Bodil Bjoernshave, RN, MHS, PhD1, Jens Korsgaard, MD, PhD2, Chris Jensen, PhD3 and Claus Vinther Nielsen, MD PhD3
From the 1Institute of Public Health, Aarhus University, 2Moelholm Private Hospital Medical Department Vejle, 3Centre for Public Health and Quality Improvement, Central Denmark Region and 4Section of Clinical Social Medicine and Rehabilitation, Institute of Public Health Aarhus University and Centre for Public Health and Quality Improvement Aarhus, Denmark
OBJECTIVE: The effect of rehabilitation for chronic obstructive pulmonary disease has been well-documented in randomized controlled trials. Evidence-based guidelines support rehabilitation programmes for chronic obstructive pulmonary disease, but knowledge of their outcome in clinical practice is limited. The aim of this study was to assess the outcome of a clinical routine rehabilitation programme for chronic obstructive pulmonary disease implemented by a Danish regional hospital.
Material and methods: Changes in walk-distance (6-min walk-distance test; 6MWD), dyspnoea (Medical Research Council dyspnoea scale; MRC) and health-related quality of life (Short-Form 36; SF-36) were compared between and within completers and non-completers from baseline to the end of clinical routine rehabilitation, and at 6 and 12 months. The 8-week clinical routine rehabilitation comprised bi-weekly 90-min sessions of patient education and physical training.
RESULTS: The hospital treated 521 patients during the study period. Of these, 175 were invited to join the study, 148 participated at baseline, and 98 at the 12-month follow-up. Completers’ 6MWD was sustained from baseline to the end of clinical routine rehabilitation, but had declined by 12 months. Dyspnoea and health-related quality of life did not change. Seventy-five percent of completers felt better or much better after rehabilitation.
CONCLUSION: The failure of completers to achieve expected outcomes shows a need for a stronger implementation effort and continuous quality control. Successful implementation in clinical routine requires targeted recruitment and overall programme improvement in general, and a stronger focus on physical training and staff competences.
Key words: COPD; completing; drop-out; inclusion; outcome; rehabilitation; selection.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Bodil Bjoernshave, Sødalvej 20 DK-8830 Tjele, Denmark. E-mail: bodil.bjoernshave.noe@viborg.rm.dk
Accepted Apr 8, 2013; Epub ahead of print Aug 8, 2013
Introduction
The effect of pulmonary rehabilitation has been documented in many randomized controlled trials (RCTs), which have informed the rehabilitation approach adopted in clinical practice. Evidence for the effect of pulmonary rehabilitation was summarized in meta-analyses by the Cochrane Collaboration in 2007 (1). Rehabilitation is recommended as an important element in the management of chronic obstructive pulmonary disease (COPD), aiming “to reduce symptoms, improve quality of life, and increase physical and emotional participation in everyday life activities”. The Danish COPD management programmes (2–6) that guide rehabilitation in clinical routine are in agreement with international guidelines (7–9).
The question is therefore no longer “Should patients with COPD receive rehabilitation?”, but rather “How should rehabilitation be delivered to patients with COPD?”, and “Which components form the basis for successful rehabilitation programmes?” (10).
It is well-known that a large proportion of patients with COPD either decline to take part in rehabilitation or drop-out. Thus, an RCT from our group reported that only approximately one-third of the patients completed rehabilitation (11). It has been speculated that completion is determined, to some extent, by the characteristics of those who participate in rehabilitation programmes. Cote et al. (12) found that those who declined to take part in rehabilitation were smokers and were more sick, measured with the BODE index1, than those who accepted the invitation (13). Young et al. (14) found that “non-adherent patients”, defined as drop-outs and those who declined to participate, were likely to be divorced, live in rented accommodation, and be smokers, compared with “adherent patients”.

1Bode Index (predicts COPD by integrating body mass index (BMI, B), airflow obstruction (O) as measured by the FEV1 (% predicted), dyspnoea (D) assessed by the modified Medical Research Council (MMRC) score, and exercise tolerance (E) measured by 6 minute walking distance. The variables are graded and summed to give a total score between 0 and 10)
Sabit et al. (15) reported that current smoking, a greater number of previous hospital admissions, higher Medical Research Council dyspnoea scale (MRC) score, or enduring a long journey, were risk factors for low attendance.
Emphasizing the need for improving the current clinical rehabilitation routines, and for such practices to be firmly founded on scientific evidence, a group of healthcare professionals at Horsens Regional Hospital in Denmark pioneered a multidisciplinary COPD rehabilitation programme. The goal of this programme was to ensure targeted inclusion and to increase attender completion rates of clinical routine rehabilitation (CRR) in COPD patients. The existence of this CRR programme affords research with an opportunity to evaluate how the programme performs in clinical practice, whether patient characteristics predict completion, and how completers differ from non-completers in terms of CRR outcomes. Focusing on patient characteristics, we recently published the first part of the study of the Horsens COPD rehabilitation programme (16). We hypothesized that completers of rehabilitation would be those who were better off in terms of comorbidities and socioeconomic characteristics, but found that completers of CRR did not differ from non-completers, and that patient characteristics (socioeconomic factors, dyspnoea, functional capacity, smoking, co-morbidity and hospitalization) did not predict completion.
The previous study therefore focused on a detailed analysis of the complex inclusion process for CRR.
The present paper analyses the clinical effect in relation to pulmonary rehabilitation in clinical routine, by comparing those who complete CRR with various subgroups of non-completers. The aims were: first, to determine completers’ and non-completers’ outcome 8 weeks and 6 and 12 months after CRR programme completion; secondly, to explore completers’ attitudes to, and experience with, rehabilitation. We hypothesized that completers would improve in terms of 6-min walk-distance (6MWD), health-related quality of life (HRQoL), and decreased dyspnoea, whereas non-completers would not.
Material and Methods
A total of 175 patients was invited to participate in the present study. Permission was given by the Danish Data Protection Agency. CVR-nr 11-88-37-29 Journal number 2008-41-2294 (www.datatilsynet.dk). The patient flow is shown in Fig. 1.
Fig. 1. Patient flow. CRR: clinical routine rehabilitation.
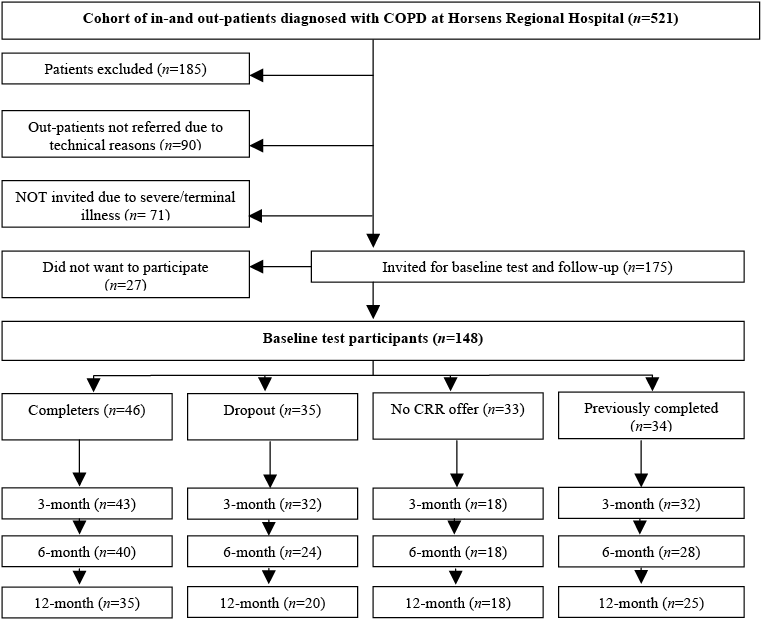
The study was designed as a prospective, explorative follow-up study including Danish COPD patients (ICD-10 DJ44X) treated as inpatients or outpatients from 1 September 2008 until 30 April 2009 at Horsens Regional Hospital.
Exclusion criteria were: patients who had participated in CRR within the preceding year or participated in the pilot test. Patients treated with long-term oxygen, or who had severe illness or were in the terminal stage of their disease.
A total of 521 patients were diagnosed with COPD during the study period. Of these, 185 patients were excluded due to: COPD diagnosis withdrawn (65), death (33), moved away (13), participated in CRR within the preceding year (22), participated in the pilot test (4), treated with long-term oxygen (48). Furthermore, 90 outpatients were not identified for technical reasons. This group differed from the patients identified, by being younger and having better lung function, and counted statistically more non-smokers, than the patients referred for baseline test.
Patients who were deemed not able to cope with the study tests due to severe illness or terminal stage of their disease were excluded (n = 71): very severe mobility disorders (24), severe ischaemic heart disease complications due to stroke, severe psychiatric disorders (38), lived in rest-homes (7), did not understand Danish (2). Those 71 patients were older and MRC scores of severe dyspnoea were statistically significantly higher than those deemed able to participate. A total of 175 patients was invited for the baseline test. Of these, 27 declined to participate, leaving a final total of 148 patients, who formed the study population.
Clinical routine rehabilitation
Some of the study participants were offered CRR, others were not. According to hospital guidelines, the inclusion criteria were patients with a forced expiratory volume in the first second (FEV1) below 50% of the predicted value, or dyspnoea equivalent to the MRC dyspnoea scale ≥ 3 (Fig. 2). Patients who satisfied these criteria should be offered 8 weeks of CRR, with 90-min sessions twice a week. Every session consisted of patient education and physical training, where intensity was increased as tolerated. The CRR programme (2) at the hospital was observed within its real-life context. The investigator had no influence on the CRR programme or on who participated.
Fig. 2. Recruitment of clinical routine rehabilitation participants for the chronic obstructive pulmonary disease (COPD) disease management programme at Horsens Hospital. FEV1: forced expiratory volume in the first second.
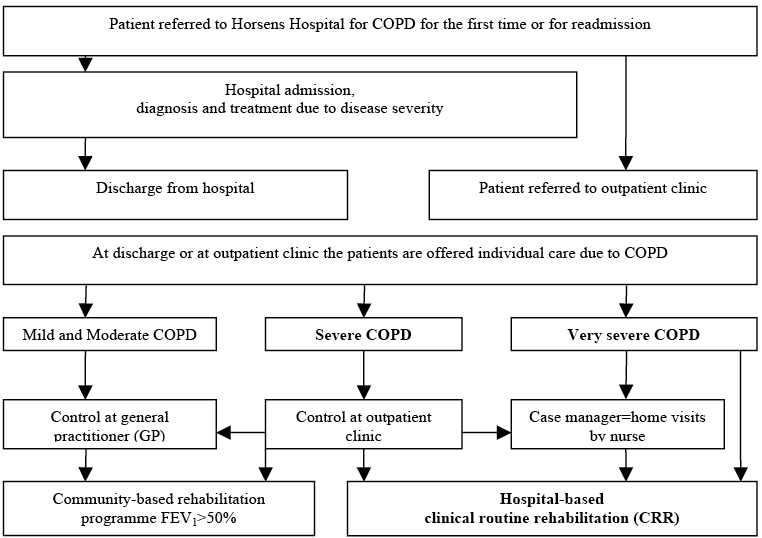
Completers and non-completers of clinical routine rehabilitation
Completers were defined as patients who completed the 8-week CRR programme during the study period. Completers were tested at baseline (the beginning of CRR), at the end of CRR (8 weeks after baseline), and 6 and 12 months after baseline.
Non-completers were divided into 3 subgroups, defined as: (i) drop-outs, i.e. patients who started CRR, but did not complete the 8-week programme; (ii) patients with no CRR offer (NRO) were those patients with FEV1 above 50% predicted. In addition, we discovered, in our previous study (16), that some were not offered CRR for unknown reasons; (iii) patients who had previously completed CRR (PC) at least 12 months prior to the baseline test.
The subgroups of non-completers were tested at baseline, 8–12 weeks after baseline, defined as the “3-month follow-up”, and 6 and 12 months after baseline.
Data collection
At baseline, patient characteristics and self-reported co-morbidity were registered.
At baseline and follow-up FEV1, MRC (17), 6MWD (18, 19) and SF-36 (20) were measured.
FEV1 (% predicted value) was measured with spirometry (Vitalograph 2120 nr 10122, Vitalograph®, UK) without prior bronchodilator inhalation, and the best of 3 measurements was registered.
The patients answered the MRC (17) dyspnoea questionnaire by marking the category that best expressed their dyspnoea:
• not troubled with breathlessness except when undertaking strenuous exercise;
• troubled by shortness of breath when hurrying, or when walking up a slight hill;
• walks slower than people of the same age due to breathlessness, or has to stop for breath when walking at own pace on the level;
• stops for breath after walking approximately 100 m or after a few minutes on the level;
• too breathless to leave the house, or breathless when dressing or undressing.
Walking distance was measured with the 6MWD test according to American Thoracic Society Guidelines (18) and the Danish manual (19).
The test measures the distance that a patient can quickly walk over a period of 6 min. It is self-paced and assesses the sub-maximal level of functional capacity. The patients chose their own intensity and were allowed to stop and rest during the test.
Health-related quality of life (HRQoL) was measured with the Medical Outcome Study Short Form 36 Health Survey Questionnaire (SF-36) and analysed according to the Danish manual (20).
The SF-36 consists of 36 items forming 2 summary scores: Physical Component Score (PCS) and Mental Component Score (MCS). Each scale goes from 0 (poor health) to 100 (good health), and the minimal clinically important difference was set to 10 points.
Depression was obtained by means of the Case Finding Questionnaire for Common Mental Disorders (CMDQ) (21).
Data on socio-economic factors and hospitalization were obtained from national databases (E-Health (www.esundhed.dk) and Statistics Denmark (www.dst.dk)). CRR completers answered a number of open questions at the end of the CRR concerning their attitudes towards the programme and the perceived outcome. This questionnaire was constructed for the present study to cover important aspects of CRR from the patients’ perspective.
Statistical analysis
Power calculation. Based on our previous study (11), we expected a 35% improvement in completers’ 6MWD (standard deviation; SD 50%) and no improvement in non-completers’ 6MWD. The significance level was set at 5% and power at 80%. The required sample size was estimated as 32 patients in each group.
Analyses. For normally distributed continuous variables, means and 95% confidence intervals (CIs) were used, and differences between and within groups were tested by paired and non-paired t-tests.
For categorical variables, proportions were used and the differences between groups were tested using Kruskal–Wallis equality of populations rank test, and differences within groups were tested using Wilcoxon signed-rank test.
The 5-point MRC scale was transformed into 3 categories: 1 and 2 equivalent to mild, 3 equivalent to moderate, and 4 and 5 equivalent to severe dyspnoea.
Statistical analyses were performed using Stata version 11 (StataCorp., College Station, Texas, USA).
Results
A total of 148 patients participated at baseline, 125 attended follow-up at the end of CRR/3-month follow-up, 110 at the 6-month follow-up, and 98 at the 12-month follow-up (Fig. 1).
Table I shows the patient characteristics, co-morbidities and hospitalization at baseline by group. The proportions of patients within the groups with one or more co-morbidities ranged from 80% to 91%, with the lowest proportion among completers. Depression was self-reported by approximately 20%; yet more than 50% tested positive in the questionnaire. Approximately 40% of baseline participants were current smokers. The proportion of patients hospitalized within 12 months after the baseline test ranged from 24% to 34% and was highest among those who started CRR, but did not complete the 8-week programme.
|
Table I. Patient characteristics, co-morbidities and hospitalizations for baseline participants (n = 148) by group |
|||||
|
Completersa (n = 46) |
Drop-outb (n = 35) |
NROc (n = 33) |
PCd (n = 34) |
p-value |
|
|
Female, % |
54 |
54 |
67 |
47 |
0.45 |
|
Age, years, mean (95% CI) |
68 (65–70) |
67 (64–70) |
69 (65–73) |
68 (65–71) |
0.89 |
|
Body mass index, mean (95% CI) |
24 (22–25) |
24 (22–26) |
26 (24–28) |
26 (23–28) |
0.16 |
|
Pack years of smoking, mean (95% CI) |
42 (37–48) |
42 (36–47) |
38 (31–45) |
43 (36–50) |
0.67 |
|
Current smoker, % |
50 |
54 |
55 |
41 |
0.66 |
|
Self-reported living alone,% |
13 |
3 |
15 |
3 |
0.13 |
|
Own their place of residence, % |
31 |
49 |
52 |
44 |
0.27 |
|
School primary or less, % |
89 |
97 |
97 |
91 |
0.39 |
|
Education level short or none, % |
84 |
97 |
94 |
88 |
0.24 |
|
Vaccination yes, % |
74 |
83 |
76 |
88 |
0.39 |
|
Proportion 1 or more co-morbidity, % |
80 |
83 |
91 |
85 |
0.61 |
|
Ischaemic heart disease (IHD), % |
55 |
50 |
70 |
41 |
0.13 |
|
Musculoskeletal problems, % |
64 |
74 |
61 |
72 |
0.64 |
|
Diabetes, % |
7 |
9 |
9 |
15 |
0.70 |
|
Self-reported depression, % |
23 |
18 |
21 |
27 |
0.85 |
|
General Depression Scale Positive, % |
55 |
54 |
50 |
53 |
0.97 |
|
Hospitalized 12 months from baseline, % |
26 |
34 |
24 |
27 |
0.80 |
|
More than 1 hospitalization 12 months from baseline, % |
58 |
58 |
63 |
11 |
0.09 |
|
Hospitalized prior to baseline, % |
33 |
46 |
79 |
18 |
0.00 |
|
Distance to hospital less than 15 km, % |
65 |
60 |
72 |
64 |
0.67 |
|
aPatients who completed the 8-week CRR (clinical routine rehabilitation) programme during the study period. bPatients who started CRR, but did not complete the 8-week CRR programme. cPatients with no CRR offer. dPatients who had previously completed CRR at least 12 months prior to the baseline test. CI: confidence interval. |
|||||
As expected, given the decision not to refer patients with a FEV1 above 50% to CRR, the patients with no CRR offered had a statistically significantly better lung function; however, those with NRO had the shortest walk distance at baseline (Table II).
Among completers there was no improvement in 6MWD from baseline to the end of CRR (Table II). However, their 6MWD declined statistically significantly from baseline to the 12-month follow-up (p = 0.007). The 6MWD did not change statistically significantly among the other groups. The Physical Component Score and the Mental Component Score of the SF-36 questionnaire showed no clinically relevant change in any of the groups (Table II). Among completers, the proportion of patients with moderate and severe dyspnoea increased from baseline to follow-up, while the proportion with mild dyspnoea increased among drop-outs, although the changes were not statistically significant (Table II).
|
Table II. Forced expiratory volume in the first second, 6-min walk-distance, quality of life (Mental Component Score and Physical Component Score) and Medical Research Council dyspnoea scale at baseline, at the end of clinical routine rehabilitation/3 month and at 12-month follow-up |
|||||||
|
Outcome |
Group |
Baseline test |
At the end of CRR/3 monthsa |
p-valueb |
6 months |
12 months |
p-valuec |
|
FEV1 (%) Mean (95% CI) |
Completers |
37 (32–42) |
38 (35–43) |
0.26 |
37 (33–42) |
37 (33–42) |
0.87 |
|
Drop-out |
37 (34–41) |
36 (32–41) |
0.59 |
38 (33–43) |
38 (33–43) |
0.20 |
|
|
NRO |
56 (52–60) |
56 (51–62) |
0.87 |
53 (47–58) |
51(44–58) |
0.15 |
|
|
PC |
36 (31–41) |
36 (31–41) |
0.98 |
36 (30–42) |
34 (29–40) |
0.1 |
|
|
6MWD (m) Mean (95% CI) |
Completers |
408 (374–441) |
413 (374–453) |
0.60 |
357 (299–414) |
336 (269–403) |
0.007 |
|
Drop-out |
363 (310–416) |
361 (297–425) |
0.89 |
375 (300–450) |
401 (342–459) |
1.0 |
|
|
NRO |
362 (310–415) |
372 (330–414) |
0.47 |
325 (243–406) |
363 (293–433) |
1.0 |
|
|
PC |
385 (351–419) |
324 (277–370) |
0.04 |
331 (272–390) |
336 (272–399) |
0.05 |
|
|
MCS Mean (95% CI) |
Completers |
56 (53–59) |
56 (53–59) |
0.68 |
54 (51–58) |
54 (50–57) |
0.32 |
|
Drop-out |
57 (53–62) |
55 (51–59) |
0.17 |
56 (54–59) |
57 (53–60) |
0.81 |
|
|
NRO |
53 (46–59) |
54 (49–59) |
0.60 |
55 (50–61) |
50 (41–58) |
0.39 |
|
|
PC |
56 (54–60) |
55 (53–59) |
0.66 |
54 (50:58) |
58 (53–61) |
0.52 |
|
|
PCS Mean (95% CI) |
Completers |
37 (33–40) |
36 (32–39) |
0.48 |
36 (32–40) |
37 (33–42) |
0.81 |
|
Drop-out |
39 (35–43) |
36 (33–39) |
0.54 |
40 (36–45) |
40 (35–44) |
0.55 |
|
|
NRO |
37 (33–43) |
40 (37–44) |
0.29 |
41 (37–45) |
40 (30–49) |
0.45 |
|
|
PC |
37 (34–41) |
36 (33–40) |
0.58 |
36 (32–40) |
36 (31–41) |
0.31 |
|
|
MRC Mild/moderate/severe, % |
Completers |
68/29/3 |
57/36/7 |
0.40 |
44/46/10 |
54/34/12 |
0.18 |
|
Drop-out |
65/25/10 |
56/28/16 |
0.50 |
54/33/13 |
70/15/15 |
1.00 |
|
|
NRO |
56/39/5 |
44/50/6 |
0.16 |
50/33/17 |
61/33/6 |
0.56 |
|
|
PC |
64/24/12 |
44/31/25 |
0.25 |
46/29/25 |
44/28/28 |
0.02 |
|
|
aCompleters were tested at the end of the CRR programme 8 weeks after baseline. The other groups were tested within 3 months after baseline. bWithin-group analyses based on participants at baseline and “the end of CRR/3 months”. cWithin-group analyses based on participants at baseline and 12 months follow-up within group differences. Paired t-test (FEV1, 6MWD, MCS, PCS) Wilcoxon signed-rank test (MRC proportions). MRC: Medical Research Council dyspnoea scale; FEV1 (%): FEV1 % of predicted value; 6MWD: 6-min walk-distance; MRC%: Medical Research Council dyspnoea questionnaire proportion with mild/moderate/severe dyspnoea; MCS and PCS: Mental and Physical Component scores from the health-related quality of life Short-Forum 36 (SF-36) questionnaire; PC: patients who had previously completed CRR at least 12 months prior to the baseline test. |
|||||||
The questionnaire concerning attitudes towards CRR was answered by 41 (89%) completers (Table III). More than 85% answered that CRR had a positive influence on their mood, their motivation for making changes in their everyday life, and their COPD coping. A total of 86% answered that their physical performance had improved. Almost everyone was satisfied with the CRR programme, and almost 75% answered that they felt somewhat or very much better.
|
Table III. Questionnaire concerning attitudes toward rehabilitation and subjective outcomes answered by 41/46 (89%) among completers at the end of the rehabilitation |
|
|
% |
|
|
Did you experience the rehabilitation programme… |
|
|
to have an influence on your mood? Yes/Unchanged |
85/15 |
|
to have an influence on your motivation for making changes in daily activities? Yes/Unchanged |
90/10 |
|
to give knowledge concerning COPD? Yes/Unchanged |
95/5 |
|
to influence your community life/participation in social life? Yes/Unchanged |
61/39 |
|
to influence your ability to cope with COPD in everyday life? Yes/Unchanged |
85/15 |
|
to increase your physical performance? Yes/Unchanged |
86/14 |
|
to influence your ability to cope with activities of daily living? Yes/Unchanged |
66/34 |
|
to influence your ability to cope with breathlessness? Yes/Unchanged |
93/7 |
|
Compared with the time before rehabilitation how do you feel now? Very much better/Somewhat better/The same |
34/42/24 |
|
What is your overall opinion about the rehabilitation programme? Excellent/Very good/Good/Do not know |
63/30/7/0 |
|
COPD: chronic obstructive pulmonary disease. |
|
A total of 50 patients (34%) were lost to the 12-month follow-up. Among completers 11/46 (24%) were lost to follow-up, among drop-outs 15/35 (43%), NRO 15/33 (45%) and PC 9/34 (26%).
The reasons for loss to follow-up were: death (n = 6 patients), treatment with long-term oxygen (n = 6), hip fracture/fall (n = 3), dementia (n = 2), COPD diagnosis withdrawn (n = 4), did not have the strength/did not want to continue (n = 18), or did not show up even though 2–3 appointments were made (n = 11). Those lost to follow-up were analysed separately from those who were followed up, and no systematic differences that might suggest bias due to differential loss to follow-up were found. We explored the differences between follow-up patients and those lost to follow-up. These differences were subtle, but the latter tended to have shorter 6MWD, and the proportion of patients with severe dyspnoea in this group appeared to be larger.
Discussion
We followed a cohort of COPD patients treated at Horsens Regional Hospital in Denmark to investigate outcomes in relation to rehabilitation in clinical routine. Contrary to our hypothesis, completers experienced no improvements in 6MWD from baseline to the end of the 8-week CRR programme. We were not surprised that the 6MWD had declined at 12-month follow-up. To provide long-term functional benefits the patients must first benefit from the programme and, secondly, maintain activity (22) and, as such, the programme must include follow-up rehabilitation to support that physical activities become a part of daily routine (23). There was no follow-up rehabilitation included in the CRR programme at the hospital.
There were no improvements in HRQoL, even though HRQoL is considered a primary outcome in rehabilitation (1–7). Contrary to our expectations (2, 22, 23), the MRC dyspnoea score did not improve. We found no between-group differences in any of the main outcomes; 6MWD, SF-36 or MRC.
These results are important for clinical practice, health professionals and decision-makers. Extensive evidence documents the effect of COPD rehabilitation in RCTs; yet, our results show that obtaining a similar effect in clinical practice is no straightforward task. Our findings emphasize the need for careful selection of rehabilitation candidates, close monitoring of rehabilitation attenders’ performance, and better designed CRR programmes and methodologies.
Three main issues will be discussed to explain our results; the inclusion of participants in CRR and their characteristics, the quality of the CRR programme and the outcome measurements used in CRR.
Inclusion of participants in clinical routine rehabilitation
We found that only a minority of the eligible patients were eventually included in CRR; a finding also made in our previously published review of RCTs (24). However, the CRR inclusion process differs fundamentally from the sampling procedures in RCTs. We previously reported that RCTs are generally not particularly meticulous in explaining their selection criteria, and only a few provide detailed characteristics of the general population from which their study sample is drawn. We found that, on average, three-quarters of the patients who are likely to be suitable for rehabilitation seem to have been deselected, probably in a biased way, due to sampling, exclusion criteria, and drop-outs. It may therefore be argued that most RCT study populations are not sufficiently representative of the COPD rehabilitation target population, and this affects the external validity and may inhibit the implementation and effects of rehabilitation programmes in clinical practice.
Therefore, keen attention should be paid to patients’ characteristics and how these may affect the possibility to reap benefit from rehabilitation. Our study explored the effect of such characteristics. An unexpected finding of the first part of our study (16) was that the patients’ characteristics did not predict completion. In contrast to the findings of our review (24), we found that the small fraction of patients who did complete the rehabilitation programme were not better off than the non-completers. Contrary to RCTs, in CRR the most suitable rehabilitation candidates were not favoured; completers were equally “poor” in terms of comorbidities, smoking and socioeconomic factors compared with non-completers. A lesson that may be learned from this is that participants in RCTs and CRRs differ.
The formal criteria for including patients for hospital-based CRR were few: an FEV1<50% combined with an estimated MRC ≥ 3. In contrast, the formal inclusion and exclusion criteria (24) used in RCTs imply that patients selected for RCTs are largely without comorbidities. We compared RCT and CRR rehabilitation candidates on the suspicion that RCT completers might differ from CRR completers in terms of co-morbidities, and socioeconomic factors, and found that the characteristics of the latter did, indeed, differ from those of the former. Hence, approximately 80% of the CRR attenders had at least one additional disease, and it is well-known that comorbidity affects the efficacy of rehabilitation (25, 26). The evidence-based guidelines for rehabilitation must be implemented in a manner that takes the existence of comorbidity into account and that the RCT should also include patients with comorbidity to better mirror real-life scenarios.
Moreover, financial resources for the CRR programme were limited, and this made it necessary for the healthcare professionals to select candidates from among those who met the hospital’s formal inclusion criteria. Four courses were held during the study period, which afforded approximately 80 patients with the chance of attending the CRR course; 46 eventually completed the course. Under these circumstances, the formal inclusion criteria (FEV1 and MRC) were not particularly well-chosen because these criteria cannot be used either to predict attenders’ ability to complete (27) or their motivation for completion.
To complicate things further, our study discovered that 15 patients who were offered rehabilitation had an FEV1 above 50%. This inconsistency also shows that the formal criteria are difficult to use in practice.
In all, our results draw attention to the resources used for CRR rehabilitation in Denmark, and they suggest that the time may be ripe for a redefinition of the referral criteria and for an adjustment to patients with several co-morbidities via broader-based patient assessment prior to enrolment.
Quality of the rehabilitation intervention
Given that the CRR brought no improvement, we may reasonably raise the question as to why resources should be allocated to a programme that does not work.
Given the seriousness of the COPD burden, a more relevant question may, however, be what could be done to improve the rehabilitation effort targeting patients with COPD in clinical practice.
Besides issues in relation to inclusion, we think that the absence of any improvements owing to the CRR testifies to intervention insufficiency. The present CRR programme ran for 8 weeks, with 45-min training sessions twice a week. However, exercise intensity was “increased as tolerated” and no intensity measurement was attempted. The patient education part of the programme was occasionally extended, which left little time for physical training. In addition, the CRR training intervention is far less streamlined than an RCT intervention; it involves less coaching, and CRR participants may be disadvantaged in terms of motivation, programme adherence and ability to follow programme guidelines and to tolerate exercise (28–31). Poorly prescribed exercise regimes may cause patients to complete training sessions at suboptimal activity levels. The intensity, duration and frequency of the training performed may therefore simply have been insufficient to achieve improvements (10, 28–30, 32–35). Furthermore, the practical and physical conditions might not provide optimal conditions for training. In future, the monitoring of exercise intensity and the support of home training should be improved. Up-to-date equipment, such as easily applicable heart rate monitors, was not available, although this would have been relevant (10, 31). Each patient could have been equipped with a heart rate monitor and encouraged to do training, e.g. by cell phone, wiis (a home video games console), or other electronic support, and could have been supervised closely (10).
Furthermore, an individualized intervention designed to meet the patient’s needs and ability in a more systematic way would probably also have achieved better results. There may also be a need for more staff training, as competences and dedication to all parts of the CRR programme may help improve its efficiency (36).
Methods used in clinical routine rehabilitation
Despite the lack of improvement in objectively measured outcomes, completers perceived subjective improvements. Although the fraction of completers was small, and the fraction that answered the questionnaire was even smaller, this is of clinical relevance. The difference between objective measurements and subjective outcomes may imply that patients achieved improvements relevant to them, meaning that the failure to respond in terms of physiological outcomes does not necessarily imply that the CRR was unsuccessful (10). This implies that patient-oriented outcomes should be used in CRR.
Methodological considerations
Conducting an observational study of a CRR already implemented at a hospital affords the present study with data obtained from a real-life context. We believe that the findings made at the hospital included in the present study could be representative of CRR findings at other regional hospitals (37).
The 6MWD test was relevant and sensitive (18, 38, 39). Health-related HRQoL was measured with the SF-36, which is a validated generic questionnaire that focuses on broad aspects of HRQoL and health status (20). We chose the SF-36 because this instrument is used in monitoring chronic care across a wide range of disease conditions in our region. However, there might be a risk that the instrument was not sufficiently sensitive to detect changes in the present patient population (20). Dyspnoea was assessed by means of the MRC dyspnoea scale (17). This questionnaire is commonly used in RCTs on rehabilitation and at the hospital of the present study, but the MRC may not be sufficiently sensitive to detect changes over time.
Misclassification can occur, as some patients with an FEV1 ≥ 50% of the predicted value were offered rehabilitation even though the formal criterion was that only patients with a FEV1 < 50% should be offered rehabilitation. On the other hand, patients with a FEV1 < 50% did not always receive an offer, although they did meet the formal criteria. The changes in 6MWD, HRQoL and dyspnoea in relation to CRR can therefore be biased due to misclassification. However, the FEV1 does not precisely predict 6MWD, HRQoL or dyspnoea (27, 40, 41), and we therefore cannot be sure in which direction this misclassification might affect the findings.
We did not find that the known confounders, e.g. lung function, smoking and comorbidities, were distributed differentially between the groups, and the confounders were therefore not likely to affect the differences between the groups.
Conclusion
The completion rate was low, and the inclusion of participants for CRR did not always follow strict criteria. Completers of CRR did not differ from non-completers at baseline and did not improve in terms of 6MWD, HRQoL or MRC, despite their subjective feeling of improvement. The 6MWD was sustained from baseline to the end of CRR, but had fallen at the 12-month follow-up.
Despite convincing documentation for the effect of rehabilitation, the implementation of evidence-based rehabilitation programmes in clinical routine is severely challenged, even in selected patient populations and when orchestrated by determined staff. These CRR results at best showed no improvements, at worst a decline. New practice-based studies are needed to focus on the contexts in which CRR targets broad populations of patients with COPD. Successful implementation in clinical routine requires targeted recruitment and overall programme improvement in general, and a stronger focus on physical training and staff competences.
ACKNOWLEDGEMENTS
The authors would like to thank the staff at the Medical Department, Horsens Regional Hospital, Denmark, for collection data and general supervision.
We also thank Niels Trolle Andersen, Statistician, Department of Bio-statistics, Institute of Public Health, Aarhus University, Denmark, for supervising the statistical analysis, and Jakob Hjort, Anne-Marie Jensen, and Elinborg Thorsteinsson, Department of Data-Management, Centre for Public Health, Central Denmark Region, for supervising the data-management.
The authors declare no conflicts of interest.
References
