre-treatment Mean (SD) [95% CI] |
Post-treatment Mean (SD) [95% CI] |
p-value |
pa |
pb |
|||
|
Intense |
7.325 (1.4) [6.7–8.1] |
4.55 (2.06) [3.5–5.5] |
0.000* |
6.9 (1.63) [6.1–7.6] |
5.9 (1.89) [5.0–6.7] |
0.006* |
0.407 |
0.034* |
||
|
Sharp |
4.45 (3.68) [2.9–6.4] |
3.35 (3.08) [1.9–4.7] |
0.018* |
3.9 (3.32) [2.3–5.4] |
3.25 (3.18) [1.7–4.7] |
0.101 |
0.545 |
0.822 |
||
|
Hot |
4.42 (3.81) [2.5–6.2] |
2.65 (3) [1.2–4.0] |
0.005* |
6.02 (2.30) [4.9–7.1] |
4.95 (2.37) [3.8–6.0] |
0.000* |
0.234 |
0.019* |
||
|
Dull |
4.47 (3.09) [3.2–6.1] |
1.85 (2.92) [0.4–3.2] |
0.002* |
4.42 (3.27) [2.8–5.9] |
3.8 (3.05) [2.3–5.2] |
0.090 |
0.902 |
0.037* |
||
|
Cold |
1.3 (2.68) [–0.1–2.3] |
0.7 (1.59) [–0.04–1.4] |
0.063 |
3 (3.37) [1.4–4.5] |
2.5 (2.87) [1.1–3.8] |
0.156 |
0.050* |
0.025* |
||
|
Sensitive |
3.7 (3.11) [2.4–5.3] |
2.4 (2.46) [1.2–3.5] |
0.014* |
4.65 (2.72) [3.3–5.9] |
3.35 (2.35) [2.2–4.4] |
0.021* |
0.305 |
0.21 |
||
|
Itching |
0.95 (2.06) [–0.1–1.8] |
0.25 (0.72) [–0.08–0.5] |
0.066 |
1.65 (2.35) [0.5–2.7] |
1.8 (2.38) [0.6–2.9] |
0.854 |
0.215 |
0.02* |
||
|
Unpleasant |
6.2 (2.28) [5.1–7.2] |
3.45 (3.03) [2.0–4.8] |
0.001* |
5.7 (3.36) [4.1–7.2] |
4.35 (3.01) [2.9–5.7] |
0.009* |
0.584 |
0.317 |
||
|
Deep |
5.55 (3.36) [3.7–7.0] |
3.9 (3.42) [2.3–5.4] |
0.014* |
5.7 (3.42) [4.0–7.3] |
5.2 (2.63) [3.9–6.4] |
0.123 |
0.848 |
0.160 |
||
|
Superficial |
2 (3.24) [0.5–3] |
1 (2) [0.06–1.9] |
0.027* |
1.45 (1.82) [0.5–2.3] |
1.9 (2.53) [0.7–3] |
0.40 |
0.870 |
0.156 |
||
|
*Wilcoxon signed-rank tests were used for within-group analyses. Mann-Whitney U tests were used for between-group analyses. The level of significance was set at p < 0.05. ap-values of pre-treatment comparisons between PNP and CNP groups; bp-values of post-treatment comparisons between PNP and CNP groups. SD: standard deviation; 95% CI: 95% confidence interval. |
||||||||||
BPI was used to assess how the presence and intensity of pain interfered with the patients’ general activities, moods, walking abilities, normal work, relationships with others, sleep, and enjoyment of life. The pre-treatment and post-treatment values were similar between the groups, except for the post-treatment values of “walking ability” and “enjoyment of life”. The PNP group showed greater improvement in these two items than the CNP group (p < 0.05). Both groups presented significant improvements in BPI values after TENS therapy (p < 0.05), except for the “relationships with others” quality in the CNP group (Table IV).
|
Table IV. Pre- and post-transcutaneous electrical nerve stimulation (TENS) treatment brief pain inventory values of patients with peripheral (PNP)or central neuropathic pain (CNP) |
||||||||||
|
PNP |
CNP |
Between-groups |
||||||||
|
Pre-treatment Mean (SD) [95% CI] |
Post-treatment Mean (SD) [95% CI] |
p-value |
Pre-treatment Mean (SD) [95% CI] |
Post-treatment Mean (SD) [95% CI] |
p-value |
pa |
pb |
|||
|
General activity |
6.75 (2.12) [5.7–7.7] |
4.3 (2.62) [3.0–5.5] |
0.003* |
6.675 (2.59) [5.4–7.8] |
5.3 (2.89) [3.9–6.5] |
0.003* |
0.935 |
0.239 |
||
|
Mood |
5.6 (3.03) [4.1–7.0] |
2.7 (3.18) [1.2–4.1] |
0.001* |
5.375 (3.39) [3.7–6.9] |
4.3 (2.72) [3.0–5.5] |
0.04* |
0.817 |
0.086 |
||
|
Walking ability |
4.9 (3.71) [3.1–6.6] |
2.8 (3.02) [1.3–4.2] |
0.003* |
6.15 (2.91) [4.7–7.5] |
4.9 (3.08) [3.4–6.3] |
0.011* |
0.343 |
0.04* |
||
|
Normal work |
6.05 (2.96) [4.6–7.4] |
3.8 (2.65) [2.5–5.0] |
0.002* |
5.05 (3.14) [3.5–6.5] |
4.05 (3.24) [2.5–5.5] |
0.026* |
0.332 |
0.774 |
||
|
Relationships with other people |
4.02 (2.84) [2.6–5.3] |
1.5 (2.04) [0.5–2.4] |
0.004* |
3.45 (2.74) [2.1–4.7] |
2.5 (2.5) [1.3–3.6] |
0.092 |
0.575 |
0.212 |
||
|
Sleep |
4.42 (3.27) [2.8–5.9] |
2 (2.83) [0.6–3.3] |
0.003* |
4 (3.46) [2.3–5.6] |
2.9 (2.85) [1.5–4.2] |
0.016* |
0.722 |
0.249 |
||
|
Enjoyment of life |
4.15 (3.12) [2.6–5.6] |
1.5 (2.72) [0.2–2.7] |
0.001* |
4.35 (3.12) [2.8–5.8] |
3.4 (2.48) [2.2–4.5] |
0.01* |
0.794 |
0.011* |
||
|
*Wilcoxon signed-rank tests were used for within-group analyses. Mann-Whitney U tests were used for between-group analyses. The level of significance was set at p < 0.05. ap-values of pre-treatment comparisons between PNP and CNP groups; bp-values of post-treatment comparisons between PNP and CNP groups. SD: standard deviation; 95% CI: 95% confidence interval. |
||||||||||
Pre-treatment and post-treatment light touch sensation values were different between the groups (p < 0.05). More specifically, light touch sensations improved after TENS therapy in the PNP group, but did not change significantly in the CNP group (Table I).
Six patients (30%) with PNP and 17 patients (85%) with CNP presented with loss of heat sensation at the beginning of the study. One patient (5%) from the PNP group improved after treatment, but none of the patients from the CNP group presented improvement.
Allodynia was initially detected in 10 (50%) patients of the PNP group; this number decreased to 7 patients (35%) after the TENS therapy. Furthermore, the number of patients in the PNP group with hyperalgesia decreased from 7 (35%) to 6 (30%) after TENS therapy. However, none of the CNP patients with allodynia or hyperalgesia improved after treatment (Table I).
Discussion
The most important results of this controlled clinical study were that the pain intensities in both groups decreased significantly following TENS therapy, and that the PNP group presented more obvious overall improvements than the CNP group. The mean pain intensity decreased by 38% in the PNP group and by 15% in the CNP group. Recent studies have stated that any improvements on pain scales that are greater than 30% are significant (21). The 38% decrease in mean pain intensity that we observed in the PNP group suggests that TENS therapies are viable treatments and should be considered for PNP patients.
There are a limited number of published studies that compare the effects of treatments on patients with PNP or CNP. In a study that investigated the effectiveness of oral opioid therapies in 81 patients (58 PNP, 23 CNP), Rowbotham et al. (22) found that both groups improved to a similar extent. However, the authors indicated that, even though there was a mean improvement of 36% with high-intensity treatment of the CNP group, the pain was not alleviated for many of the patients. In fact, 24% of the patients in the CNP group were unable to complete the therapy due to intense and frequent adverse effects.
We also found that light touch sensations improved after TENS therapy in the PNP group, but did not change significantly in the CNP group. One mechanism potentially underlying CNP is that the CNP patients probably could not perceive the current sufficiently because of their sensation loss, and thus received less benefit from the TENS therapy. Another possible reason for the TENS treatments being less beneficial for the CNP group may be “pain memory”. Uludağ (23) stated that functional mechanisms of the nervous system become harder to elucidate when moving from peripheral nerves to the spinal cord and then to higher cortical levels. This is because many factors, such as cognition and past experiences, are integrated with the ascending neural processes. Therefore, memories of past experiences can cause complications in the conscious assessment of the current painful conditions. In our study these complications may have been worse in the CNP group than the PNP group, which would provide another explanation for the results in the CNP group.
The significant reductions in pain intensity assessed using the VAS in the PNP group were compatible with previous reports. Almost all of these previous studies were conducted on patients who had diabetic neuropathic pain. This is one of the first studies conducted on a disease group that encompassed PNP aetiologies other than diabetes mellitus.
Importantly, we also found that TENS treatments alleviated pain in CNP patients. Although the effects of TENS therapies were more effective in the PNP patients, the 15% reduction in pain intensity observed in CNP patients is important. There are currently no fully accepted treatment modalities for CNP patients, and most of the medical treatments incur adverse effects at a significant rate. The adverse effects of current treatments for CNP can include dry mouth, sedation, imbalance, hypertension, nausea, vomiting, constipation, and weight gain (24). Importantly, no adverse effects were observed during this study. Therefore, TENS therapies may be effective treatment options for CNP patients. Previous studies have assessed the effectiveness of TENS therapies for CNP patients with spinal cord injuries (SCI). Fattal et al. (25) evaluated the efficacies of physical therapeutics used to treat neuropathic pain in patients with SCI. They stated that some practices, including TENS therapies, lacked any proven effectiveness. However, they also mentioned that TENS therapies can alleviate pain through segmental deafferentation effects.
Based on the within-group NPS score analyses, both the CNP and PNP groups had lower scores in “intense”, “hot”, “sensitive”, and “unpleasant” statements after TENS therapy. Those lower scores indicate that patients experienced less discomfort after being subjected to TENS therapies. Eight qualities were significantly improved in the PNP group, and 4 were improved in the CNP group. The between-group comparisons revealed that the TENS therapy more effectively improved pain qualities in the PNP group. These NPS results were consistent with the VAS findings that showed different levels of pain alleviation between the groups. Although the improvements in the CNP group were less substantial than in the PNP group, overall TENS effectively improved pain quality parameters in both groups.
All BPI parameters, except for “relationships with other people” in the CNP group, were significantly improved by TENS therapy in both groups. These findings parallel the effects of TENS on pain alleviation and are important because the negative effects of pain on common feelings and functions can have a significant influence on daily life.
The between-group comparisons of the BPI scores demonstrated no differences except for the “walking ability” and “enjoyment of life” parameters. Both of these parameters had better scores in the PNP group. This difference between-groups was anticipated because of the chronic and progressive nature of CNP diseases.
Allodynia and hyperalgesia are the two common sensation disorders in patients with neuropathic pain (9). Previous studies have demonstrated that TENS is effective for decreasing mechanic hyperalgesia. Ainsworth et al. (26) found that TENS alleviates primary mechanic hyperalgesia caused by joint inflammation. Cheing & Luck (27) studied the effects of high-frequency TENS in patients with hyperalgesia and hand pains. They found that TENS resulted in significant decreases in pain intensities, as evaluated by VAS on the 1st, 4th, 7th, and 11th days of treatment. They also found improvements in touch tolerance measures. We found that TENS improved hyperalgesia in only one PNP patient and allodynia in 3 PNP patients. These findings suggest that different current parameters and/or electrode placements should be tried on patients with hyperalgesia and allodynia in order to optimize TENS parameters for such sensation disorders.
There is controversy about which TENS frequency provides the most beneficial treatment. Both high-frequency (HI) and low-frequency (LO) TENS are used to treat neuropathic pain patients. HI TENS affects muscarinic receptors through a µ-opioid receptor-dependent mechanism, and LF TENS treatments alleviate secondary allodynia through serotonergic, muscarinic, and µ-opioid receptor-dependent mechanisms (28, 29). Norrbrink (29) investigated the effectiveness of LO and HI TENS in 24 SCI patients, and found that neither current type significantly improved the pain intensity ratings. However, 29% and 38% of the patients that received HI TENS and LO TENS, respectively, reported favourable effects on a 5-point global pain-relief scale. Thus, Norrbrink stated that “we still have very little support for the choice of the frequency”. Warke et al. (30) investigated the hypoalgesic effects of HI and LO TENS in 90 patients with MS. The patients were randomly assigned to LO, HI, or placebo TENS groups, and outcome measures were recorded at several time-points. They found that the greatest pain reduction effects of HI TENS occur during the initial 6 weeks of treatment. By contrast, LO demonstrates positive long-term results at 32 weeks. They conclude that neither HI nor LO TENS is more beneficial than the other. In our study it may be a limitation that there was no LO TENS application, especially for the patients who had a decreased skin sensibility. However, we chose to use HI TENS because it is well-tolerated by patients. HI TENS stimulates Aβ fibres, whereas LO TENS stimulates Aδ and C fibres. Since high-intensity currents or bursts of currents are necessary to stimulate the Aδ and C fibres, LO TENS treatments can be painful and unpleasant experiences for patients (31).
Impaired sensation is generally considered a precaution for TENS. However, Tyson (32) suggested that TENS may be used as a sensory stimulus to rehabilitate sensory function. In addition, Donnelan & Caldwell (33) and Yozbatiran et al. (34) reported no unpleasant sensations when they used TENS to treat stroke patients with sensory impairments. Furthermore, Laufer & Elboim-Gabyzon (35) suggested in a review study that TENS can easily be applied and tolerated if the stimulations are at a pleasant sensory level. They analysed 15 articles and a total of 446 stroke patients and found that no adverse effects were reported following any of the TENS interventions. We did not observe an important adverse effects of TENS treatment in this study. Only one patient (entrapment neuropathy) reported an increased sensation of numbness, on initial application of the TENS at the first 3 treatment sessions; however, he did not want to withdraw from treatment. The patients with sensory impairments tolerated the TENS treatments as well as the other patients. This may be due to the frequency selection (HI) and the current level used. The current was increased from the “strong” level to the “not painful and not unpleasant” level.
Studies have shown that adverse effects of TENS are rare. In addition, these studies may have used inappropriate techniques. Köke et al. (36) reported the following adverse effects of TENS: skin irritation (17/180 patients, 9.4%), adherence problems of electrodes (22/180 patients, 12.2%), and problems attaching electrodes (4/180 patients, 2.2%). Four patients withdrew from the study due to these adverse effects. Norrbrink (29) assessed the effectiveness of LO and HI TENS therapies in 24 patients with SCI. They reported that 3 patients experienced discomfort or increased pain during the treatments. We did not observe any important adverse effects of TENS treatment in this study. Only one patient (entrapment neuropathy) reported an increased sensation of numbness just at the time of TENS application at the first 3 treatment sessions. However, he did not want to stop treatment. This may be due to the HI TENS frequency selection and the current levels that we used. We adapted the current strength and it was increased throughout the sessions until the patient felt that it was at a “strong but not painful and not unpleasant” level.
An important limitation of our study is the absence of a third group with no-treatment or sham TENS. The European Federation of Neurological Societies (EFNS) states that TENS is superior to placebo. This is based on 9 controlled trials with data extracted from 200 neuropathic pain patients. Trial reports suggest that TENS is more beneficial than placebo for painful diabetic neuropathy, peripheral mononeuropathies of traumatic origin, painful cervical radiculopathy, and chronic pain that includes neuropathic elements (7). However, this information does not justify the lack of control group in our study.
Our clinical experience shows that neuropathic pain is one of the more troublesome pain conditions, especially when it is accompanied by abnormal sensations. Neuropathic pain treatments aim only to convert pain from dull to tolerable levels and not to remove it (26). The results from the trial with CNP and PNP patients reported here are promising. Both groups showed improvements in pain intensities, pain characteristics, pain qualities, and functional capacities. The PNP patients presented more favourable results than the CNP patients. Therefore, TENS therapies can be used in clinical practice, either as an alternative treatment or as a supportive method.
REFERENCES
