Silvia Sterzi, MD1, Alfredo Cesario, MD2,3, Giacomo Cusumano, MD2, Valentina Dall’Armi, MSc4, Luisa Maria Lapenna, MD5, Vittorio Cardaci, MD3, Pierluigi Novellis, MD2, Filippo Lococo, MD6, Giuseppe Maria Corbo, MD7, Stefano Cafarotti, MD2, Stefano Margaritora, MD2 and Pierluigi Granone, MD2
From the 1Department of Rehabilitative Medicine, Campus Biomedico University, 2Division of General Thoracic Surgery,Catholic University, 3Scientific Direction, IRCSS San Raffaele Pisana 4Unit of Clinical and Molecular Epidemiology, IRCSS San Raffaele Pisana, 5Department of Rehabilitative Medicine, IRCSS San Raffaele Pisana, Rome, 6Unit of Thoracic Surgery, IRCCS Santa Maria Nuova Hospital, Reggio Emilia and 7Department of Pulmonary Pathophysiology, Catholic University, Rome, Italy
Introduction: Post-operative pulmonary rehabilitation in patients who have undergone surgery for lung cancer is a subject of open debate. Clinical practice in this setting is based on the results of observational trials, such as the one described here. Prospective randomized controlled trials have been registered and recruitment is ongoing.
METHODS: From 2005 to 2008, 110 patients with surgical non-small cell lung cancer were entered into a post-operative inpatient pulmonary rehabilitation programme for 3 weeks. All patients were evaluated for pulmonary function after surgery (time 0; T0) and at the end of pulmonary rehabilitation programme (time 1; T1). Statistical analysis focused on improvement in pulmonary function parameters and physical performance in the 6-min walking test (6MWT). Mixed models multiple linear regression was used to identify parameters related to the primary end-points of this research.
RESULTS: Patients’ mean age was 70.1 years (standard deviation (SD) 8.5 years); male/female ratio 73/37. A total of 94 patients underwent lobectomy, 8 underwent pneumonectomy, and the remaining 8 underwent bilobectomy. Among the analysed parameters a significant improvement could be detected only with regards to the 6MWT (257.4 (SD 112.2) at T0 and 382.8 (SD 11.09) at T1).
CONCLUSION: Post-operative pulmonary rehabilitation in patients with surgical non-small cell lung cancer is effective in terms of exercise tolerance.
Key words: pulmonary rehabilitation; NSCLC; pulmonary function; effort tolerance; functional modifications; surgery.
J Rehabil Med 2013; 45: 00–00
Corresponding address: Filippo Lococo, Unit of Thoracic Surgery, IRCCS Santa Maria Nuova Hospital, Vial risorgimento, n 80, 42121 Reggio Emilia, Italy. E-mail: filippo_lococo@yahoo.it
Accepted Mar 14, 2013, Epub ahead of print Jul 4, 2013
INTRODUCTION
Lung cancer is the most common solid neoplasm in the world and surgery remains the mainstay of therapy. However, surgery itself is the direct cause of pulmonary function impairment, which dramatically reduces patients’ tolerance to exercise and their quality of life (QoL) (1, 2). This is particularly true for those patients undergoing multimodality approaches and/or who have co-morbidity with chronic obstructive pulmonary disease (COPD).
Pulmonary rehabilitation (PR) is established as a universally accepted therapeutic approach in COPD (3) and several groups, including our own (4, 5), have tested the impact of PR on patients with surgical non-small cell lung cancer (NSCLC) in the pre-operative and post-operative setting. On the basis of the preliminary results of these and other observational studies, the European Respiratory Society and European Society of Thoracic Surgeons (ESTS) (6) have voiced the need to foster additional research in this field, since a clear benefit for patients who have undergone pulmonary rehabilitation after surgery for NSCLC has been homogeneously reported. We report here the observational data for 110 patients operated for NSCLC and rehabilitated in the immediate post-operative period.
MATERIAL AND METHODS
Population
We retrospectively reviewed the clinical evidence of a cohort of 110 consecutive patients who underwent PR after resection for NSCLC in the period between September 2005 and June 2009. Patients who underwent surgery at the Department of Thoracic Surgery of the Catholic University were transferred to the Department of Rehabilitative Medicine of the “Campus Bio-Medico” University, both located in Rome, Italy. In the rehabilitation scenario, a 2–3 week rehabilitative protocol was administered. Given the lack of established evidence recommending in-patient PR after lung resection, we gave all resected eligible patients the choice to be included in our PR programme. Inclusion criteria were: (i) surgically treated NSCLC; (ii) ability to understand and comply with the PR programme and use the necessary equipment; and (iii) motivation to participate in a full in-patient PR programme. All patients received a standardized surgical approach (lateral muscle sparing thoracotomy) and supportive pharmacological treatment (pain control), and those (all treated with early physiotherapeutic interventions up to discharge from the surgical unit) who agreed to participate were admitted to the PR protocol.
Pulmonary function evaluation
The PR protocols were coherent with those adopted in (4). Subjects participated in 5 daily sessions/week (3-h supervised sessions). The PR programme consisted of: (i) incremental exercise up to 30 min of continuous cycling at 70–80% of maximum work-load achieved on an incremental cycle-ergometer test carried out at admission. At rest, subjects were asked to indicate their perceived breathlessness/ dyspnoea and leg fatigue by pointing to a number or phrase on a 10-point modified Borg scale; (ii) abdominal muscle activities, in-breathing resistive sessions, treadmill, upper and lower extremities training and full arm circling; (iii) educational sessions conducted twice a week (pulmonary physiopathology, pharmacology of patients’ medications, dietary counselling, relaxation and stress management techniques, energy conservation principles and breathing re-training).
Among the parameters of interest, dynamic and static lung volumes, blood gases analysis and exercise tolerance, as measured by the 6-min walking test (6MWT), were measured before discharge from the thoracic surgery unit and at the end of the PR during pneumological follow-up visits. In particular, the following features were considered: (i) standard spirometric data (forced expiratory volume in the 1st s; FEV1, forced vital capacity; FVC, forced expiratory flow at 25–75%; FEF25–75%); (ii) arterial blood gases (ABGs) analysis; and (iii) 6MWT with recording of distance walked.
Statistical analysis
The role of several demographic, clinical and surgical factors on the distance walked at the 6MWT and on all the spirometric and ABGs measurements were explored by means of mixed-effects multiple linear regression analysis. In particular, the following explanatory variables were considered in the regression modelling as potential confounders of the effect of the rehabilitation: age, body mass index (BMI), type of surgery, Borg scale score, days to rehabilitation, length of hospital stay, and baseline score (different for each outcome). A random intercept and slope were also fitted. The likelihood ratio test was applied to select the best-fitting model. The goodness of fit of the regression models was assessed with the following criteria: (i) a reasonably low residual variance; (ii) standardized residuals within a range of ± 2 (few exceptions allowed within the range of ± 4); and (iii) a good approximation of the fitted data to the observed data.
The critical limit for significance was set at the 5% level. Bonferroni adjustment was applied. All statistical analyses were performed in STATA/SE Release 10.0.
RESULTS
Descriptive statistics
The mean period of time that the patients spent in the rehabilitation unit was 17.7 days (standard deviation; SD 5.2 days). The sample distribution according to the main demographic, clinical and surgical characteristics is summarized in Table I.
|
Table I. Frequency distribution of the sample for the main demographic, clinical and surgical characteristics |
||
|
|
|
n (%) |
|
Gender (n = 110) |
||
|
Female |
37 (33.6) |
|
|
Male |
73 (66.4) |
|
|
BMI (n = 110) |
||
|
< 25 |
52 (47.3) |
|
|
≥ 25 to < 30 |
47 (42.7) |
|
|
≥ 30 |
11 (10.00) |
|
|
Age (n = 110) |
||
|
< 65 years |
26 (23.6) |
|
|
≥ 65 to ≤ 70 years |
28 (25.45) |
|
|
> 70 to ≤ 76 years |
27 (24.55) |
|
|
≥ 76 |
29 (26.4) |
|
|
Days to rehabilitation (n = 110) |
||
|
≤ 7 days |
40 (36.4) |
|
|
> 7 to < 12 days |
42 (38.2) |
|
|
≥ 12 days |
28 (25.45) |
|
|
Length of stay (n = 110) |
||
|
≤ 15 days |
38 (34.55) |
|
|
> 15 to ≤ 20 days |
42 (38.2) |
|
|
> 20 days |
30 (27.3) |
|
|
Resection (n = 110) |
||
|
Lobectomy |
94 (85.45) |
|
|
Bilobectomy |
8 (7.3) |
|
|
Pneumonectomy |
8 (7.3) |
|
|
BMI: body mass index. |
||
An improvement of mean of 125 m in the 6MWT was observed from the beginning to the end of the rehabilitation protocol on the overall sample of 110 patients. A slight overall improvement was also detected in the measurements of ABGs. On the contrary, the serial pulmonary functional analysis showed controversial results; in fact, if on the one hand a certain positive change was found concerning the FEF25–75% values recorded before (T0) and after the rehabilitation treatment (T1), on the other hand, a moderate worsening of the pulmonary function (from T0-evaluation to T1-evaluation) was detected in terms of FEV1 and FVC percentages of the predicted volumes. Table II summarizes measures of central tendency and dispersion for the performance at the 6MWT, and for the main spirometric and ABGs measurements, recorded before (T0) and after the rehabilitation treatment (T1).
|
Table II. Measures of central tendency and dispersion of the main spirometric and emogasanalytic features, and of tolerance to exercise |
|||
|
|
|
n |
Observed Mean (SD) |
|
6-min walking test, mm |
|||
|
Distance walked pre-rehabilitation |
110 |
257.4 (112.4) |
|
|
Distance walked post-rehabilitation |
110 |
382.4 (111.1) |
|
|
Forced expiratory volume/1s, % |
|||
|
Percentage of the predicted pre-rehabilitation |
80 |
72.4 (20.8) |
|
|
Percentage of the predicted post-rehabilitation |
67 |
63.9 (22.1) |
|
|
Forced expiratory vital capacity, % |
|||
|
Percentage of the predicted pre-rehabilitation |
80 |
76.1 (27.7) |
|
|
Percentage of the predicted post-rehabilitation |
67 |
71.0 (18.6) |
|
|
Forced expiratory flow, 25–75% of FVC |
|||
|
Percentage of the predicted pre-rehabilitation |
80 |
47.4 (26.2) |
|
|
Percentage of the predicted post-rehabilitation |
67 |
50.4 (25.2) |
|
|
pH |
|||
|
Pre-rehabilitation |
87 |
7.4 (0.04) |
|
|
Post-rehabilitation |
65 |
7.4 (0.03) |
|
|
pO2, mmHg |
|||
|
Pre-rehabilitation |
91 |
80.4 (12.6) |
|
|
Post-rehabilitation |
69 |
82.4 (9.9) |
|
|
pCO2, mmHg |
|||
|
Pre-rehabilitation |
91 |
36.9 (4.6) |
|
|
Post-rehabilitation |
69 |
37.8 (4.5) |
|
|
SD: standard deviation. |
|||
The improvement observed in the performance at the 6MWT was confirmed by the mixed models regression approach. More specifically, the sample was post-stratified into 4 classes according to the level of baseline performance: class 1: < 180 m; class 2: from 180 to < 250 m; class 3: from 250 to < 330 m; and class 4: 330 m or more. The effect of the rehabilitation on the distance walked at the 6MWT was found to be different depending on the baseline performance. In particular, it was estimated that the difference in improvement between patients in class 1 and 4 was a mean of 70.9 m (SD 19.3), with the largest progress in class 1 (p < 0.0001). Table III shows the effect of the rehabilitation for the 4 performance classes. The linear mixed model fitted time (pre–post rehabilitation), baseline waiting time distribution in 4 classes, their interaction and age as fixed effects, and intercept and time slope as random effects; Fig.1a shows the fitted regression lines for the 4 classes.
|
Table III. Effect of the rehabilitation programme on the distance (m) walked during the 6-min walk test (6MWT), according to the pre-rehabilitation performance. Mean distance walked (standard deviation; SD) pre- and post-rehabilitation, observed change (SD), and estimated pre–post change (effect of the rehabilitation, standard error) |
||||||||
|
Distance walked pre-rehabilitation |
n |
Pre-rehabilitation Mean (SD) |
Post-rehabilitation Mean (SD) |
Change Mean (SD) |
Effect of rehabilitationa Mean (SE) |
p-value |
p-value |
p-value |
|
< 180 m |
26 |
112.4 (43.0) |
254.8 (90.7) |
145.0 (98.0) |
Reference |
Reference |
||
|
≥ 180 m to < 250 m |
26 |
216.7 (21.2) |
391.6 (89.6) |
174.8 (88.9) |
32.4 (20.3) |
0.11 |
Reference |
|
|
≥ 250 m to < 330 m |
26 |
291.3 (14.7) |
410.7 (70.3) |
119.4 (68.0) |
–23.1 (20.3) |
0.25 |
0.0061* |
Reference |
|
≥ 330 m |
32 |
384.1 (66.9) |
455.6 (79.7) |
71.5 (44.0) |
–70.9 (19.3) |
< 0.0001* |
< 0.0001* |
0.0129 |
|
*Statistical significance: p < 0.0083 (6 non-independent multiple comparisons: Bonferroni correction applied). aResults from a linear mixed model regression, accounting for a random intercept and a random slope; estimates adjusted for age. |
||||||||
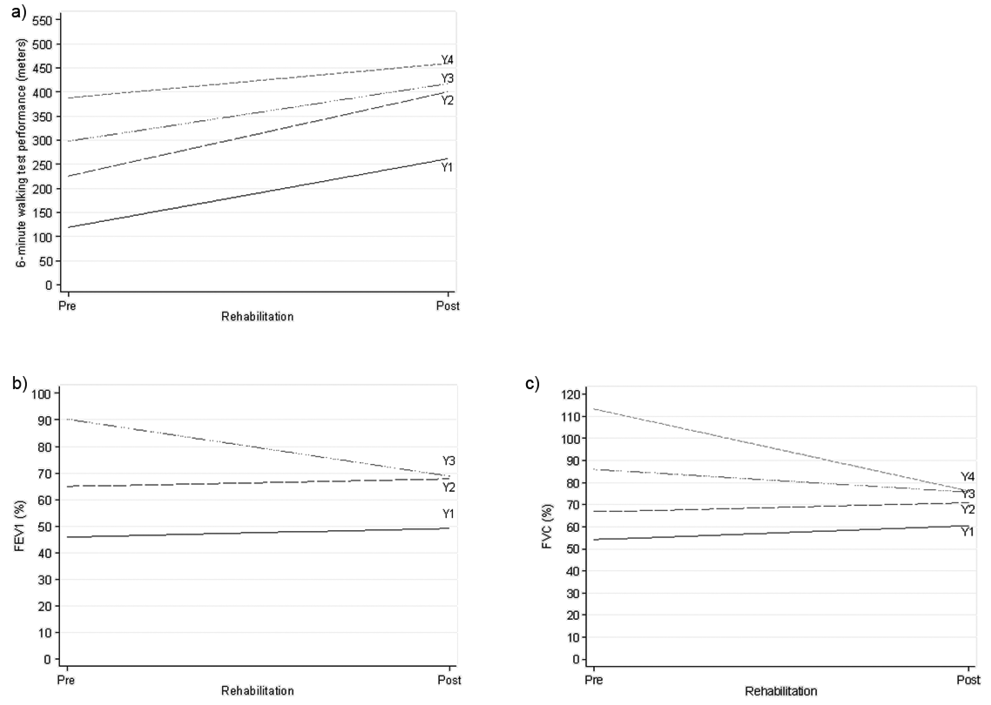
Fig. 1. Graphical representation of the effect induced by the rehabilitation program estimated by a mixed modelling approach on exercise tolerance and lung volume capacity in surgical Non-Small Lung Cancer patients. a) Effect of the rehabilitation program on the performance at the 6-minute walking test for different levels of performance (WTD) prior to the start of the rehabiltitation. (Y1–Y4 indicate the fitted regression lines from the mixed multiple linear regression model for the 4 classes of the baseline WTD. In the order, class 1: (< 180 m); class 2: (≥ 180 m; < 250 m); class 3: (≥ 250 m; < 330 m); class 4 (≥ 330 m)). b) Effect of the rehabilitation program on the FEV1 (%) for different levels of FEV1 (%) prior to the start of the rehabilitation. (Y1–Y4 indicate the fitted regression lines from the mixed multiple linear regression model for the 3 classes of the baseline FEV1 (%). In the order, class 1: (< 60%); class 2 (≥ 60%; < 80%); class 3: (≥ 80%)). c) Effect of the rehabilitation program on the FVC (%) for different levels of FVC (%) prior to the start of the rehabiltitation. (Y1–Y4 indicate the fitted regression lines from the mixed multiple linear regression model for the 4 classes of the baseline (FVC (%). In the order, class 1: (≤ 60%); class 2 (> 60%; < 80%); class 3: (≥ 80%; < 100); class 4 (≥ 100)).
A more detailed analysis of the spirometric measurements revealed different change patterns in the volume of FEV1 for different baseline levels of this parameter (see Table IV and Fig. 1b). In particular, an improvement, although minimal, could be observed for patients who had a FEV1 < 60% and for those who had a volume ≥ 60% and < 80%. Nevertheless, the difference in the improvement between these 2 groups (–0.5, standard error (SE) 4.4%) was not statistically significant (p = 0.91). Interestingly, a negative change in the volume of FEV1 from pre- to post-rehabilitation was found in patients who had a baseline value ≥ 80%. Furthermore, 3 classes were identified according to the lapse of time between the surgery and the admission into our rehabilitation clinic: c1: ≤ 7 days; c2: 8–11 days; c3: ≥ 12 days. With reference to c1, c2 had a FEV1 volume lower by 0.55, SE 2.3%, clearly not significant, while c3 had a FEV1 of 4.4, SE 2.7% lower (borderline significance: p = 0.10; results not in Table). Fixed effects fitted in the model were: time (pre–post rehabilitation), baseline FEV1 in 3 classes, their interaction, days-to-rehabilitation and age; random effects were: intercept and time slope.
|
Table IV. Effect of the rehabilitation programme on the forced expiratory volume in 1 s (FEV1 (%)), according to the pre-rehabilitation FEV1. Mean % (standard deviation; SD) pre- and post-rehabilitation, observed change (SD), and estimated pre–post change (effect of the rehabilitation; standard error; SE) |
|||||||
|
FEV1 (%) pre-rehabilitation |
n |
Pre-rehabilitation Mean (SD) |
Post-rehabilitation Mean (SD) |
Change Mean (SD) |
Effect of rehabilitationa Mean (SE) |
p-value |
p-value |
|
< 60% |
26 |
50.3 (7.1) |
53.8 (13.9) |
0.6 (15.8) |
Reference |
Reference |
|
|
≥ 60% to < 80% |
25 |
69.3 (6.0) |
72.14 (15.0) |
2.7 (13.2) |
–0.5 (4.4) |
0.91 |
Reference |
|
≥ 80% |
29 |
94.8 (13.1) |
73.4 (17.7) |
–28.2 (29.7) |
–24.7 (4.4) |
< 0.0001* |
< 0.0001* |
|
*Statistical significance: p < 0.0167 (3 non-independent multiple comparisons: Bonferroni correction applied). aResults from a linear mixed model regression, accounting for a random intercept and a random slope; estimates adjusted for age and days-to-rehabilitation. |
|||||||
Similarly to FEV1 (%), the regression analysis on the FVC volumes revealed the presence of an interaction between the time with respect to the application of the rehabilitation programme and the different levels of FVC prior to the start of the rehabilitation (see Table VI and Fig. 1c). In particular, 4 groups of patients were identified with regards to their baseline FVC volume: class 1: < 60%; class 2: 60–79%; class 3: 80–99%; class 4: ≥ 100% (Table V). The mean FVC volume of patients in classes 1 and 2 improved mildly from baseline to post-rehabilitation, while that of patients in classes 3 and 4 decreased. As expected, no significant differences between the pre-to-post-rehabilitation change was found when comparing the first 2 classes (–2.1% (SD 4.8), p = 0.66), whereas statistical significance emerged when comparing classes 3 and 4 with class 1 (class 3: –16.5% (SD 5.2), p = 0.0010; class 4: –43.3% (SD 5.4), p < 0.0001). Fixed effects for the fitted model were: time (pre–post rehabilitation), baseline FVC in 4 classes, their interaction, days-to-rehabilitation and age; random effects were: intercept and time slope.
|
Table V. Effect of the rehabilitation programme on the forced vital capacity (FVC (%)), according to the pre-rehabilitation FVC. Mean % (standard deviation; SD) pre- and post-rehabilitation, observed change (SD), and estimated pre-post change (effect of the rehabilitation standard error; SE) |
||||||||
|
FVC (%) pre-rehabilitation |
n |
Pre- rehabilitation Mean (SD) |
Post- rehabilitation Mean (SD) |
Change Mean (SD) |
Effect of rehabilitationa Mean (SE) |
p-value |
p-value |
p-value |
|
≤ 60% |
15 |
53.7 (3.9) |
61.2 (13.9) |
17.1 (31.8) |
Reference |
Reference |
||
|
> 60% to < 80% |
26 |
68.9 (6.2) |
73.4 (15.6) |
4.1 (13.3) |
–2.1 (4.8) |
0.66 |
Reference |
|
|
≥ 80% to < 100 |
19 |
86.8 (4.9) |
75.5 (10.1) |
–10.1 (12.9) |
–16.5 (5.2) |
0.0010* |
0.0017* |
Reference |
|
≥ 100% |
16 |
114.7 (10.7) |
76.9 (22.6) |
–35.6 (19.0) |
–43.3 (5.4) |
< 0.0001* |
< 0.0001* |
< 0.0001* |
|
*Statistical significance: p < 0.0083 (6 non-independent multiple comparisons: Bonferroni correction applied). aResults from a linear mixed model regression, accounting for a random intercept and a random slope; estimates adjusted for age and days-to-rehabilitation. |
||||||||
Furthermore, we observed a substantial stability in the pO2 and pCO2, also confirmed by the regression analysis, which showed no statistically significant pre–post rehabilitation change in either parameter. However, a significant difference was found between the groups of patients who stayed in the rehabilitation unit for 16–20 days, and for more than 20 days, where the latter was estimated to have a pCO2 of 1.4 mmHg (SD 0.9) lower than the other group. Some borderline (0.05 < p < 0.10) evidence of a predictive role of the BMI, with values ≥ 25, and of the lapse of time between surgery and rehabilitation, for higher pCO2 values was also found (results not shown).
DISCUSSION
Previous experiences including our own (4, 8, 9), reported the beneficial effects of PR on pulmonary function and effort tolerance in NSCLC surgical and non-surgical patients. In the post-operative setting, in particular, we have observed that the benefits of rehabilitation are directly proportional to its duration and this is particularly true if the results are benchmarked to the exercise tolerance (Table II). This evidence would indeed advocate for long-term rehabilitative protocols, ideally considered as the bridge from surgery to the “as complete as possible” self-sufficiency (8, 9).
Pulmonary function
Pulmonary function analysis showed a substantial stability of volumes and air-flows and a marginal improvement in the blood-gases analysis parameters. These results are not unexpected, but they remain inconclusive. The removal of lung parenchyma, as anatomically performed during lung cancer surgery, dramatically reduces pulmonary function proportionally to the entity of resection (1, 9). Thus far, rehabilitation programmes, including the one adopted by ourselves, have shown a limited effect on the volumes and air-flows to an anatomical situation in which the overall amount of lung parenchyma is reduced (4, 10). It is also speculated that the minimal modifications constantly reported may be associated with the improvement in rib-cage mechanics and endurance of respiratory muscles. Moreover, in this setting other factors, as such as smoking cessation and optimal use of anti-inflammatory drugs and bronchial dilators, could play a role by reducing the amount of reversible small airway obstruction, therefore improving the expiratory flows. This aspect (as reflected in our findings), as well as the improvement at the level of gas exchanges, are most evident in those patients in whom the performance is low at baseline (FEV1 and FVC less than 80%). This observation is in line with previously reported results (4).
Effort tolerance
The improvement in exercise tolerance following a PR programme is a timely and well-established finding in patients with COPD (6), but this has not been sufficiently investigated in the few studies focused on post-operative rehabilitation in patients with lung cancer. In particular, by analysing the effects of an 8-week multidisciplinary in-patient rehabilitation programme, Spruit et al. (5) have indicated that a statistically significant improvement in 6MWT can happen at peak cycling load. In the same way, in a study by Jones et al. (11) the positive effects on VO2peak and QOL of aerobic training has been demonstrated to be within reach of a programme comprising 3 cycle ergometer sessions/week for 14 weeks post-operatively. Finally, in our previous studies (4, 7), in which the rehabilitation programme consisted of 5 weekly sessions of 3 h each, patients who underwent rehabilitation showed a significant improvement in terms of 6MWT without significant changes in terms of volumes and flows.
The present study further supports the clinical value of the rehabilitation programmes as a useful tool of exercise tolerance improvement, suggesting that early and continuous rehabilitative interventions speed up the recovery of functionality in surgical patients who have undergone anatomical resections for NSCLC.
The co-existence of ameliorated exercise tolerance with the absent (or marginal) lung function improvement following PR remains not entirely understood (10). We speculate that factors other than lung function should be considered. Patients with lung cancer, in fact, are typically current or former smokers, and their mean age is 65 years; they commonly present with other concomitant smoking-related chronic diseases that may affect effort tolerance in a complex phenotype (COPD, ischaemic heart disease, chronic heart failure). Furthermore, it must be taken into account that these types of patients are physically unfit and have a poor nutritional status. Thus, PR may give an important support in the global health status, not solely on the pulmonary function. Further information may emerge from the evaluation of QOL and psychological characteristics in relation to effort tolerance, and these merit further investigation.
In conclusion, high-intensity, inpatient, supervised aerobic exercise training among NSCLC patients who have recently undergone surgical intervention is beneficial. Pulmonary volumes and airflows remain stable after rehabilitation treatment; however, patients who show worse functional levels at baseline gain, in proportion, more than patients who have a better status at baseline.
Additional evidence is needed to assess the entire spectrum of PR potentialities in this setting, especially regarding the control of local and systemic treatment side-effects and the beneficial impact on the QOL of patients with NSCLC, who are challenged by a terrible disease and subject to very aggressive therapeutic schedules (12).
Acknowledgements
The authors certify that no party having a direct interest in the results of the research supporting this article has been or will be conferred a benefit by us or by any organization with which we are associated.
The authors declare no conflicts of interest
REFERENCES
