Alison K. Godbolt, MBChB, MD1,2, Catharina Nygren DeBoussard, MD, PhD1, Maud Stenberg, MD3, Marie Lindgren, MD4, Trandur Ulfarsson, MD5 and Jörgen Borg, MD1
From the 1Department of Clinical Sciences, Karolinska Institute and University Department of Rehabilitation Medicine Stockholm, Danderyd Hospital, Stockholm, 2formerly of Department of Rehabilitation Medicine, University Hospital Uppsala and Uppsala University, Uppsala, 3Department of Rehabilitation Medicine, Norrlands University Hospital, Umeå, 4Clinical Department of Rehabilitation Medicine, County Council of Östergötland, Linköping and 5Department of Rehabilitation Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden
BACKGROUND: Very severe traumatic brain injury may cause disorders of consciousness in the form of coma, unresponsive wakefulness syndrome (also known as vegetative state) or minimally conscious state. Previous studies of outcome for these patients largely pre-date the 2002 definition of minimally conscious state.
OBJECTIVES: To establish the numbers of patients with disorder of consciousness at 3 weeks, 3 months and 1 year after severe traumatic brain injury, and to relate conscious state 3 weeks after injury to outcomes at 1 year.
DESIGN: Multi-centre, prospective, observational study of severe traumatic brain injury. Inclusion criteria: lowest (non-sedated) Glasgow Coma Scale 3–8 during the first 24 h; requirement for neurosurgical intensive care; age 18–65 years; alive 3 weeks after injury. Diagnosis of coma, unresponsive wakefulness syndrome, minimally conscious state or emerged from minimally conscious state was based on clinical and Coma Recovery Scale Revised assessments 3 weeks, 3 months and 1 year after injury. One-year outcome was measured with Glasgow Outcome Scale Extended (GOSE).
RESULTS: A total of 103 patients was included in the study. Of these, 81% were followed up to 1 year (76% alive, 5% dead). Three weeks after injury 36 were in coma, unresponsive wakefulness syndrome or minimally conscious state and 11 were anaesthetized. Numbers of patients who had emerged from minimally conscious state 1 year after injury, according to status at 3 weeks were: coma (0/6), unresponsive wakefulness syndrome (9/17), minimally conscious state (13/13), anaesthetized (9/11). Outcome at 1 year was good (GOSE > 4) for half of patients in minimally conscious state or anaesthetized at 3 weeks, but for none of the patients in coma or unresponsive wakefulness syndrome. These differences in outcome were not revealed by prognostic predictions based on acute data.
CONCLUSION: Patients in minimally conscious state or anaesthetized 3 weeks after injury have a better prognosis than patients in coma or unresponsive wakefulness syndrome, which could not be explained by acute prognostic models.
Key words: traumatic brain injury; prognosis; vegetative state; minimally conscious state; outcome; care pathways.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Alison K. Godbolt, Department of Clinical Sciences, Karolinska Institute and University Department of Rehabilitation Medicine Stockholm, Danderyd Hospital, Stockholm. E-mail: alison.godbolt@ki.se
Accepted February 19, 2013
INTRODUCTION
Some patients survive severe traumatic brain injury (S-TBI) and emerge from coma to a state with preserved sleep-wake cycles, but no evidence of awareness of self or environment, and, as such, no evidence of consciousness. The description of this state as “vegetative state” (1), proposed 40 years ago, has in recent years been seen as derogatory to patients. A new term, “unresponsive wakefulness syndrome” (UWS) (2) has recently been proposed for the same condition and will be used here.
After S-TBI, patients may alternatively show clearly discernible, but inconsistent, signs of consciousness; for example, sustained visual tracking, localization of painful stimuli, and/or attempts at communication, without these reaching a functional level. This state is described as minimally conscious state (MCS) (3).
Brain-injured patients may or may not recover from UWS to consciousness, and the time-course for this recovery may vary from hours or days (in which case it may not be meaningful to describe the clinical progression in terms of UWS) to years (4). Patients pass through MCS for a shorter or longer period, before sometimes emerging from the minimally conscious state (EMCS).
An understanding of the natural history of recovery from S-TBI is a prerequisite for optimizing care for these patients. Care pathways for patients in UWS after S-TBI typically involve several transfers between healthcare and other facilities, at various time-points after injury, but there is a lack of consensus on what is optimal. Rehabilitation interventions may or may not begin as early as in the neurointensive care unit (5). Admission to neurorehabilitation units in some countries requires that the patient is able to participate actively in rehabilitation interventions, and therefore this may, by definition, exclude patients in UWS.
Although there are no such formal barriers to access to rehabilitation in Sweden, there is a lack of consensus regarding the appropriateness of rehabilitation admission for patients in UWS (6). Health insurance is universal, with decisions on admission to rehabilitation units, and on length of stay, made largely by rehabilitation physicians, according to local criteria. However, there are no defined care-pathways or national guidelines regarding the care of patients with impaired consciousness after S-TBI, in-patient beds for acquired brain injury rehabilitation are limited, and (with the exception of a single two-bed unit in one centre) S-TBI patients with disorders of consciousness receive rehabilitation within the same services as patients with less severe acquired brain injury. Experience is that, when beds are limited, patients with disorders of consciousness compete for admission with patients who may more obviously benefit from rehabilitation, and in practice may have difficulties accessing services. Implementation of specific rehabilitation interventions (medication, sensory stimulation programmes, orthotics, physiotherapy) may be according to a structured programme in some units, but otherwise may be largely up to the interest and experience of healthcare personal involved.
Integral to decisions on care pathways is an understanding of the natural course of UWS after S-TBI. A 1994 meta-analysis performed by the Multi-Society Task Force on persistent vegetative state, synthesized data on recovery from UWS in 434 patients reported in 6 articles (7). Outcomes were expressed in terms of the relatively crude Glasgow Outcome Scale, with the method of assessment either not stated (8–11) or based on standard neurological examination and interview with the family (12). Information on participation rates was incomplete in some studies. Of those in UWS (then “vegetative state”) 1 month after traumatic brain injury, 33% recovered consciousness by 3 months, 46% by 6 months, and 52% by 1 year. However, this report pre-dated the definition of MCS in 2002 (3), with probable inclusion of some patients who would today be diagnosed as being in MCS and not UWS.
Since publication of the Task Force study, it has been shown that misdiagnosis of UWS (VS) may occur in up to 40% of patients (13), when standardized assessment instruments are not used. Developments in neurosurgical intensive care and neurorehabilitation during the past 20 years may also impact on recovery.
This study was based on a subset of data from a prospective observational study of S-TBI (the “PROBRAIN” study). The objective was to provide updated data on the rates of occurrence of coma, UWS and MCS after S-TBI, and to assess the extent to which state of consciousness 3 weeks after injury is related to outcome at 1 year. It is hoped that these data will inform the planning and provision of acute, rehabilitation and social care for patients suffering S-TBI, and inform discussions with relatives.
METHODS
We performed a prospective, multicentre, observational study of patients with severe traumatic brain injury. Inclusion criteria were: (i) severe, non-penetrating, traumatic brain injury, with a lowest non-sedated Glasgow Coma Score (GCS) score of 3–8 or Reaction Level Scale (14) score (RLS) of 4–8 in the first 24 h after injury; (ii) age at injury 18–65 years; (iii) injury requiring neurosurgical intensive care, or collaborative care with a neurosurgeon in another intensive care unit.
Exclusion criteria were: death or expected death within 3 weeks of injury.
The 8-point RLS (Table I) is widely used in Sweden, and in some emergency departments and neurosurgical units is used instead of the GCS: RLS criteria were therefore necessary to allow recruitment of patients from those centres using this scale, and thus to avoid selection bias. Scores on the GCS of 3–8 and on the RLS of 8–4 reflect similar severity of injury (15), the RLS having been shown to have somewhat better inter-rater reliability than the GCS (16). RLS scoring is in the opposite direction to GCS scoring, with the highest RLS score of 8 reflecting the most severe injuries.
|
Table I. Reaction level scale (RLS) |
|
|
1 |
Alert, with no delay in response (responds without stimulus). |
|
2 |
Drowsy or confused, but responds to light stimulation. |
|
3 |
Very drowsy or confused, but responds to strong stimulation. |
|
4 |
Unconscious; localizes (moves a hand towards) a painful stimulus but does not ward it off. |
|
5 |
Unconscious; makes withdrawing movements following a painful stimulus. |
|
6 |
Unconscious; stereotypic flexion movements following painful stimuli |
|
7 |
Unconscious; stereotypic extension movements following painful stimuli. |
|
8 |
Unconscious; no response to painful stimuli. |
|
Patients with RLS > 3 are unconscious. |
|
Patients were recruited prospectively by rehabilitation physicians from January 2010 until June 2011, with extended recruitment until December 2011 at 2 centres. Results from the main recruitment period until June 2011 are reported here. The participating centres provide neurosurgical care to more than 80% of the population of Sweden, and the population of Iceland (total approximately 4.7 million adults aged 18–65 years). Neurosurgical intensive care units at 6 (out of a possible 7) centres in Sweden and Iceland were contacted on a weekly basis to identify eligible patients. The patient gave informed consent in cases where he/she had capacity. In the majority of cases the patient lacked capacity and the patient’s nearest relative gave consent to inclusion. The study was reviewed by the regional ethics review board in Stockholm.
After inclusion, acute data were obtained from medical records. Patients were then assessed prospectively, at 3 time-points, 3 weeks (18–24 days), 3 months (75–105 days) and 1 year (350–420) days after injury. Assessments took place in the patient’s current care setting where possible (which in some cases was in the patient’s home), or in a local out-patient department. Inclusion and follow-up was therefore designed to be independent of any decisions regarding care-pathways and admission to in-patient rehabilitation.
Assessments were performed by rehabilitation physicians with assistance from rehabilitation nurses, psychologists, physiotherapists and occupational therapists. Assessments at each of the 3 time-points included both clinical examination and a battery of standardized instruments, allowing description of the patient’s condition according to the framework of the International Classification of Functioning, Disability and Health (ICF): bodily structure and function, activities and participation.
Instruments relevant to this sub-study included the Coma Recovery Scale Revised (CRS-R) (17), and the Glasgow Outcome Scale Extended (GOSE) (18). The CRS-R was recently recommended by the American Congress of Rehabilitation Medicine for the assessment of possible disorders of consciousness (DOC), has good reliability and validity (19), and was administered in all patients where a DOC was suspected on the basis of lack of functional communication and/or functional object use, with the exception of patients who remained sedated or anaesthetized. The GOSE has good inter-rater reliability (18) and validity (20), and is an established measure of global outcome after traumatic brain injury.
Patient age and acute markers of injury severity are known to impact on outcome, and possible differences in outcome according to conscious state 3 weeks after injury would be of lesser interest if different outcomes could already have been predicted using acute data. The CRASH acute prognostic model (21) is an externally validated acute prognostic model, based on data from 10,008 patients worldwide. CRASH incorporates 10 acute variables: age, pupil reaction, acute GCS, country, presence or absence of major extracranial injury, presence or absence of 5 specified acute CT-brain findings. We used the online calculator for the CRASH prognostic model (available at: http://www.crash2.lshtm.ac.uk/Risk%20calculator/index.html) to calculate percentage risk of an unfavourable outcome (equivalent to GOSE 1–4) at 6 months, for each patient, after conversion of RLS scores for those patients not assessed with the GCS. Conversion used was RLS8 = GCS3, RLS7 = GCS4, RLS6 = GCS5, RLS5 = GCS6, RLS4 = GCS7. Ordering of severity with the RLS and GCS has been shown to be consistent (22), the RLS and GCS are highly correlated (r = –0.94), and assess similar behavioural features reflecting consciousness (15).
Data were analysed with SPSS version 20.
RESULTS
A total of 103 patients were recruited from 6 neurosurgical intensive care units in Sweden and Iceland, and acute data entered for 102 patients (one patient withdrew consent). Three weeks after injury 102 patients continued in the study. Three months after injury, 3 (3%) patients had died, 4 (4%) had withdrawn from the study and 96 continued (93%). One year after injury 5 (5%) patients had died, 18 (17%) had withdrawn, 78 (76%) continued, and data on study status was missing for one. Patients who withdrew were similar to those who continued in terms of median age (34 compared with 42 years, Mann-Whitney test, p = 0.55) and median acute GCS or RLS-derived GCS (4 compared with 5, Mann-Whitney test, p = ” M0.18). Demographic details and summary statistics on severity of injury are given in Table II.
For acute assessment of level of consciousness, the GCS alone was available for 27 patients, RLS alone for 43, both scales for 30 patients, data was missing for 2 patients: where both scales were available the GCS is reported. The median lowest GCS of 5 (n = 57) and median lowest RLS of 5 (n = 43), median duration of artificial ventilation of 13 days and median length of intensive care of 17.5 days reflect that, as a group, these patients had brain injuries towards the more “severe” end of the group generally defined as having S-TBI. Most injuries were due to transport accidents and falls. Due to a minor protocol violation one patient was included shortly before their 18th birthday.
|
Table II. Patient characteristics |
|
|
Age at injury, years, median, (range) |
41 (17–65) |
|
Worst un-sedated GCS (3–15) first 24 h (n = 58) Or Worst un-sedated RLS (8–1) first 24 h in patients not assessed with GCS (n = 42) |
5 (3–8) 5 (8–4) |
|
Cause of injury, % |
|
|
Transport accident |
42 |
|
Fall |
44 |
|
Other |
11 |
|
Missing data |
4 |
|
Length of stay in intensive care, days median (range) |
17.5 (1–54) |
|
Duration of ventilation, days, median (range) |
13 (range 0–36, with 1 outlier at 101 days) |
|
Economic support at time of injury, % |
|
|
Employed/self-employed |
50 |
|
Study grant |
7 |
|
Unemployment benefit or social support |
8 |
|
Sick pay |
17 |
|
Other |
7 |
|
Unknown |
12 |
|
Previous brain injury requiring hospitalization, % |
15 |
|
Known drug or alcohol misuse at time of injury, % |
26 |
|
Gender, men/women/missing, n |
69/25/9 |
|
GCS: Glasgow Coma Score; RLS: Reaction Level Scale. |
|
Outcomes 1 year after injury
GOSE 1 year after injury was 1 (dead, n = 5), 2 (vegetative state, n = 6), 3 (lower severe disability, n = 22), 4 (upper severe disability, n = 6), 5 (lower moderate disability, n = 10), 6 (upper moderate disability, n = 0), 7 (lower good recovery, n = 19), 8 (upper good recovery, n = 12), missing (n = 3). Data on the relationship between conscious state at 3 weeks and outcome at 1 year is given in Fig. 1.
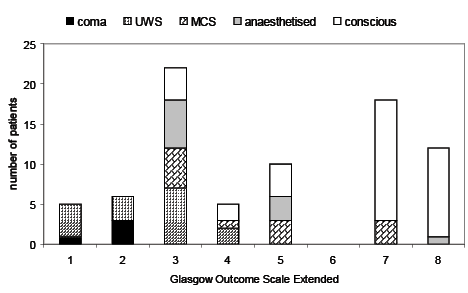
Fig. 1. Outcome at one year in relation to conscious state 3 weeks after injury. Each bar shows the number of patients with each Glasgow Outcome Scale Extended level 1 year after injury. Within each bar, the conscious level of patients 3 weeks after injury is shown by different patterns of shading, as indicated below the figure title. 1: dead; 2: vegetative state; 3: lower severe disability; 4: higher severe disability; 5: lower moderate disability; 6: upper moderate disability; 7: lower good recovery; 8: upper good recovery.
Disorders of consciousness
Three weeks after injury 17 patients were in UWS, 13 in MCS, 6 in coma and 11 sedated/anaesthetized. Outcomes are summarized in Table III. Trajectories of recovery are summarized in Table IV.
|
Table III. Outcome one year after injury related to conscious state 3 weeks after injury |
||||||
|
GOSE, 1 year after injury |
Conscious state 3 weeks after injury |
|||||
|
Cons-cious |
Anaes-thetized |
Coma |
UWS |
MCS |
Not assessable/ missing data |
|
|
1 = dead |
0 |
0 |
1 |
4 |
0 |
0 |
|
2 = vegetative state |
0 |
0 |
3 |
3 |
0 |
0 |
|
3 = lower severe disability |
4 |
6 |
0 |
7 |
5 |
0 |
|
4 = upper severe disability |
2 |
0 |
0 |
2 |
1 |
1 |
|
5 = lower moderate disability |
4 |
3 |
0 |
0 |
3 |
0 |
|
6 = upper moderate disability |
0 |
0 |
0 |
0 |
0 |
0 |
|
7 = lower good recovery |
15 |
0 |
0 |
0 |
3 |
1 |
|
8 = upper good recovery |
11 |
1 |
0 |
0 |
0 |
0 |
|
Grand total |
36 |
10a (+1) |
4a (+2) |
16 (+1) |
12 (+1) |
2 |
|
aGOSE data at 1 year missing for 5 patients. Number of additional patients in each category with missing GOSE data is given in brackets. GOSE: Glasgow Outcome Scale Extended; UWS: unresponsive wakefulness syndrome (i.e. vegetative state); MCS: minimally conscious state. |
||||||
|
Table IV. Recovery for patients with low levels of consciousness 3 weeks after injury |
|||
|
Status 3 weeks after injury |
Follow-up status |
Three months after injury, n |
One year after injury, n |
|
UWS (n = 17) |
EMCS |
4 |
9 |
|
MCS |
6 |
1 |
|
|
UWS |
5 |
2 |
|
|
Dead |
2 |
4 |
|
|
Missing data |
0 |
1 |
|
|
MCS (n = 13) |
EMCS |
13 |
13 |
|
Coma (n = 6) |
UWS |
4 |
4 |
|
MCS |
1 |
1 |
|
|
EMCS |
0 |
0 |
|
|
Dead |
1 |
1 |
|
|
Withdrawn |
0 |
0 |
|
|
Anaesthetized/sedated (n = 11) |
UWS |
1 |
0 |
|
MCS |
3 |
2 |
|
|
EMCS |
7 |
9 |
|
|
Dead |
0 |
0 |
|
|
Withdrawn |
0 |
0 |
|
|
EMCS: emerged from the minimally conscious state; MCS: minimally conscious state; UWS: unresponsive wakefulness syndrome. |
|||
Patients in unresponsive wakefulness syndrome 3 weeks after injury. Of the 17 patients in UWS at 3 weeks, by 3 months, 5 remained in UWS, 6 had improved to MCS, 4 had emerged from MCS, and 2 were dead. One year after injury, 2 remained in UWS, 1 was in MCS, 9 had emerged from MCS, 4 were dead, and data on 1 was missing.
Outcome 1 year after injury for these patients, according to the GOSE, was 1 (dead, n = 4), 2 (vegetative state, n = 3), 3 (lower severe disability, n = 7), 4 (upper severe disability, n = 2), missing data (n = 1). Note, that GOSE level 2, associated with the description “vegetative state”, includes in fact some patients in MCS, explaining the apparent discrepancy.
Scores on the CRS-R (maximum 23) at first assessment, 3 weeks after injury, for patients found to be in UWS, ranged from 0 to 7. Correlation between CRS-R score at 3 weeks and outcome at 1 year for these patients, according to the GOSE, was poor ,with a correlation co-efficient of 0.29.
Patients in minimally conscious state 3 weeks after injury. Of those in MCS at 3 weeks, all 13 had emerged from MCS at 3 months. These patients had scored a median of 12 points (range 6–19 of a possible maximum 23 points) on the CRS-R at 3 weeks.
GOSE 1 year after injury for these 13 patients was 1 (dead, n = 0), 2 (vegetative state, n = 0), 3 (lower severe disability, n = 5), 4 (upper severe disability, n = 1), 5 (lower moderate disability, n = 3), 6 (upper moderate disability, n = 0), 7 (lower good recovery, n = 3), 8 (upper good recovery, n = 0), missing data (n = 1). Correlation between CRS-R score at 3 weeks and outcome at 1 year for these patients, according to the GOSE, was also weak, with a correlation co-efficient of –0.19.
One year after injury, 4 of these patients were living at home without assistance, 8 were at home with assistance, and 1 was in a nursing home. One patient was working full-time (and also driving).
Patients in coma or sedated/anaesthetized, 3 weeks after injury. Of the 6 patients in coma (i.e. not sedated, but no eye opening) at 3 weeks, by 3 months, 4 were in UWS, 1 was in MCS, none were better than MCS, and 1 was dead. These figures were unchanged at follow-up 1 year after injury.
At the 3-week assessment, rehabilitation physicians recorded whether patients were sedated/anaesthetized, after review of the current drug regime. Of the 11 patients who were sedated/anaesthetized 3 weeks after injury, by 3 months, 1 was in UWS, 3 were in MCS, and 7 were better than MCS. One year after injury none of these initially sedated patients remained in UWS, 2 were in MCS, and 8 were better than MCS (Table IV).
Consideration of possible confounders
We considered whether the better one-year outcome of patients in MCS compared with UWS (3 weeks after injury) could have been predicted from acute variables of prognostic significance. There was no significant difference in the percentage risk of an unfavourable outcome at 6 months, as assessed with the CRASH model, between patients in UWS 3 weeks after injury compared with those in MCS (median risk of unfavourable outcome 81% for patients in UWS (range 47–98%) and 75% for patients in MCS (range 47–97%), Mann-Whitney test not significant, p = 0.81), showing that the differences in outcome between these groups could not have been predicted using existing acute prognostic models.
The need to derive GCS scores from RLS score for those patients not assessed with GCS could have introduced some error, with possible overestimation of the risk of unfavourable outcome as calculated with CRASH for patients with RLS 4, for whom it can be debated where an appropriate conversion is to GCS 7 (as initially performed) or GCS 8 (16). To exclude any impact of this possible error on the above finding, the CRASH-risk was re-calculated using an alternative conversion of RLS4 = GCS8. This resulted in unchanged median risk of unfavourable outcome for both UWS and MCS groups, but with a slightly modified range for UWS of 43–98% (previously 47–98%).
Outcome predictions with CRASH are relatively crude: unfavourable outcome is defined as death, vegetative state (UWS) or severe disability according to the earlier Glasgow Outcome Scale (corresponding to GOSE 1–4). By this definition of unfavourable outcome, all of our study patients in UWS at 3 weeks, but only half (n = 6) of the patients in MCS at 3 weeks, had an unfavourable outcome at one year. This difference in outcome between UWS and MCS was statistically significant (Fisher’s exact test, p = 0.01).
Medication use in patients with UWS or MCS. Some evidence has existed for many years regarding beneficial effects of amantadine (with dopaminergic and NMDA effects) in aiding recovery of consciousness after profound acquired brain injury (including traumatic injury). Recently, a large, well-performed, multicentre, randomized controlled trial demonstrated a clear effect of amantadine in speeding improvement in patients with disorders of consciousness after S-TBI (23). There is also some, less robust, support for the use of other dopaminergic agents. It is therefore relevant to consider medication use in study patients when interpreting our findings.
Very few patients were treated with dopaminergic drugs at the time of study assessments. Of patients in UWS at any point during the study, none were receiving such drugs at the 3-week assessment; at the 3-month assessment 1 patient (in UWS at 3 weeks, MCS at 3 months, and EMCS at one year) was receiving Madopar (levodopa/benserazide combination), and one patient (coma at 3 weeks, UWS at 3 months, UWS at 1 year) was receiving amantadine at 3 months but not at 1 year. One further patient (UWS at all study time-points) was receiving amantadine at the 1-year assessment, but not earlier. One patient initially in MCS had emerged from MCS at 3 months, before later receiving Madopar, which was noted at the 1-year assessment. In summary, the impact of drug use in altering patterns of recovery from post-traumatic disorders of consciousness in study patients is probably minimal.
There are practical barriers to the use of amantadine in Sweden, which may explain the low rates of its use: it is not registered with the national Medical Products Agency, and physicians are required to apply for a special license before it can be prescribed. The period of recruitment to this study was also before the publication of the most robust study on amantadine (23).
Possible confounding from other treatments. To our knowledge, no attempt has been made in Sweden to use deep brain stimulation to treat patients in UWS or MCS. Despite promising case reports (24), and case series (25), there has been no randomized controlled trial, and in Sweden the evidence has not been considered strong enough to support introduction into routine clinical practice.
Admission to specialized rehabilitation units. Of the 15 patients in UWS at 3 weeks who survived at least to 3 months, 14 were admitted to an inpatient specialized rehabilitation unit (missing data for 1 patient). Rehabilitation admission occurred a mean of 62 days after injury (standard deviation (SD) 46, range 26–198 days). All of the 13 patients in MCS 3 weeks after injury were admitted to inpatient rehabilitation units, a mean of 44 days after injury (SD 18, range 17–78).
Participation rates. The number of patients recruited corresponds to an assumed annual incidence of S-TBI, with survival of at least 3 weeks, of 14 per million, had all eligible patients had been identified and recruited. This is very similar to the reported incidence of approximately 15 per million population (26) from a previous retrospective study based on review of medical records of patients with S-TBI treated at 3 centres in Sweden, and suggests that participation rates were sufficiently high that the sample can be considered representative.
DISCUSSION
Rates of recovery from post-traumatic UWS in this study are, at first sight, remarkably similar to those reported in the Multi-Society Task Force study nearly 20 years ago. Our first assessment was slightly earlier than that in the original task force (3 weeks rather than 1 month), and despite this study spanning 80% of the population of Sweden and 100% of the population of Iceland, patient numbers were relatively small, necessitating some caution in interpretation.
Comparing figures from the current study with those from the 1994 Task Force (given in brackets), 24% (33%) of patients in UWS 3 weeks (1 month) after injury had emerged to full consciousness (EMCS) at 3 months, and 53% (52%) at 1 year.
However, MCS had not been defined at the time of the Task Force report, and it is likely that most MCS patients would have been included in the vegetative state group in the Task Force report. Neither did the original studies behind the Multi-Society task force use standardized scales in diagnosis of vegetative state/UWS, which have been shown to improve diagnostic accuracy (13). If, instead, one compares outcomes for all patients with either UWS (vegetative state) or MCS early after injury, 57% (Task Force 33%) of patients in UWS/MCS 3 weeks (1 month) after injury had recovered consciousness (EMCS) at 3 months, and 73% (52%) at 1 year. This is probably a fairer reflection of developments in neurosurgical and neurorehabilitative care in the past decades.
Long-term outcome for patients in UWS 3 weeks after injury was, however, poor, with the best GOSE level being upper severe disability. Such patients, according to the GOSE can, however, be left alone, unsupervised, for some periods during the day. Definitions of poor outcomes are always relative. Outcomes were similarly poor for patients who showed no eye opening at 3 weeks, as such classified as being in coma.
Reports have recently appeared in the literature on outcomes for selected groups of patients with disorders of consciousness from the point at which they are admitted to specialized rehabilitation programmes. Katz et al. (27) reported a retrospective review of outcomes in 36 patients admitted to a slow-to-recover rehabilitation unit, of whom the 22 with traumatic injuries (8 in UWS at admission, 14 in MCS) were admitted a mean of 37 days after injury. Seven of the 8 UWS patients improved to MCS and 45% (number not stated) later emerged from MCS. Although follow-up periods differ, the figure of 45% improving to better than MCS is not dissimilar to our figure of 53% 1 year after injury. It should be emphasized that such estimates are, of necessity, based on small numbers of patients and some margin of error is to be expected.
Outcome was better for patients in MCS 3 weeks after injury, suggesting that it is important to distinguish between UWS and MCS when considering prognosis. This distinction is not easy, with reports of misdiagnosis in 40% of patients even in the hands of teams experienced in the assessment of patients in low responsive states (13). More than one-third of patients in MCS 3 weeks after injury were living independently at home 1 year after injury. One patient had returned to work and had regained their driving licence. Katz et al. (27) reported similar findings for MCS patients: all of their patients admitted in MCS after TBI emerged from MCS during rehabilitation. Identification of patients in MCS rather than UWS, via standardized assessment of conscious level at 3 weeks post-injury, gave additional prognostic information that was not apparent from acute-stage predictions using the CRASH-model.
Outcome was also better for patients who were sedated/anaesthetized at 3 weeks. Our data do not allow analysis of the possible reasons for this, but is could be that sedation is continued when treatable factors, such as raised intracranial pressure, are present, which, if successfully controlled, result in a better outcome than for patients for whom sedation was not judged appropriate, probably due to the absence of such treatable factors.
It is encouraging that all patients with UWS or MCS 3 weeks after injury were later admitted to inpatient rehabilitation units. We cannot exclude that contact with study personnel had some impact on this: rehabilitation medicine in Sweden is a relatively small profession, and physicians involved in the study are also clinically active, with admitting rights to rehabilitation units. However, the extended time before admission is suboptimal. Recent evidence from Norway (5) has shown that early initiation of an unbroken chain of rehabilitation improves outcomes after S-TBI. The Norwegian study involved rehabilitation physicians integrated into the intensive care unit, a model with at present does not exist in Sweden. Cardiovascular instability and other medical complications in the post-acute phase after S-TBI may preclude earlier transfer to specialized rehabilitation units, which are often geographically distant from neurosurgical intensive care units. Integrating rehabilitation physicians and paramedical staff into the intensive care team would seem to offer a solution.
Study limitations
Confirming a diagnosis of UWS or MCS requires repeated assessments over a period of time (19), which were not possible within the study design, given that patients were assessed in whichever care setting was current at the study time-points. In some cases this required study personal to travel long distances to the patient, which made repeated assessments over time impossible. However, the use of the CRS-R is a strength, and has been shown to improve diagnostic accuracy (13). A degree of misclassification is, however, possible, but is probably of a much lesser degree than that in the original Task Force report.
Our follow-up rate of 81% patients (76% living, 5% dead), 1 year after injury, is satisfactory, considering that of necessity patients were initially included in the study with the consent of the nearest relative, as S-TBI causes patients to lack capacity in the acute phase after injury. In this context, it is noteworthy that only 18% of patients withdrew consent to further follow-up.
Some degree of error is possible due to derivation of acute GCS scores from RLS scores for those patients not assessed with GCS. This could have caused some slight overestimation of injury severity, particularly for patients with RLS 4–5. Proponents of the RLS in Sweden highlight its superior inter-rater reliability compared with the GCS, and the avoidance of the GCS’s problems with scoring for intubated patients. However, the exclusive use of the RLS does complicate application of established prognostic models, such as CRASH, and hampers direct application of evidence from studies of patients assessed with the GCS.
Another possible source of error is the use of radiology reports to assess the CT-criteria for the CRASH model. If certain features were not reported, there is some uncertainty as to whether they were absent or simply not reported. However, it is unlikely that major abnormalities will have been omitted from radiology reports. The protocol for the CRASH study (28) did not state how or by whom the CT criteria should be assessed, and it is reasonable to assume similar errors would have been possible during that study. We consider our use of radiology reports to be a reasonable, although imperfect, method. A re-review of CT-brain images by independent neuro-radiologists is currently underway in order to assess the degree to which this could have impacted on predictions using the CRASH model.
The CRASH prognostic model predicts outcome 6 months after injury. We assessed outcome 1 year after injury, as recovery may continue at least until this time-point in severely injured patients. These differing time-frames could explain why differences in outcome between patients in UWS and MCS 3 weeks after injury were not predicted by CRASH. However, it seems unlikely that new acute prognostic models will be developed considering outcome at 1 year, given the practical difficulties involved in longer-term follow-up of the very large numbers of patients needed.
Implications for application of Swedish law
In Sweden, patients with severe cognitive and physical disability after S-TBI in adulthood have rights defined in law to suitable, specified, support in the community, including 24-h care at home, if desired, with a higher level of support for those with cognitive impairments equivalent to learning disability (“The law on support and service for certain people with disabilities”, LSS). However, the law requires that impairments are permanent, and certified as such by doctors and psychologists. Statements of permanence have, by tradition, not been considered possible until 6 months after injury, with the consequence that optimal care placement is often not possible before this time. Our data show that patients in UWS 3 weeks after injury will have, at best, severe disability at 1 year, and early certification that severe disability will persist at least for 1 year after injury is justified.
Conclusion
The approximate annual incidence of post-traumatic disorders of consciousness (PT-DOC) persisting for at least 3 months, was 3 per million working age people a year (based on 20 patients in our study, recruited over 18 months, from a population of 4.7 million). More transient PT-DOC occurred in 5 per million working-age people (present 3 weeks after injury, but not at 3 months) and longer lasting PT-DOC, persisting 1 year after injury had an incidence of 1.4 per million working age people per year.
With these small numbers of patients spread throughout a geographically large country, development of national standards for post-acute and rehabilitation care for these patients is necessary to ensure a good standard of care for all. Such standards already exist in some European countries (e.g. Scotland (29)). Some centralization of care and/or development of a disorders of consciousness network should be considered to enable dissemination of expertise, implementation of standards, and to promote further research.
Based on our figures, one can further calculate that each year, in Sweden, approximately 14 patients of working age will develop coma or UWS in the post-acute phase after S-TBI, and that all of these patients can be expected to have severe disability 1 year after injury, even if approximately half of them will recover consciousness.
Development of a continuous chain of rehabilitation after S-TBI, which has been shown to improve outcomes, but was not in place for any patients in this study, should be prioritized.
ACKNOWLEDGEMENTS
The authors would like to thank the patients and their relatives, the PROBRAIN collaborators*, the clinical staff of our units, and our neurosurgical colleagues for allowing recruitment of their patients. This study was supported by grant 060833 from AFA insurance. The funders had no access to data and no input to study design or data analysis. AKG has received support from ALF-grants from Uppsala University Hospital and Danderyd Hospital. We also thank Dr Anna Tölli, Danderyd Hospital and Karolinska Institute, Dr Kristina Lindgren, Karlstad Hospital, Dr Björn Johansson, University Hospital Uppsala and Dr Christer Tengvar, University Hospital Uppsala, who assisted in assessment of patients. Paramedical staff contributing to the study included Stina Gunnarsson and Marina Byström Odhe, (Linköping), Anna-Lisa Nilsson (Umeå), Marie Sandgren (Stockholm), Staffan Stenson (Uppsala), and Siv Svensson (Gothenburg). Seija Lund was nurse coordinator in Stockholm and Ingrid Morberg in Gothenburg. Lisbet Broman gave advice on statistical aspects.
*PROBRAIN collaborators: the authors, together with Dr Britt-Marie Stålnacke, Department of Rehabilitation Medicine, Norrlands University Hospital, Umeå, Sweden, Dr Marianne Lannsjö, Department of Rehabilitation Medicine, Sandviken Hospital and Institute of Neuroscience, Uppsala University Hospital, Sweden, Dr Gudrun Karlsdottir, Department of Rehabilitation Medicine, Landspitalinn, University Hospital, Reykjavik, Iceland.
REFERENCES
