Shasha Li, MD1,2, Muke Zhou, MD3, Bo Yu, MD4, Zhenxing Ma, MD3, Sihan Chen, MS3, Qiyong Gong, PhD5,6, Li He, MD3, Xiaoqi Huang, PhD5, Su Lui, PhD5, Xiaotong Wang, MS7, Dong Zhou, MD3 and Chengqi He, MD1
From the 1Department of Rehabilitation Medicine, West China Hospital of Sichuan University, Chengdu, Sichuan, 2Department of Rehabilitation Medicine, the Second Affiliated Hospital of Wenzhou Medical College, Wenzhou, Zhejiang, 3Department of Neurology, West China Hospital of Sichuan University, 4Department of Paediatric, Sichuan Province of Women and Children Hospital, Chengdu, 5Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Sichuan, P. R. China, 6Division of Medical Imaging, Faculty of Medicine, University of Liverpool, Liverpool, UK, 7Department of Neurology, the Second Affiliated Hospital of Wenzhou Medical College, Wenzhou, Zhejiang, P. R. China
OBJECTIVE: Neuroimaging studies in stroke patients provide substantial evidence for the involvement of widespread cortical and subcortical regions in the control of swallowing. Although the affective network and the default mode network are functionally related to “autonomic” and “volitional” swallowing, little is known about their functional changes in dysphagic stroke patients.
METHODS: Unbiased seeds functional connectivity analysis was used to study the connectivity patterns of these resting-state networks. Resting-state functional magnetic resonance imaging was performed in stroke patients with (n = 12) and without dysphagia (n = 12).
RESULTS: Compared with healthy controls, stroke patients with and without dysphagia had decreased functional connectivity in the default mode network and the affective network. Moreover, stroke patients with dysphagia also had decreased functional connectivity in both the default mode network and the affective network relative to patients without dysphagia.
CONCLUSION: The difference in the extent of impairment in the default mode network and affective network of stroke patients with and without dysphagia may lead to improved understanding of the neuropathophysiological mechanism and rehabilitation of dysphagia.
Key words: stroke; deglutition disorders; magnetic resonance imaging; dysphagia; affective network; functional connectivity.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Chengqi He, Department of Rehabilitation Medicine, West China Hospital, Sichuan University, No. 37 Guo-xue-xiang-Street 610041 Chengdu, Sichuan, P. R. China. E-mail: hecq88@163.com
Accepted Aug 27, 2013; Epub ahead of print Nov 8, 2013
INTRODUCTION
Neurological oropharyngeal dysphagia is a common clinical problem in stroke patients. Dysphagia occurs in approximately 25–50% of acute stroke patients, but frequently resolves within days (1). For a smaller subset of individuals, swallowing difficulties persist. The brain stem and motor cortex are hubs that are necessary for swallowing (2, 3). In the past few years, a number of neuroimaging studies have suggested that multiple cortical and subcortical regions are involved in the control of swallowing (4–8). One human brain network, the affective network (AN), comprises a corticolimbic circuit in charge of negative emotional arousal or regulation (9–11) and is involved in autonomic and visceral functions that may be related to “autonomic” and “volitional” swallowing (12, 13). Our previous task-based functional magnetic resonance imaging (fMRI) study showed abnormal activation in some regions overlapping the AN, such as the medial prefrontal and insular cortices (14). Thus, we hypothesized that the AN may be altered in stroke patients. In addition, we are interested in the default mode network (DMN), which consists of brain regions deactivated during external-oriented tasks, but shows the highest activation in the resting state, and involves the most fundamental brain functions (15, 16). The DMN has been demonstrated to be affected in a wide spectrum of brain disorders (17–19); therefore, we speculate that it may also be abnormal in stroke patients.
In the past decade, resting-state fMRI (RS-fMRI) has become an important paradigm during which no specific task is required. Due to the simplicity and reliability of RS-fMRI data, this modality has been adopted in a large number of clinical studies (18, 20, 21). With RS-fMRI, the human brain can be separated into several networks, and the functional integration within these networks can be quantified by the correlation of blood oxygenation level-dependent (BOLD) signal fluctuation between brain regions. This paradigm was adopted in the current study to investigate the functional connectivity of brain networks in stroke patients.
Because dysphagia frequently resolves within a few days after stroke, it might be a good model to explore the rehabilitation mechanisms of the brain. In the current study, we hypothesized that the functional architecture of the DMN and AN are altered in stroke patients, and, furthermore, we speculated that this alteration in patients with dysphagia might be more serious than in patients without dysphagia.
PATIENTS AND METHODS
Participants
The study protocol was approved by the institutional ethics committee of Sichuan University. Written informed consent was obtained from all participants prior to commencing the research.
Patients admitted to the Stroke Center of West China Hospital from January 2010 to July 2012 were included in the study. Inclusion criteria were: first and only hemispheric ischaemic stroke in the distribution of the middle cerebral artery, with symptoms lasting at least 24 h, and with objective lesions observable via cerebral imaging (computed tomography and/or magnetic resonance imaging). During the initial consultation, the following information was obtained from each patient: sex, age at which the stroke occurred, time elapsed since the stroke, type of stroke, and the location and laterality of the lesion. A total of 24 stroke patients were enrolled and diagnosed with their first ischaemic stroke, as defined according to World Health Organization (WHO) criteria (sudden onset of neurological deficit persisting for more than 24 h) (22). All patients were assessed for swallowing dysfunction within 24 h of stroke onset by a qualified speech and language therapist. The therapist used the Logemann clinical indicators of dysphagia (23): coughing, oral residue, delayed swallowing, reduced laryngeal elevation (observed subjectively through placing one finger on the hyoid and one on the thyroid during swallowing), throat clearing and choking. To confirm the diagnosis, patients underwent a videofluoroscopic swallowing examination (VFSS Imager, Model IAB12LD/HG12, Shimadzu Corporation, Kyoto, Japan). Those patients with at least one of the following symptoms met the criteria for a diagnosis of dysphagia (24): (i) food residue occupying more than 50% of the epiglottic vallecula or pyriform sinus space after swallowing; (ii) subglottic aspiration; (iii) pharyngeal transit time longer than 2 s (operationally defined as the time taken for the bolus to move from the point at which the pharyngeal swallow is triggered to the cricopharyngeal sphincter); and (iv) impaired cricopharyngeal muscle relaxation. Twelve patients meeting these criteria were enrolled in the study. This group consisted of 6 females and 6 males, mean age 65.2 years (standard deviation; SD 4.3) (age range 58–72 years). Twelve stroke patients who did not demonstrate difficulties in the initial clinical evaluation of swallowing were enrolled as a comparison group (7 males and 5 females, mean age 66.5 years (SD 5.2)). Exclusion criteria were: prior cerebrovascular disease; pre-existing neurological or psychiatric disorders (including a history of seizures, global cognitive impairment, aphasia, neglect, substantial sensory disturbances, severe depression, or claustrophobia); use of an electrically sensitive biomedical device (e.g. cardiac pacemaker or cochlear implant); metal clips in the brain; or pneumonia at the time of enrolment.
The patients had each sustained a middle cerebral artery infarction up to 3 days prior to admission. The time from stroke onset to the start of the fMRI study ranged from 2 to 3 days. Based on the results of the initial VFSS, 5 patients were diagnosed with both oral and pharyngeal dysfunction, 3 with only oral dysfunction, and 4 with only pharyngeal dysfunction. Of these patients, 2 presented with mild initial P-A scale scores (score of 2), 7 presented with moderate P-A scale scores (score of 3–5), and 3 presented with severe P-A scale scores (score of 6), indicating at least one aspiration episode (2, 9).
Twelve stroke patients were defined as having normal swallowing function with P-A scale scores of 0. There was no significant difference in the distribution of brain infarction between all patients with lesions in the left and right hemispheres. In addition, there was no significant difference in the degree of severity of dysphagia between left and right hemispheric stroke patients (p > 0.05).
An age- and gender-matched group of healthy older volunteers (n = 12; 6 females and 6 males; mean age 65.8 years (SD 3.3); age range 60–70 years) served as the control group. All control subjects had a normal neurological examination, no history of a stroke, and no significant active neurological problems. They were also free of systemic diseases and neurological disorders. All subjects were strongly right-handed according to the Edinburgh Handedness Inventory. The study adhered to the MRI safety depositional guidelines established by the United States Food and Drug Administration for clinical scanners.
Data acquisition
Patients and healthy controls (HC) underwent scanning using a GE Signa EXCITE 3 BT MR system (GE Healthcare, Milwaukee, USA). During scanning, participants were instructed to relax with their eyes closed without falling asleep. Foam padding was used to minimize head motion. Resting-state functional images were obtained using a single-shot, gradient-recalled echo planar imaging sequence (205 volumes, repetition time 2000 ms, echo time 30 ms, flip angle 90º, field of view 240×240 mm2, interslice gap 0 mm, voxel size 3.75 × 3.75 × 5 mm3, 30 transverse slices aligned along the anterior–posterior commissure).
Data pre-processing
Pre-processing of functional images was carried out using the DPARSF (http://www.restfmri.net) and SPM8 (http://www.fil.ion.ucl.ac.uk/spm) toolkit. The first 5 images were excluded to ensure steady-state longitudinal magnetization, and then the remaining images were corrected for temporal differences and head motion. No translation or rotation parameters in any given data-set exceeded ± 1.5 mm or ± 1.5º, and there were no differences between groups for both parameters (2-sample 2-tailed t-test, p > 0.05). Because our patients all had unilateral cerebral hemispheric ischaemic stroke lesion, we flipped all right lesion patients’ fMRI images to make them appear as all left lesions. Specifically, considering the anatomically asymmetric nature of human brain, a symmetrical template image was produced by averaging the Montreal Neurological Institute (MNI) template and its mirror copy reversed in the sagittal plane (25). The fMRI data from the patient with right lesion were first normalized to this modified template at a 3 × 3 × 3 mm3 resolution, and the right-left flipped to obtain the mirror copies. The fMRI data from the patient with left lesion and HC were warped into a standard stereotaxic space at a 3 × 3 × 3 mm3 resolution using the MNI echo-planar imaging template. Then, the images were spatially smoothed with an 8-mm full-width half-maximum (FWHM) isotropic Gaussian kernel.
Seed-based functional connectivity analysis
To compare functional connectivity network of the DMN and AN between the patients and HC, 6 regions were selected as seeds to identify 2 networks (each had 3 seeds). The posterior cingulate cortex (PCC) (MNI coordinates: 0, –56, 30), left angular gyrus (AG) (–45, –66, 30), and right AG (45, –66, 30) were selected for detecting the DMN (26). The anterior cingulate cortex (ACC) (10, 35, –2), left amygdala (–24, 0, –18) and right amygdala (24, 0, –18) were selected for detecting the AN (27, 28).
To remove possible spurious sources of variance, the time series were pre-processed as described previously (26). (i) Temporal band-pass filtering (pass band 0.01–0.08 Hz) was conducted to reduce the effects of low-frequency drift and high-frequency noise. (ii) The time series was further corrected through linear regression to eliminate the effect of 6 head motion parameters obtained in the realigning step and the effect of the signals from cerebro-spinal fluid (CSF), white matter and global brain signal. A temporal correlation analysis was conducted between the seeds and each voxel in the whole brain. The resulting r maps were converted using Fisher’s r-to-z transformation to improve the Gaussianity of their distribution. Finally, the individual Z maps were entered to random effect 1-sample t-test in SPM8. Age and sex were used as confounding variables in all statistical analyses. A statistical map of significant functional connectivity was created for each seed. The significance level was set at p < 0.05. Conjunction analysis (26, 29) was used to combine the 3 Z maps with similar spatial patterns of each network. First, the individual Z maps were combined within each network. This conjunction analysis was used for observation of the brain network module patterns. The voxels whose functional connectivity values survived at a threshold at p < 0.05 were corrected for multiple comparisons using the false discovery rate (FDR)-criterion (30). The mean was masked by using a conservative conjunction procedure. Voxels were included in the mask only if they were significantly correlated or anti-correlated with at least 2 of the 3 seed regions.
To examine the difference among 3 groups, 2-sample t-test in SPM8 was used for each paired group within a mask. These specific masks were created by combining the voxels for each pairing (stroke patients with dysphagia and HC, stroke patients without dysphagia and HC, stroke patients without and with dysphagia) that were obtained from 1-sample t-test results. These group-difference comparisons were restricted to corresponding positive functional connectivity voxels. The significance level was set at p < 0.05, FDR-corrected.
RESULTS
Functional connectivity of the default mode network
To assess the alterations in resting-state functional connectivity on the DMN, 3 brain regions were chosen as seeds (PCC and bilateral AG). To construct unbiased resting-state functional connectivity maps, conjunction analysis was used to combine the correlation maps of the 3 seeds. The population-averaged correlation maps were generated for each of the networks and each of the 3 groups by using random effects analysis across the population. A significant group difference in the main pattern of connectivity is shown in Table I.
The main pattern of connectivity of the DMN between HC and stroke patients was similar by visual inspection (Fig. 1A). The DMN network included the PCC, bilateral AG, ventral and dorsal medial prefrontal (MPFC), inferior temporal cortex, medial temporal cortex and medial cerebellum, consistent with previous studies (26). The between-group difference in the DMN was obtained using 2-sample t-test (p < 0.05, FDR-corrected) (Fig. 1B). Compared with the HC, a significantly decreased positive correlation was found in stroke patients without and with dysphagia in PCC, bilateral AG and bilateral prefrontal cortex. These brain regions showed a marked increased in functional connectivity in the DMN of stroke patients without dysphagia compared with patients with dysphagia.
|
Table I. Brain regions showing significant group difference |
||||||
|
Brain area |
BA |
x |
y |
z |
T-value |
Cluster size (voxels) |
|
Dysphagia vs HC |
||||||
|
AN |
||||||
|
ParaHipp, Putamen_R |
34 |
15 |
–3 |
–21 |
–7.7 |
196 |
|
ParaHipp, Putamen_L |
38 |
–24 |
12 |
–27 |
–7.49 |
569 |
|
Frontal_Sup_L |
11 |
–6 |
51 |
–12 |
–6.7 |
11 |
|
Cingulum_Mid_L |
32 |
–3 |
–6 |
36 |
–2.84 |
47 |
|
DMN |
||||||
|
Frontal_Mid_L |
8 |
–30 |
3 |
63 |
–14.7 |
354 |
|
Frontal_Mid_R |
9 |
30 |
18 |
39 |
–8.26 |
373 |
|
Parietal_Inf_R |
40 |
57 |
–54 |
39 |
–9.65 |
734 |
|
Parietal_Inf_L |
39 |
–39 |
–54 |
30 |
–5.58 |
187 |
|
Precuneus_R |
7 |
15 |
–72 |
36 |
–14.1 |
936 |
|
Non-dysphagia vs HC |
||||||
|
AN |
||||||
|
ParaHipp_L |
25 |
–18 |
–15 |
–24 |
–5.16 |
30 |
|
Putamen_R |
21 |
18 |
–6 |
–7.34 |
322 |
|
|
Putamen_L |
–30 |
–6 |
–3 |
–7.24 |
352 |
|
|
Cingulum_Ant_R |
32 |
6 |
45 |
9 |
–8.38 |
163 |
|
Cingulum_Mid_R |
24 |
6 |
–12 |
36 |
–2.81 |
18 |
|
DMN |
||||||
|
Frontal_Mid_R |
6 |
30 |
9 |
54 |
–9.49 |
281 |
|
Frontal_Mid_L |
6 |
–30 |
6 |
66 |
–10.9 |
368 |
|
Frontal_Sup_R |
10 |
30 |
51 |
12 |
–2.52 |
23 |
|
Frontal_Inf_R |
47 |
45 |
24 |
21 |
–3.74 |
29 |
|
Parietal_Inf_L |
39/40 |
–33 |
–57 |
36 |
–4.99 |
140 |
|
Parietal_Inf_R |
39/40 |
42 |
–51 |
36 |
–5.37 |
248 |
|
Cingulum_Pos_L |
31 |
–18 |
–57 |
12 |
–6.75 |
127 |
|
Non-dysphagia vs dysphagia |
||||||
|
AN |
||||||
|
Frontal_Inf_L |
47 |
–27 |
15 |
–15 |
3.41 |
22 |
|
ParaHipp_R |
34 |
18 |
–12 |
–21 |
4.31 |
13 |
|
Amygdala_L |
34 |
–30 |
–3 |
–21 |
4.9 |
30 |
|
Hippocampus_L |
36 |
–27 |
–27 |
–6 |
4.67 |
56 |
|
Temporal_Sup_L |
41 |
–36 |
–15 |
–3 |
2.53 |
29 |
|
DMN |
||||||
|
Precuneus_L |
7 |
–18 |
–66 |
27 |
3.17 |
25 |
|
Precuneus_R |
7 |
9 |
–45 |
6 |
18.16 |
993 |
|
Parietal_Inf_R |
39/40 |
45 |
–63 |
24 |
5.67 |
272 |
|
Parietal_Inf_L |
39/40 |
–51 |
–66 |
33 |
3.93 |
169 |
|
Frontal_Sup_L |
9 |
–9 |
45 |
30 |
4.26 |
159 |
|
Frontal_Mid_L |
8 |
–36 |
24 |
27 |
18.29 |
293 |
|
Frontal_Mid_R |
9 |
45 |
27 |
39 |
9.52 |
219 |
|
BA: Brodmann area; ParaHipp: ParaHippcampus Inf: inferior; Sup: superior; Mid: middle; Pos: posterior; AN: affective network; DMN: default mode network; HC: healthy control; R: right; L: left. |
||||||
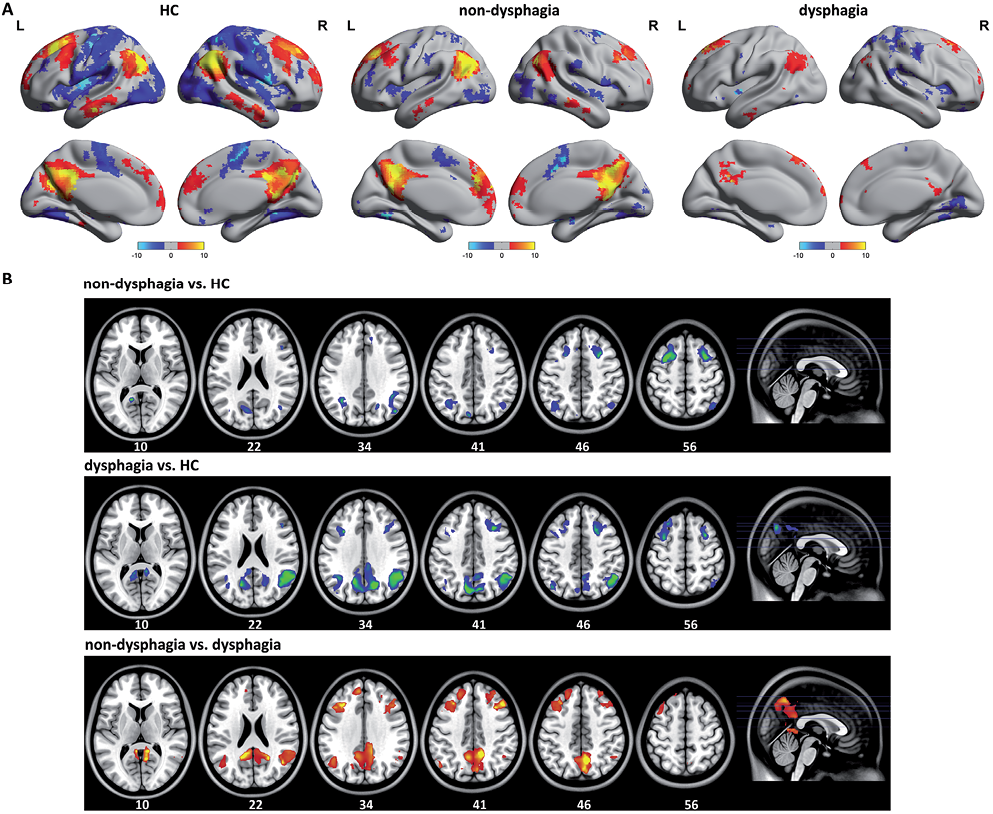
Fig. 1. A) The main pattern of default mode network (DMN) correlation maps of the healthy controls (HC) and stroke patients without dysphagia. The DMN included regions in the posterior cingulate cortex (PCC), bilateral angular gyrus (AG), ventral and dorsal medial prefrontal (MPFC), inferior temporal cortex, medial temporal cortex and medial cerebellum. B) Compared with the HC, stroke patients with dysphagia showed diffuse impact on widely distributed the DMN connectivity pattern. A significantly decreased positive correlation was found in stroke patients without and with dysphagia in PCC, bilateral AG and bilateral profrontal cortex. R: right; L: left.
Functional connectivity of the affective network
The functional connectivity pattern of the AN included the amygdala, ventromedial prefrontal cortex and perigenual cingulate cortex, which comprise a corticolimbic circuit (Fig. 2A). The between-group difference in the AN was obtained using a 2-sample t-test (p < 0.05, FDR-corrected) (Fig. 2B). Compared with the HC, a significantly decreased functional connectivity was found in stroke patients without and with dysphagia in the perigenual cingulate cortex, bilateral amygdala, pallidum and putamen. The bilateral amygdala showed increased functional connectivity in stroke patients without dysphagia compared with patients with dysphagia.
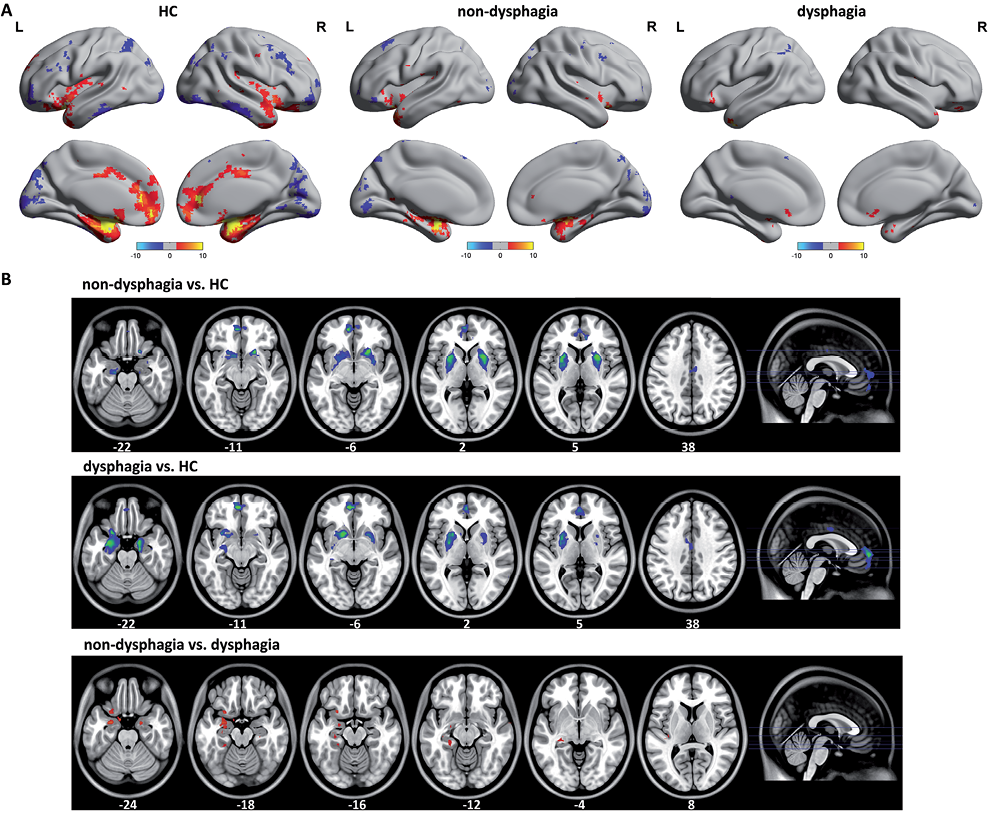
Fig. 2. A) The functional connectivity pattern of the affective network (AN) included the amygdala, ventromedial profrontal cortex and perigenual cingulate cortex, which comprise a corticolimbic circuit. The stroke patients with dysphagia showed widely diffuse impairments on functional connectivity organization in the AN. B) Compared with the healthy controls (HC), a significantly decreased functional connectivity was found in stroke patients without and with dysphagia in perigenual cingulate cortex, bilateral amygdala, pallidum and putamen. The bilateral amygdalae were found to have marked increased functional connectivity in stroke patients without dysphagia than in with dysphagia. R: right; L: left.
DISCUSSION
This study aimed to determine whether intrinsic functional connectivity changes in the AN and DMN were present in stroke patients with and without dysphagia. To our knowledge, this is the first study to investigate the functional architecture of these networks in relation to swallowing ability in stroke patients. Our findings indicate that both patients with and without dysphagia showed distinctive changes in the functional connectivity patterns in both the DMN and the AN. Patients with dysphagia demonstrated greater disruption than those without swallowing difficulties.
Our previous study based on task-related fMRI indicated that unilateral stroke of either cerebral hemisphere can cause dysphagia (14). This finding suggests that effective recovery is associated with cerebral activation related to the cortical swallowing representation in the compensating hemisphere or recruited areas of the intact hemisphere. The recent development of the resting functional connectivity approach to fMRI has provided a window to observe intrinsic brain activity in health and disease beyond the task-related fMRI study. Resting state brain networks are often shown to present abnormalities in many neuropsychiatric disorders (17, 18). These abnormalities relate mainly to the alterations of coherent intrinsic neuronal activity of BOLD fluctuations observed in the resting state by fMRI. Little is known, however, about the intrinsic functional connectivity changes in the resting state network in stroke patients with and without dysphagia. Here, we provide evidence that the resting-state functional architecture is impaired in relation to swallowing ability in stroke patients. The DMN involves a number of fundamental brain functions, such as emotional state and self-related mental representations (31). Although the DMN has been reported to show dysfunction in stroke patients (32) as our current findings suggest, it is unlikely to be an alteration of brain function specific to stroke because similar changes commonly appear in many other disorders (17, 18). The alteration in DMN may relate to the uncomfortable state or chronic pain of stroke patients (33). This subjective feeling may be more serious for patients with dysphagia than for patients without dysphagia and may be indicative of the more severe disruption that was found in the former group. The functional change in the AN in patient groups was similar to that in the DMN. This may also largely be related to the uncomfortable state of patients, because the AN is partly in charge of negative emotional responses (11). The function connectivity impairment in AN was found in disorders with aversive affective experiences, such as major depression (28, 34, 35) mood and anxiety disorders (36). In addition, the AN is important in fear, vigilance, and autonomic and visceral regulation. This may explain why the AN impairment in patients with dysphagia was worse than in patients without dysphagia. Specifically, we found lower functional connectivity within bilateral amygdalae of stroke patients without dysphagia than in patients with dysphagia. It is known that amygdala-vMPFC dysfunction may have particular relevance to aggressive traits and behaviour (37, 38). Our findings indicated that the intrinsic functional connectivity of amygdala modified symptoms of negative affect between stroke patients with and without dysphagia. One may argue that this is due to the difference in the extent of the deficit between the 2 patient groups, but the National Institutes of Health Stroke Scale score was not significantly different between groups (p > 0.05).
Resting-state fMRI has been widely applied to clinical studies due to its simplicity. The relation between brain function change in resting state and traditional task fMRI has been discussed (39). The task activation could be partly explained by resting state brain activity, but in pathological conditions, resting-state rather than task fMRI could be more sensitive to the function alteration (40). Thus, these 2 modalities are closely related, although they sometimes show different results.
The study limitations were as follows: first, we flipped some patients’ images to make all patients’ lesions appear to be present in the same hemisphere. Considering the functional and structural asymmetry between hemispheres, it would be better to recruit more patients with lesions on the same side, rather than to flip and process their images. Secondly, more comprehensive neuropsychological tests are needed to verify whether the neuroimaging findings are associated with psychological changes in the patients. Finally, a larger sample size should be studied.
In conclusion, the current findings demonstrate significant alterations in intrinsic functional connectivity in both the AN and the DMN in relation to swallowing ability in stroke patients. The impairment in patients with dysphagia was worse than in patients without dysphagia. These findings suggest that dysphagia-related abnormal brain activity is not restricted to the motor network.
Acknowledgements
The authors would like to thank all of the participants for their cooperation. The authors acknowledge support from the State Key Laboratory of Biotherapy and HMRCC, West China Hospital, Sichuan University.
This research was supported by National Basic Research Program of China (973 Program numbers 2011CB707803 and 2007CB512305), the Natural Science Foundation of China (Grant numbers 81000852, 81030027, 30625024 and 30900361), the National High Technology Program of China (863 Program number 2008AA02Z408), the PCSIRT project, the Supporting Project of Science & Technology of Sichuan Province (grant number 2012SZ0140) and the project and the Research Foundation of Zhejiang Province (grant number 201022896).
The authors declare no conflicts of interest.
REFERENCES
