Dielise D. Iucksch, MSc1, Vera L. Israel, PhD2, Danieli I. R. Ribas, MSc3 and Elisangela F. Manffra, Dr. rer. nat.4
From the 1Centro Hospitalar de Reabilitação Ana Carolina Moura Xavier, Curitiba, Brazil, 2Universidade Federal do Paraná, Setor Litoral, Matinhos, 3Faculdades Integradas do Brasil, Curitiba and 4Programa de Pós-Graduação em Tecnologia em Saúde, Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba, Brazil
OBJECTIVE: To describe the kinematic gait characteristics of individuals with incomplete chronic spinal cord injury in a water environment and to compare these characteristics with those of healthy individuals.
DESIGN: Cross-sectional study.
SUBJECTS: Nineteen adults divided into 2 groups: individuals (n = 9) with incomplete chronic spinal cord injury (> 1 year), American Spinal Injury Association Impairment Scale (AIS) C or D; and a second group (n = 10) of healthy young adults. The groups were paired up according to body mass and height.
METHODS: Participants walked at a self-selected speed in a heated pool with water at the level of the xiphoid process. Participants with spinal cord injury were allowed to hold the researcher’s hands. The body segment and joint angle coordinates in the sagittal plane were retrieved with SIMI Motion software. Temporal-spatial variables and joint ranges of motion were compared between groups.
RESULTS: Duration of stance phase, stride length and speed differed significantly (p < 0.05) between groups. The ranges of joint motion were not significantly different (p > 0.05), and the joint angle patterns were qualitatively similar between groups.
CONCLUSION: The physical properties of water provided the required time for reorganization of gait phases and allowed all individuals with spinal cord injury to walk in the water environment.
Key words: incomplete spinal cord injury; human gait; kinematics; water environment; rehabilitation; hydrotherapy.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Dielise Debona Iucksch, Quintino Bocaiúva Street, 329, 80035-090 Curitiba – PR, Brazil. E-mail: dielise@gmail.com
Accepted Mar 18, 2013, Epub ahead of print Jul 4, 2013
INTRODUCTION
Recovery of walking function is regarded by individuals with spinal cord injury (SCI) as one of the main goals of rehabilitation, irrespective of the severity or duration of SCI, or of age at the time of injury (1). Accordingly, gait training strategies for people with SCI, such as functional electrical stimulation (2) and treadmill training with partial weight support with manual help (3) or robotic devices (4), have been investigated in recent decades. More recently, gait training in water has drawn research attention, and qualitative improvements in motor function and gait speed following water training have been reported (5).
Warm water is an interesting therapeutic resource because it promotes muscle relaxation and apparent reduction in body weight (6, 7–9), allowing the individual to exploit motor experiences without the need for assistive devices. The water environment makes it easier for the person with SCI to move their limbs, allowing the execution of motor skills that are not usually possible on dry land. In clinical practice, it is common to observe that SCI patients who are not able to walk without assistance on land can walk in water with some movement compensations and body adjustments (10). Intuitively, this phenomenon is due to the physical properties of water, such as buoyancy and hydrostatic pressure (6), which might favour the execution of residual movements. However, the actual biomechanical gait characteristics of SCI patients in water compared with those of healthy individuals are not known.
In the case of healthy individuals, there is strong evidence to suggest that the physical properties of water lead to changes in gait characteristics. It has been observed that walking in water is associated with longer stride duration, shorter stride length and slower gait speed (11, 12) compared with walking on dry land. The magnitude of the ground reaction forces and the impact force measured in water are smaller than on land (11, 13, 14). Moreover, electromyographic activity during walking in water is reduced in comparison with on dry land (11, 15, 16). It was also observed that the water environment favours knee flexion and ankle neutrality (17). Nevertheless, the range of motion of lower limb joints was similar in both environments (11, 18).
In summary, these studies reveal lower strength requirements and, consequently, less muscular effort in water to attain the same ranges of motion achieved on dry land at self-selected velocities. Because muscular weakness is an important limiting factor for individuals with incomplete SCI, it is possible that in water the characteristics of their gait would resemble that of non-impaired individuals. Therefore, the objective of this study was to describe the gait kinematics of subjects with SCI and compare them with those of healthy individuals. We believe that this description is a first step towards a better understanding of the motor patterns and strategies adopted by subjects with SCI to walk in water. Moreover, therapists could benefit from this description because it complements their intuitive clinical knowledge and might provide parameters for analysing and discussing patient performance.
Kinematic description of gait is one of the methods applied in SCI gait research and provides the outcome measures of clinical trials (19) that allow the assessment of clinical protocols. It has been suggested that clinicians could benefit from incorporating motion analysis in their practice for diagnosis, setting objectives, intervention planning and monitoring patient progress in a more precise way (20). Applying motion analysis methods for gait research in water is technically more difficult than on dry land (18). Thus, no kinematic or kinetic data for individuals with incomplete SCI walking in water, as opposed to dry land, are available in the literature.
METHODS
This was a cross-sectional study conducted with 19 adult volunteers who were divided into 2 groups: a spinal cord group (SCG) composed of 9 individuals (8 men and 1 woman) with incomplete chronic SCI and a non-injured group (NIG) composed of 10 males. Inclusion criteria for the SCG were: adult volunteers, incomplete SCI for more than 1 year, level of bone injury between C4 and L1, American Spinal Injury Association Impairment Scale (AIS) C or D (21), spasticity grade between 1 and 4 according to the Ashworth Scale (22) and clinically and surgically stable. Individuals with lower limb pathologies that were not related to SCI were excluded, as were those who had contraindications for water activities. The NIG included young adult males with a normal gait pattern and no impairments of the orthopaedic, nervous and cardiopulmonary systems. The groups were paired up based on body mass and height to assure that neurological issues, and not body composition, would be the determinant factors of group differences.
This trial was approved by PUCPR Ethics Committee (0001400/08). All individuals were informed about the study procedures and objectives and gave their written informed consent.
The mean (standard deviation; SD) for age, height and mass for the SCG were 39 years (SD 14.2 years), 1.70 m (SD 0.07 m) and 67 kg (SD 9.5 kg), respectively. The individuals belonging to the SCG were assessed according to the AIS (21). The Walking Index for Spinal Cord Injury (WISCI II) (23, 24) was used to assess gait on dry land, thus determining the need for orthotic or physical assistance during walking. Spasticity of the lower limbs was graded by the Ashworth Scale (22). The results of these assessments are summarized in Tables I and II. The NIG individuals presented a mean age of 24.4 years (SD 3.5), 1.71 m (SD 0.04) of height and body mass of 66 kg (SD 4.1).
|
Table I. Characteristics of the spinal cord group (SCG) subjects (n = 9) |
||||||
|
Participant |
Age, years |
Post-injury time, years |
Bone injury level |
AIS |
WISCI |
FIM motor |
|
P1 |
48 |
1.1 |
C5, 6, 7 |
D |
20 |
89 |
|
P2 |
43 |
6.0 |
C6 |
D |
16 |
88 |
|
P3 |
23 |
1.5 |
C5, 6 |
D |
12 |
88 |
|
P4 |
53 |
21.0 |
T3, T4 |
D |
12 |
86 |
|
P5 |
31 |
5.0 |
C4, 5 |
D |
20 |
88 |
|
P6 |
57 |
33.0 |
T12, L1 |
D |
19 |
88 |
|
P7 |
18 |
2.3 |
C5 |
D |
20 |
91 |
|
P8 |
51 |
10.0 |
T12, L1 |
C |
12 |
85 |
|
P9 |
29 |
1.8 |
T12, L1 |
C |
16 |
90 |
|
AIS: American Spinal Injury Association Impairment Scale; WISCI: Walking Index for Spinal Cord Injury; FIM: Functional Independence Measure. |
||||||
|
Table II. Muscle strength of lower limbs of spinal cord group (SCG) participants graded according to American Spinal Injury Association Impairment Scale (AIS) scale |
||||||||||||
|
Participant |
Hip flexors |
Knee extensor |
Knee flexor |
Ankle dorsiflexor |
Ankle plantar flexors |
Long toe extensor |
||||||
|
L |
R |
L |
R |
L |
R |
L |
R |
L |
R |
L |
R |
|
|
P1 |
4 |
4 |
5 |
5 |
4 |
3 |
4 |
3 |
5 |
4 |
5 |
5 |
|
P2 |
4 |
4 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
3 |
4 |
|
P3 |
5 |
5 |
5 |
5 |
5 |
5 |
4 |
4 |
4 |
4 |
4 |
5 |
|
P4 |
1 |
4 |
4 |
4 |
4 |
4 |
2 |
3 |
3 |
3 |
3 |
4 |
|
P5 |
3 |
5 |
5 |
4 |
4 |
4 |
5 |
2 |
5 |
2 |
4 |
0 |
|
P6 |
3 |
3 |
5 |
5 |
4 |
4 |
5 |
5 |
5 |
5 |
5 |
5 |
|
P7 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
|
P8 |
3 |
3 |
5 |
5 |
4 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
|
P9 |
5 |
5 |
4 |
4 |
4 |
3 |
1 |
1 |
1 |
0 |
1 |
0 |
|
L: left; R: right. |
||||||||||||





Both groups received gait assessment in the water with video analysis. Adhesive waterproof square markers were placed on the participants’ skin prior to video recording, following the 4-segment model proposed by Winter (25). The foot segment is delimited by the lateral malleolus and head of the 5th metatarsal; the leg segment by the lateral femoral condyle and lateral malleolus; the thigh by the greater trochanter and lateral femoral condyle and the head-arms-and-trunk (HAT), by the greater trochanter and 10th rib. The video recording of NIG individuals was made with the same marking, except for the HAT segment, which was delimited by the greater trochanter and acromion.
Data collection took place in a pool heated to approximately 33ºC, with the water level kept approximately at the xiphoid process. Images were obtained in the sagittal plane with a digital video camera with a frequency of acquisition of 30 Hz.
Participants went into the pool and stayed for as long as they judged necessary to become adapted to the water environment. They were then filmed as they walked over a bounded path on the pool floor that was 1 m wide and 3 m long. It was intended that SCI participants walk along the path without external assistance. However, 2 participants were not able to perform the task without help, probably due to an absence of aquatic skills. Therefore, to maintain the homogeneity of the SCG, assistance was provided to all SCI participants. The assistance consisted of support by the researcher using both hands, without the researcher interfering with the walking speed selected by the participant. The researcher kept her elbows fixed on her own waist and did not pull the subjects (Fig. 1). This type of assistance is usually provided to injured patients in hydrotherapy, especially when they do not have aquatic skills.

Fig. 1. A participant with spinal cord injury (SCI) walking with researcher support, with markers positioned on the anatomical landmarks.
All participants walked over the bounded path at least 5 times in each direction while being recorded. From these images, 3 strides taken on the participants’ right and left sides were selected for analysis. To select these strides, the first and the last recorded strides were discarded, and the 3 that were more focused and centralized within the visual field of the camera lens were chosen. The kinematic parameters of the selected strides were calculated with SIMI Motion v.6.1 software (Unterschleissheim, Germany). The spatio-temporal gait parameters and joint ranges of motion of each individual were obtained by calculating the mean of the 3 selected strides.
The Shapiro-Wilk test was used to check the normality of data distribution. To verify whether external assistance or the side influenced gait parameters of those SCI participants who could walk with and without assistance analysis of variance (ANOVA) analysis was applied. Comparisons between the groups were performed with the unpaired Student’s t-test or Mann-Whitney test, according to data normality. The limit for statistical significance was set to 0.05.
RESULTS
Mean values of spatio-temporal gait parameters and joint ranges of motion obtained from individuals of the SCG who could walk with and without assistance did not differ between these two conditions. Thus, instead of excluding the 2 individuals who could not walk without assistance from the analysis, we will describe and discuss only the results obtained with assistance. No difference between sides was found either.
As seen in Table III, SCG participants walked with a significantly shorter stride length (34%) and significantly slower walking speed (62%) than those in the NIG. Only two subjects in the SCG could walk with a stride time within the range of the NIG, and none of those subjects had a walking speed matching those of the NIG. Regarding the duration of the stance phase, only two SCG participants (P6 and P7) had values within the NIG range. These 2 subjects also had the highest strength levels in the group (Table II).
Despite all of the differences in the spatio-temporal parameters, most SCG participants had joint range of motion (ROM) close to the NIG values. Indeed, joint ROMs were not significantly different between groups (Table III).
|
Table III. Spatio-temporal parameters and angular ranges of motion during gait in the water environment of spinal cord group (SCG) and non-injured group (NIG) participants |
||||||||
|
Group |
Participant |
Stride time (s) |
Stance (%) |
Stride length (m) |
Speed (m/s) |
Hip ROM (◦) |
Knee ROM (◦) |
Ankle ROM (◦) |
|
SCG |
1 |
2.72 |
66 |
0.37 |
0.14 |
10.19 |
15.50 |
14.06 |
|
2 |
3.83 |
69 |
0.80 |
0.21 |
31.36 |
40.43 |
18.96 |
|
|
3 |
4.57 |
68 |
0.67 |
0.15 |
56.28 |
88.35 |
18.70 |
|
|
4 |
6.00 |
81 |
0.84 |
0.14 |
44.07 |
91.02 |
27.37 |
|
|
5 |
4.51 |
75 |
0.75 |
0.17 |
21.70 |
22.11 |
25.12 |
|
|
6 |
3.94 |
63 |
0.46 |
0.12 |
18.72 |
50.98 |
15.75 |
|
|
7 |
3.91 |
62 |
0.99 |
0.25 |
32.48 |
78.07 |
25.79 |
|
|
8 |
3.92 |
67 |
0.50 |
0.13 |
62.38 |
87.50 |
40.34 |
|
|
9 |
3.17 |
68 |
0.70 |
0.22 |
34.51 |
58.11 |
–1.22 |
|
|
Median |
3.92 |
0.68 |
0.70 |
0.15 |
32.48 |
58.11 |
18.96 |
|
|
Min–Max |
2.72–6.00 |
62–81 |
0.37–0.99 |
0.12–0.25 |
10.19–62.38 |
15.50–91.02 |
–1.22–40.34 |
|
|
NIG |
Median |
2.71 |
59 |
1.08 |
0.40 |
39.13 |
71.69 |
31.16 |
|
Min–Max |
2.22–3.45 |
53–64 |
0.96–1.26 |
0.31–0.53 |
25.45–61.16 |
55.44–126.11 |
20.55–39.80 |
|
|
p-value |
0.0005a |
– |
< 0.0001a |
< 0.0001a |
0.498a |
0.540b |
0.088a |
|
|
aStudent’s t-test; bMann-Whitney test. ROM: range of motion. |
||||||||
In an attempt to describe the motion patterns of SCI in a water environment, the mean angular trajectories of the SCG are shown in Fig. 2 together with those of the NIG and the respective standard deviations. The high variability present in SCG trajectories can be easily observed.
Regarding the mean angular trajectories (Fig. 2), it was observed that the hip joint movement of the SCG begins with less flexion than the NIG, whereas the latter continues to extend until it reaches the neutral position at the end of the stance phase (at approximately 59% of the gait cycle). The minimum value of the hip joint of the SCG displays more flexion and reaches its turning point before the end of the stance phase (at 68% of the cycle). The mean knee-joint trajectories of both groups were very similar. The greatest difference occurred at the end of the stance phase, in which the NIG begins to bend the knee at approximately 60% of stride, whereas the SCG begins this movement earlier, indicating that, for the majority of the SCG sample, the knee remained flexed during the entire stance phase. The ankle joint (whose neutral position corresponds to 60º according to the biomechanical model used) was kept at approximately 10º of plantar flexion during the entire gait cycle for the SCG. Moreover, compared with the NIG, in the SCG there was maintenance of the plantar flexion position even after the beginning of the swing phase, practically without recovery towards dorsiflexion.
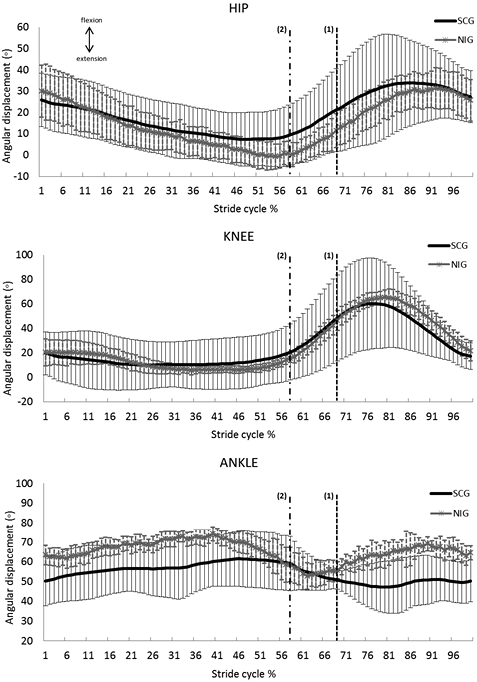
Fig. 2. Mean joint angle trajectories during gait cycle in the underwater environment of spinal cord group (SCG) (black) and non-injured group (NIG) (grey). The bars represent 1 standard deviation. Vertical dashed lines indicate the end of stance phase for SCG (1) and NIG (2).
DISCUSSION
To the best of our knowledge, this study reports for the first time the kinematic parameters of gait in water for individuals with incomplete chronic SCI and compares those parameters with those of healthy individuals.
For physiotherapists applying gait training in subjects with SCI, we believe that this study provides a methodological framework for analysing the kinematic data of their patients. Although the results shown here are far from normative data, they could be regarded as a starting point for analysing patient performance in a more objective way. Kinematic analysis has helped to determine patterns of abnormalities in the gait of SCI patients on dry land, as reported in Krawetz & Nance (26), who were able to associate weakness and spasticity with these patterns. More recently, Abel et al. (27) performed a kinematic analysis during treadmill training to monitor patients with SCI during therapy to ensure that joint range of motion would remain inside physiological limits while velocity was increased.
All individuals with SCI were capable of walking in water under the conditions of this study. This fact indicates that for people with SCI, walking in water may offer therapeutic benefits that improve motor skills aimed at increased functionality and independence. The water environment can thus be an option that allows SCI individuals to learn to generate and control the necessary forces for walking or to improve their performance.
The performance of SCG participants in water is mainly due to the physical properties of water, especially buoyancy and hydrostatic pressure, which act in a way that decreases apparent body weight and contributes to ascending movements (9, 28). These properties lead to a reduced requirement for muscle activation (15) and for the generation of joint torque (18). These reductions favour gait production in conditions with sensory-motor alterations such as those present in SCI. Buoyancy also supports hip and knee flexion, increasing foot clearance during the swing phase. It is speculated that the physical properties of water may stimulate the activation of locomotor centres (CPG) present in the spinal cord itself, producing rhythmic patterns of movement such as gait (5, 29, 30). This argument is reinforced by the results obtained in a trial with rats injured in the thoracic region, in which the animals were capable of generating a functional locomotor pattern in shallow water after the acute post-injury phase (31). The authors observed that there was improvement in gait after locomotor training in the underwater environment, even though the animals were not able to transfer the results to the land environment.
The comparison of spatio-temporal parameters of gait in the water between the two groups reveals that the SCG participants walked with a shorter stride length, a longer stance phase and slower speed than the NIG individuals. These differences might result from the motor strategies used by the SCG individuals to organize and control movement in the water to face constraints imposed by SCI. The reduced strength of the lower limbs, together with balance impairment (partially compensated by the researcher’s hand support) and the sensorial information deficit, might have led to a shorter stride length in the individuals with SCI. Another factor to be considered is spasticity, which is important for maintaining an upright position, but which may have contributed to the shorter stride length and to the longer time of the stride execution.
Although the underwater environment facilitates ascending movement, it also offers more frontal resistance, requiring greater effort to overcome the fluid viscosity and the drag force (6) to push the body forward. The viscosity of the water leads to slower movement execution because the increased speed leads to higher drag force. Thus, the speed of healthy individuals is always slower in the water environment than it is on land (11, 12, 14, 18). For the individuals with SCI, speed was even slower than for healthy individuals because individuals with SCI have less muscle strength to overcome such resistance. The SCG individuals probably had a shorter stride length because of the interaction of frontal resistance in water with the aforementioned neuromuscular conditions. Moreover, it was observed that individuals with SCI had a longer stance phase; the water environment provides more support than the land environment, which indicates an increase in the stance time and temporal reorganization compared with the NIG. This finding leads to the assumption that, compared with healthy individuals, SCI individuals develop different strategies for walking in water. Even with lower speed and shorter stride length in the NIG, also found by Barela & Duarte (32), the healthy individuals displayed the same relationship of duration in the stance and swing phases both in water and on land.
The results of this trial indicate that people with SCI follow a pattern of progression of joint angles that is qualitatively similar to that of healthy individuals, but it is important to note that gait phases are not organized in the same way as in the healthy condition. Regarding the mean angular trajectories, a certain similarity can be observed in the pattern of hip joint, knee and ankle curves between the SCG and the NIG. On average, the ankle joint in the SCG did not recover dorsiflexion in the swing phase, leading to earlier foot contact on the floor, thus contributing to a greater duration of the stance phase and to a reduction in stride speed. It is thought that this phenomenon occurs due to the SCI, which keeps the ankle from executing the correct ankle rocker movement, even though this issue is more related to body support than to propulsion in the water environment (18).
It was also observed that, on average, there was greater flexion in the hip joint in the SCG throughout the gait cycle, probably to assist limb movement in the swing phase. We must also consider the facilitation of ascending movements due to buoyancy and the effects of frontal resistance in the water environment. This resistance during gait may have led participants to bend the hip and knee joints as a way to reduce the frontal area and, consequently, the effects of the drag force (12, 16, 32). The greater control of proximal muscles present in the SCG also contributed to increased adaptations of movement at the hip.
Although the focus of this work was not on the coronal plane, we cannot refrain from mentioning some body adjustments made by the individuals with SCI, such as the extension and rotation of the trunk to allow foot release in the swing phase. This coordination involving the trunk and upper limbs is a strategy used by individuals with SCI to compensate for lower limb weakness (33). Nevertheless, in this trial, only the sagittal analysis was used and, therefore, it was not possible to quantify such adjustments.
The assistance provided by the therapist in this study might have influenced the results, but it was based on the principles of hydrotherapy, in which the patient’s body is stabilized to free his or her attention to other requirements, such as coordination and balance (34, 35). Therefore, the results shown here are those expected in the clinical conditions of hydrotherapy. Moreover, assistance without traction, as provided in this study, can be progressively modified in future studies of hydrotherapy interventions for gait rehabilitation. Quantitative or qualitative approaches for assessing these interventions could help in verifying how underwater gait recovery mechanisms are recruited. Those assessments might indicate that individuals with SCI try to apply strategies of motor control adjustments that go beyond the simple body compensations mentioned above. There are differences in postural response mechanisms between SCI and healthy individuals regarding feedback and feed forward that must be taken into account during the recovery of functional gait by SCI individuals (36) when planning gait training in water.
In spite of differences in mean joint trajectories between groups, their ranges of motion did not show significant differences, probably due to the large standard deviation in the SCG. The variability of kinematic data (parameters and trajectories) reflects the different strategies adopted by participants with SCI to accomplish gait due to their diversity in terms of injury level and muscle strength. Therefore, a kinematic pattern for gait in water of subjects with SCI could not be determined due to our small and diverse sample. What these results do reveal are the kinematic strategies utilized by the participants in this study to walk in water in spite of their limitations. The present trial had a small number of participants with SCI, composed of tetraplegic and paraplegic individuals, and caution must be taken not to generalize the results obtained. However, our study has value, both because there are only a small number of scientific trials assessing movement in pathological conditions in the water compared with the considerable number of trials assessing healthy subjects, and because of the difficulties involved in making effective kinematic analyses in the water environment.
The authors declare no conflicts of interest.
REFERENCES
