Solrun Sigurdardottir1,2, Nada Andelic2, Cecilie Roe2,3 and Anne-Kristine Schanke1,4
From the 1Department of Research, Sunnaas Rehabilitation Hospital, Nesoddtangen, 2Department of Physical Medicine and Rehabilitation, Oslo University Hospital, Ulleval, 3Faculty of Medicine, University of Oslo and 4Department of Psychology, Faculty of Social Sciences, University of Oslo, Oslo, Norway
OBJECTIVE: To determine the prevalence of depressive symptoms among individuals with traumatic brain injury (TBI) and to identify predictors of depressive symptoms and psychological distress.
DESIGN: A longitudinal study with assessments at 3 months, 1 year and 5 years after injury.
SUBJECTS: A total of 118 individuals (29% females; mean age 32.5; range 16–55 years) with mild-to-severe TBI who were hospitalized in the Trauma Referral Centre from 2005 to 2007.
METHODS: Self-report assessments using the Hospital Anxiety and Depression Scale, the Symptom Checklist 90-Revised and the Fatigue Severity Scale. Injury severity, trauma scores, pain, fatigue, substance abuse and demographic characteristics were also recorded.
RESULTS: The prevalence of depressive symptoms was 18% at 3 months, 13% at 1 year and 18% at 5 years after injury. Only 4% had persistent depressive symptoms at all time-points. At 1 year post-injury, anxiety, age, ongoing stressors and employment status predicted depressive symptoms (R2 = 0.43, p < 0.001), and ongoing stressors, employment status, fatigue and pain predicted psychological distress (R2 = 0.45, p < 0.001).
CONCLUSION: Psychosocial stressors and employment status contributed to depressive symptoms and psychological distress, whereas injury severity did not have any predictive value. The prevalence of depressive symptoms remained stable over time, emphasizing the importance of recognizing and treating depression early after the injury.
Key words: traumatic brain injury; depression; anxiety; psychosocial; fatigue; pain.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Solrun Sigurdardottir, Sunnaas Rehabilitation Hospital, 1450 Nesoddtangen, Norway. E-mail: solrun.sigurdardottir@sunnaas.no
Accepted Mars 12, 2012
INTRODUCTION
Traumatic brain injury (TBI), defined as injury to brain tissue caused by an external trauma, is a life-changing event that may result in persistent or progressive psychiatric disturbances. A significant proportion (30%) of individuals with TBI experience psychiatric disorders during the first year after TBI (1). A study found that 65% of individuals with TBI received at least one psychiatric diagnosis up to 5.5 years after injury (2). Most studies that have followed individuals with TBI for 1 year or more after injury have found that anxiety and depression are the most common symptoms reported by these individuals (3–5). The prevalence of depression reported in the literature varies from 17% to 53% (1, 6, 7), and this variation is mostly due to the use of different instruments and procedures, or to differences in the study population and design. Other potential disorders are anxiety, varying from 10% to 29% (1, 6); post-traumatic stress disorder, varying from 10% to 27% (8, 9); and substance abuse disorders, varying from 10% to 25% (1, 5, 10).
The relationships between psychiatric disorders and TBI are multidimensional, with biological, psychological, and social contributors, as demonstrated in reviews of the literature (11, 12). Most researchers consider depression after TBI to have a complex aetiology, in which acute depression is more associated with biological mechanisms (4) and chronic depression is more related to psychosocial factors (13). The literature suggests that factors such as pre-existing psychiatric or depressive disorders (4, 7), female gender (2), increasing age (14), lower education level (2, 15, 16), unemployment (15, 17, 18), pain (2, 13), and substance abuse (3, 7, 15) may play roles in the development of depression after TBI. However, there are inconsistent findings with respect to the relationships between depression and pre-existing psychiatric disorders (5, 13, 15), gender effects (4, 17) and education level (4).
Depression appears to be unrelated to TBI severity and is found in all TBI severity groups (mild, moderate, severe) during the first year or longer after injury (4, 11). One study found that 27% of individuals with moderate-to-severe TBI met the criteria for major depression 10–126 months after injury, and that neither TBI severity nor the time since injury was correlated with depression (17). However, a study of 520 veterans 50 years after head injury (16) indicated that the severity of the head injury was positively related to the lifetime risk of major depression. Several studies that investigated depression beyond 2 years after TBI have assessed patients at one time-point with no longitudinal collection of data (5, 16, 19, 20). However, longitudinal studies are few; some indicate that depression may increase over time (3, 6), in contrast to other studies (15, 21). Chronically depressed TBI individuals have been found to be more likely to have poorer psychosocial functioning (13) and to experience higher levels of psychological distress compared with their non-depressed counterparts (22). Studies have shown that concurrent psychiatric disorders predict psychosocial and functional outcomes during the first year after TBI (6). However, these relationships are unclear, as depression may either lead to, or be an effect of, poor psychosocial functioning (23).
Some of the trends in epidemiology, acute management and rehabilitation in Scandinavia have been described by Borg et al. (24), who emphasized the importance of a continuous chain of medical care after TBI. However, there have been few longitudinal studies in the areas of psychology and psychiatry in Scandinavia that illustrate the influence of specific injury-related and clinical variables on depressive symptoms after TBI. Moreover, much is known about the point prevalence of depression, but less is known about the long-term course of depressive symptoms. This longitudinal study including individuals with varying TBI severity based on clinical evaluations at 3 different time-points and assessed multiple variables, including injury severity, depressive symptoms, psychosocial stressors, fatigue and pain.
The aims of this study were as follows:
• to determine the prevalence of depressive symptoms after TBI over time (3 months, 1 year and 5 years post-injury);
• to examine changes in depressive symptoms and other symptoms of psychological distress over time (3 months, 1 year and 5 years post-injury);
• to determine if depressive symptoms and psychological distress have overlapping predictors (demographic characteristics, injury-related variables, psychosocial stressors, fatigue and pain).
METHODS
Design and participants
A longitudinal prospective study of individuals admitted to the Trauma Referral Centre of Oslo University Hospital, Ulleval, Norway, with acute TBI during the period May 2005 to May 2007 was conducted. Inclusion criteria were: (i) age 16–55 years; (ii) admission within 24 h of injury; (iii) computed tomography (CT) brain scan performed within 24 h of injury; and (iv) fluent Norwegian speaker. Individuals were excluded (non-eligible) if they had any of the following: (i) severe substance abuse (n = 14); (ii) a known severe psychiatric disorder (n = 7) or previous brain pathology (n = 6); or (iii) associated spinal cord injury (n = 3). Severe substance abuse was defined as a previous diagnosis of illicit substance abuse/dependence or alcohol abuse/dependence according to the International Classification of Diseases (ICD-10) diagnosis of substance use disorders. Severe psychiatric disorders included, for example, schizophrenia or recent attempted suicide. The initial severity of TBI was measured using the Glasgow Coma Scale (GCS) (25) score determined at admission to the emergency department at the hospital or prior to intubation at the accident site. As shown in Fig. 1, 296 individuals fulfilled the inclusion criteria. All potential individuals in the age range 16–55 years (n = 270) received a letter containing information about the study 4–6 weeks after injury. The participants (n = 118) and non-participants (n = 133) did not differ with respect to age, gender, GCS, cause of injury, loss of consciousness, or duration of post-traumatic amnesia (PTA). However, significantly more participants with moderate-to-severe TBI (n = 78) than non-participants with moderate-to-severe TBI (n = 27) had an intracranial pathology (85% vs 63%, respectively (χ2(1) = 6.0, p < 0.05). A total of 118 individuals (84 males, 34 females) were included and participated at 3 months after injury. At 1 year, 109 of the 118 originally included individuals (78 males, 31 females) participated and at 5 years 89 individuals (63 males, 26 females) completed the follow-up (see Fig. 1). Individuals lost to follow-up at 5 years were significantly more often unemployed at the time of injury (45%) than those who completed the study (13%) χ2(1) = 12.9, p<0.001, and had more often mild TBI (59%) than those who completed the study (26%) χ2(1) = 10.5, p < 0.001, but those lost to follow-up at 5 years and those who completed the study did not differ significantly with respect to other demographic, substance abuse, or injury-related variables.
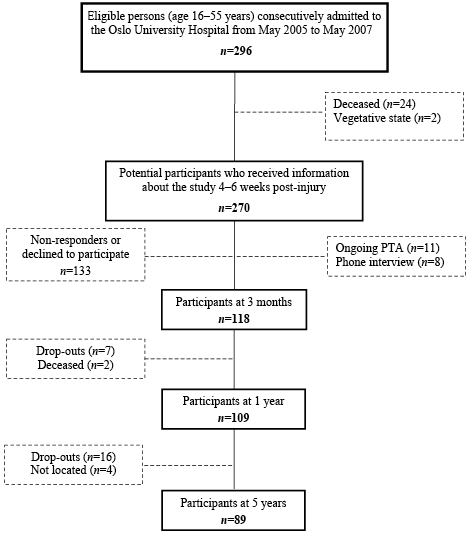
Fig. 1. Included individuals admitted to the hospital after traumatic brain injury. PTA: post-traumatic amnesia.
Evaluations were performed at 3 months, 1 year and 5 years post-injury. Most of the patients were assessed at the out-patient department of Oslo University Hospital. Those participants who received inpatient rehabilitation at 3 months or medical care follow-up at 1 year were assessed during their hospital stay at Sunnaas Rehabilitation Hospital. Participants underwent neuropsychological examinations (collected for a parallel study) before they completed a set of questionnaires. The time required to complete the examination was approximately 3 h. Written consent was obtained from all participants. No control group was used in this study. The Regional Committee for Medical Research Ethics, East-Norway, and the Norwegian Data Inspectorate approved this study according to the Declaration of Helsinki.
Measurements
Individuals with TBI were interviewed at 3 months, 1 year and 5 years after injury to provide information related to demographic characteristics (age, gender, education, and marital status), employment status and psychosocial situation. The pre-injury (for the last year) and post-injury (1 year) employment statuses were dichotomized into productive work/employment (employed full-/part-time or full-/part-time student) and unemployment (unemployed, sick leave, homemaker, disability pension, and other). An inclusive definition of employment was used that included other productive activities, such as studying, as described in our previous studies on TBI (19, 26).
A semi-structured psychological interview, based on clinical experiences and relevant literature, was developed by a research group of 5 psychologists at Sunnaas Rehabilitation Hospital (27). Previous studies have published this interview on multiple traumas and spinal cord injuries (27) and polio survivors (28). This interview was used in the current study to assess the participants’ psychosocial situations and stress loads. The pre-injury (for the last year) and post-injury (1 year) psychosocial factors assessed in this interview were rated as “Yes” or “No” for 8 items (stressors): serious illness, psychiatric illness requiring therapy, serious illness or death of a close family member, marital problems, economic problems, substance abuse, feeling isolated or lonely, and other related problems (27). Substance abuse was evaluated using the Cut down, Annoyed, Guilty, Eye-opener (CAGE cut-off ≥ 2), and responses were dichotomized into “Yes” or “No”. Individuals also completed self-report questionnaires to provide measures of depression, anxiety, psychological distress, fatigue, and pain at 3 months, 1 year and 5 years post-injury. Symptoms of depression and anxiety during the previous 7 days were measured using the validated Norwegian version of the Hospital Anxiety and Depression Scale (HADS) (29). Both HADS subscales consist of 7 items rated on a 4-point scale from 0 (no symptom) to 3 (a severe symptom). The cut-off score > 7 was used to indicate at least a mild, but significant, level of depressive symptoms. Distress symptomatology was evaluated using the validated Norwegian translation of the Symptom Checklist 90-Revised (SCL-90-R) (30). This questionnaire measures emotional distress during the previous 7 days and consists of 90 items rated on a 5-point scale from 0 (not at all) to 4 (extremely). The 90 scores are transferred to a profile sheet of 9 symptom dimensions (Somatization, Obsessive-Compulsive, Interpersonal Sensitivity, Depression, Anxiety, Hostility, Phobic Anxiety, Paranoid Ideation, and Psychoticism) and provide a Global Severity Index (GSI) that represents overall psychological distress. In this study, depression was operationalized by scores above the cut-off on 2 scales to avoid false positives: the HADS-Depression subscale (scores >7) and the SCL-90-R Depression symptom dimension (T scores ≥ 63). This operational definition does not fulfil the ICD-10 criteria for major depression requiring treatment. The severity of fatigue related to daily activities was assessed using the Norwegian translation of the Fatigue Severity Scale (FSS) (31). This scale contains 9 items rated on a 7-point scale, from 1 (strongly disagree) to 7 (strongly agree). The severity of pain during the previous 7 days was measured using a visual analogue scale for pain (VAS-P) and was rated on a 100-mm horizontal line, ranging from 0 (indicating no pain) to 100 (very severe pain).
All persons underwent CT scanning within 24 h after injury. Magnetic resonance imaging (MRI) scans were performed on 104 participants at 1-year follow-up. The trauma scores of the Abbreviated Injury Scale (AIS) (32) and the Injury Severity Score (ISS) (33) were extracted from the Trauma Registry of the Oslo University Hospital, Ulleval. An AIShead score from 3 to 5 indicates increasingly severe intracranial pathology. An ISS greater than 15 is accepted as the definition of major trauma.
Statistical analysis
Chi-square tests were conducted to analyse the frequencies of demographic characteristics, injury-related variables, and psychosocial situations and to compare the percentages of males and females who met the criteria for depression, as stated above. Data obtained from questionnaires (mean, standard deviation (SD)) were analysed by repeated-measures analysis of variance (ANOVA) (within-subjects) at 3 months, 1 year, and 5 years. A total of 89 (of the original 118) individuals participated at 5 years; a response rate of nearly 75%. This reduction affected the power of the statistical analysis and the number of potential predictors in the regression analyses. Regression analyses were therefore performed on the 1-year data of 106 participants. To reduce the effect of multiple comparisons, only two regression models were chosen. The HADS-Depression score was chosen as the dependent variable in the regression analysis because this scale is one of the most common measures of depression used in TBI studies (34). For the first model, linear regression analysis (backward selection) was conducted to explore the associations between the HADS-Depression score at 1 year and demographic variables (age, gender, and education), injury severity (ISS in the acute phase), employment status (pre-injury and at 1 year), psychosocial situation (pre-existing and at 1 year), and scores on the FSS, VAS-P, and SCL-90-R Anxiety scales. For the second model, the associations between GSI (SCL-90-R) at 1 year and demographic variables (age, gender, and education), injury severity (ISS in the acute phase), employment status (pre-injury and at 1 year), psychosocial situation (pre-existing and at 1 year), and scores on the FSS and VAS-P were assessed. The AIShead was excluded from the analyses due to strong correlations (r > 0.70) with the GCS and ISS. A sample size of 106 individuals at 1 year, including 11 predictors, had a sufficient power of 0.86 for a medium effect size (f 2 = 0.20). Significance was assumed for p-values < 0.05 for all statistical analyses (two-tailed). Data analyses were performed using PASW® Statistics 18.
RESULTS
Characteristics of participants and psychosocial stressors
Individuals’ demographic characteristics, pre- and post-injury psychosocial variables, and injury-related data are presented in Table I. The mean age at the time of injury was 32.5 years (SD 11.1) and the mean length of education 13.2 years (SD 2.5). In our sample, 66% of patients sustained a moderate-to-severe TBI (GCS 3–12) and 34% sustained a mild TBI (GCS 13–15). Transport accidents caused the injury in 46% of individuals, followed by falls (27%), assaults (19%) and other causes (8%). A considerable number of individuals had substance abuse problems and unemployment.
|
Table I. Demographics, pre-injury information and injury severity of individuals with traumatic brain injury (n = 118) |
|
|
TBI sample n (%) |
|
|
Males Females Single at the time of injury Unemployed pre-injury Unemployed at 1 yeara Substance abuse pre-injurya Substance abuse at 1 yeara Substance abuse at 5 yearsa Glasgow Coma Scale score 3–12 Glasgow Coma Scale score 13–15 Traffic accident cause of injury Abbreviated Injury Scalehead ≥ 3 Injury Severity Score ≥ 15 CT acute intracranial findings MRI intracranial findings at 1-yeara Post-traumatic amnesia > 7 days |
84 (71) 34 (29) 82 (70) 25 (21) 35 (32) 28 (24) 20 (19) 22 (25) 78 (66) 40 (34) 54 (46) 80 (68) 72 (61) 72 (61) 75 (73) 51 (43) |
|
aData missing for: unemployed 1 year post-injury (n = 10), MRI (n = 14), substance abuse pre-injury (n = 1), substance abuse at 1 year (n = 13), substance abuse at 5 years (n = 31). TBI: traumatic brain injury; CT: computed tomography; MRI: magnetic resonance imaging. |
|
Pre-existing psychosocial stressors were summed to provide an overall score, resulting in a mean score of 1.4 (SD 1.4, range 0–6, n = 117). Pre-existing stressors were as follows (from most to least frequent): serious illness or death of a close family member (27%), substance abuse CAGE > 2 (24%), psychiatric illness requiring therapy (15%), marital problems (11%), serious medical illness (11%), economic problems (10%), feeling isolated or lonely (9%), and other stressors (20%), such as being in prison (4%), prior trauma (3%) or work-related stress (3%). Psychosocial stressors at 1 year were also summed to provide an overall score used in the regression analysis, resulting in a mean score of 1.3 (SD 1.6, range 0–7, n = 105). The frequency of these stressors were as follows: psychiatric illness requiring therapy (24%), substance abuse CAGE > 2 (19%), economic problems (19%), feeling isolated or lonely (18%), serious illness or death of a close family member (17%), serious medical illness (11%), marital problems (2%), and other stressors (21%) such as waiting for a trial/serving a sentence (6%), or not having a driving licence (2%).
Frequency and predictors of depressive symptoms
The percentage of individuals reporting depressive symptoms was relatively stable over time from 18% at 3 months (n = 20), 13% at 1 year (n = 14), and 18% at 5 years post-injury (n = 16). No effects of TBI severity (mild vs moderate-to-severe), marital status or education level on depressive symptoms were observed at the 3 time-points (all p-values >0.05). The prevalence of depressive symptoms differed substantially between genders (χ2= 6.5, p = 0.011) at 1 year, but not at 3 months or 5 years. Depressive symptoms were observed in 18% of males and none of the females at 1 year. Of the 105 individuals who participated in both the 3- and 12- months follow-up assessments, 22% (n = 23) reported significant depressive symptoms at least once during the first year. Of the 83 individuals who were assessed at all time-points, 28% (n = 23) had depressive symptoms at least once during the 5-year period after the injury. Only 4% (n = 3) had persistent depressive symptoms at all time-points.
Regression analysis was performed using the HADS-Depression score at 1 year as the dependent variable. Because depression and anxiety often co-exist (4), the SCL-90-R Anxiety dimension at 3 months was included in the regression analysis. Pearson’s correlation coefficient for the association between the HADS-Depression and SCL-90-R Anxiety scores was r = 0.51, p <0.001. Table III shows that higher levels of anxiety at 3 months (SCL-90-R Anxiety dimension), a high number of ongoing psychosocial stressors, older age, being employed pre-injury and being unemployed post-injury were the main predictors of depressive symptoms. These 5 variables accounted for 43% of the variance (F5,101=14.7, p = 0.001). Post-hoc analysis using t-tests showed that individuals who had high levels of depressive symptoms at 1 year (HADS-Depression > 7 and SCL-90-R Depression ≥ 63), had significantly higher numbers of pre-existing psychosocial stressors (2.1 (SD 1.7)) compared with those with low levels of depressive symptoms (1.1 (SD 1.2)) (p < 0.01). Individuals who had high levels of depressive symptoms at 1 year also had higher numbers of ongoing psychosocial stressors (2.9 (SD 1.7)) than their counterparts (1.1 (SD 1.3)) (p < 0.001).
Changes in psychological distress
Table II presents the results obtained from the questionnaires addressing aspects of psychological distress (SCl-90-R), fatigue (FSS) and pain (VAS-P). A total of 83 individuals completed the SCL-90-R at all time-points. The raw SCL-90-R scores were converted into gender-adjusted T-scores as suggested by Derogatis (30). Repeated-measures ANOVA (within-subjects) using the Greenhouse-Geisser adjustment revealed a significant increase in the scores from 3 months to 5 years for 5 dimensions of the SCL-90-R. The FSS and VAS-Pain scores did not vary significantly over time (p > 0.05). The mean FSS scores were similar to the mean score of 3.98 for the Norwegian population (31).
|
Table II. Results of questionnaires at 3 months, 1 year and 5 years post-injury, as calculated with repeated measures of analysis of variance (n = 83) |
|||||
|
3 months Mean (SD) |
1 year Mean (SD) |
5 years Mean (SD) |
F |
p-value |
|
|
Hospital Anxiety and Depression Scale Anxiety (total score) Depression (total score) |
4.6 (3.8) 3.7 (3.5) |
4.6 (4.2) 3.2 (3.5) |
5.8 (4.5) 4.4 (3.8) |
5.89 4.51 |
0.01* 0.01* |
|
Symptom checklist-90-R (T-scores) Global Severity Index Somatization Obsessive-Compulsive Interpersonal Sensitivity Depression Anxiety Hostility Phobic Anxiety Paranoid Ideation Psychoticism |
55.6 (15.1) 57.3 (14.5) 58.4 (15.7) 50.3 (11.5) 57.3 (15.5) 55.6 (16.4) 49.9 (10.0) 56.9 (20.9) 50.7 (13.1) 51.4 (14.3) |
54.8 (12.5) 55.2 (13.8) 59.0 (15.5) 51.1 (10.4) 54.9 (13.6) 53.7 (15.4) 50.3 (9.6) 54.7 (17.2) 49.9 (9.6) 50.3 (11.0) |
58.6 (17.2) 57.0 (15.1) 62.1 (17.6) 54.8 (15.4) 59.3 (18.4) 57.2 (18.0) 54.3 (14.0) 59.3 (27.9) 53.3 (15.3) 52.7 (13.4) |
2.82 1.20 3.27 7.75 4.09 2.89 8.39 2.21 3.68 1.74 |
0.07 0.30 0.05* 0.001* 0.02* 0.06 0.001* 0.12 0.03* 0.18 |
|
Fatigue Severity Scale (total score) |
3.8 (1.8) |
3.8 (1.8) |
4.0 (1.7) |
0.45 |
0.62 |
|
Pain Visual Analogue Scale (1–100 mm) |
25 (25) |
20 (25) |
24 (27) |
1.60 |
0.21 |
|
*Significant within-subjects effect. SD: standard deviation. |
|||||
|
Table III. Regression coefficients (B,β) with 95% confidence intervals (CI) for predictors of depressive symptoms (HADS-Depression) at 1 year after injury |
||||
|
Independent variables |
B |
b |
95% CI (B) |
p-value |
|
Constant SCL-90-R Anxiety at 3 months Psychosocial stressors at 1 yeara Employment at 1 year Age at injury Employment pre-injury |
–4.55 0.08 0.70 1.93 0.06 –1.70 |
0.38 0.28 0.24 0.19 –0.18 |
–7.29 to –1.83 0.04 to 0.11 0.25 to 1.15 0.38 to 3.47 0.01 to 0.12 –3.58 to 0.19 |
0.001 0.003 0.015 0.021 0.078 |
|
R2 = 0.43, adjusted R2 = 0.40. HADS: Hospital Anxiety and Depression Scale; SCL-90-R: Symptom Checklist 90-Revised. aMedical or psychiatric illness, serious illness or death in family, marital problems, economic problems, substance abuse, feeling isolated or other problems. |
||||
Predictors of psychological distress
Regression analysis was performed using the GSI (SCL-90-R) at 1 year as the dependent variable. Table IV shows that a high number of ongoing psychosocial stressors, a low number of pre-existing stressors, being unemployed before injury, and higher levels of fatigue and pain at 3 months, emerged as the best predictors of psychological distress at 1 year, explaining 45% of the variance (F5,101=15.6, p = 0.001).
|
Table IV. Regression coefficients (B,β) with 95% confidence intervals (CI) for predictors of psychological distress (SCL-90-R: Global Severity Index) at 1 year after injury |
||||
|
Independent variables |
B |
b |
95% CI (B) |
p-value |
|
Constant Psychosocial stressors at 1 yeara Fatigue Severity Scale Employment pre-injury Psychosocial stressors pre-existinga Visual analogue scale for pain |
28.12 4.73 2.88 9.64 –2.35 1.04 |
0.43 0.31 0.23 –0.20 0.23 |
19.09 to 37.15 2.66 to 6.81 1.33 to 4.43 2.40 to 16.89 –4.65 to –0.05 –0.05 to –2.11 |
0.001 0.001 0.010 0.045 0.054 |
|
R2 = 0.45, adjusted R2 = 0.42. SCL-90-R: Symptom Checklist 90-Revised. aMedical or psychiatric illness, serious illness or death in family, marital problems, economic problems, substance abuse, feeling isolated or other problems. |
||||
Correlations between measurements are reported in Table V. The data from questionnaires investigating depressive symptoms were highly correlated with the GSI (SCL-90-R) and psychosocial stressors at 1 year (Pearson’s two-tailed correlations). Substance abuse at 1 year was correlated with psychosocial stressors (Spearman’s two-tailed correlations).
|
Table V. Correlations between measures of depressive symptoms (HADS and SCL-90-R), Global Severity Index (GSI: SCL-90-R), FSS, VAS-P, CAGE, and psychosocial stressors |
|||||||
|
GSI |
FSS |
VAS-P |
CAGE |
Stressors pre-injury |
Stressors at 1 year |
HADS-Depression |
|
|
Depression dimension (SCL-90-R) Global Severity Index (SCL-90-R) at 1 year Fatigue Severity Scale (FSS) at 3 months VAS-Pain at 3 months CAGE at 1 year Psychosocial stressors pre-injury Stressors at 1 year |
0.94*** |
0.41*** 0.45*** |
0.27*** 0.39*** 0.42*** |
0.11 0.11 0.05 –0.05 |
0.23* 0.22* 0.17 0.20* 0.22a |
0.46*** 0.48*** 0.12 0.24* 0.28b 0.59*** |
0.72*** 0.72*** 0.28** 0.31** 0.04 0.28** 0.50*** |
|
*Pearson’s correlation (p <0.05), **Pearson’s correlation (p <0.01), ***Pearson’s correlation (p <0.001). aSpearman’s correlation (p <0.05), bSpearman’s correlation (p <0.01). SCL-90-R: Symptom Checklist 90-Revised; VAS-P: visual analogue scale for pain; CAGE: Cut down, Annoyed, Guilty, Eye-opener. |
|||||||
DISCUSSION
This longitudinal study investigated the relationships between depressive symptoms and potential predictors in a sample of hospitalized individuals with mild to severe TBI. The prevalence of depressive symptoms was found to be stable over a 5-year period. High levels of depressive symptoms were a significant problem in 18% of individuals at 3 months, 13% at 1 year, and 18% at 5 years after injury. Among demographic, injury-related and clinical factors, anxiety and ongoing psychosocial stressors were the strongest predictors of depressive symptoms (HADS), together with increasing age, being employed before injury and being unemployed post-injury. Psychological distress symptoms (SCL-90-R GSI) were more strongly predicted by ongoing psychosocial stressors than by pre-existing stressors, together with pre-injury unemployment and higher levels of fatigue and pain.
In our study, the frequencies of depressive symptoms were similar to those found by Bryant et al. (1), who reported that 18% of patients had depression at 3 months and 17% had depression at 1 year after mild TBI. Our results are also consistent with those of another study (15), which found that 17% of patients had depression 3–5 years after moderate-to-severe TBI. In a recent longitudinal study, the prevalence of depression was found to be 26% at both 1 and 2 follow-up years after injury and 75% of those with depression at 1 year had significant symptoms at 2 years (6). Another study assessed depression at 3 months up to 4 years after TBI, using repeated clinical interviews 1 year apart (21), and 35% of subjects reported depression at the initial assessment, 24% at the second, and 21% at the third. A Norwegian study found a prevalence of depressive symptoms of 31% at 10 years after moderate-to-severe TBI (19). Currently, there is firm evidence that the prevalence of depression (> 30–50%) is high after TBI (1, 6, 7, 19, 21) relative to the 12-month prevalence rates of 4.2%–10.3% for the general population (35, 36). In this study, 22% of patients developed high levels of depressive symptoms at least once during the first year after injury and 28% at least once during the 5-year period after injury. Overall, 4% reported high levels of depressive symptoms at all time-points (chronic cases). Other studies found that 33–53% of subjects met the criteria for depression during the first year after TBI (4, 7) and 46–52% during the first 5 years (5, 13), with 14% defined as chronic cases (13). The results of our study are consistent with previous findings, indicating that injury severity (ISS) does not predict depressive symptoms (1, 13, 15, 17).
According to the literature, females are expected to experience depression more often than males (35). However, in this study males reported more depressive symptoms than females at 1 year. Another study found this same gender difference in the TBI population (15) and regarded it as a finding of chance. Several possibilities could explain this difference. First, males may not be receiving medical or psychological treatment for their depression during the first year, in contrast to females, who may receive more attention and treatment for their depression. Secondly, the number of females in this study was small at one year (n = 31) and, by chance, none had experienced depressive symptoms.
Anxiety was expected to contribute to depressive symptoms, as a high frequency of depression and anxiety co-morbidity (73.5%) was documented in the study by Whelan-Goodinson et al. (5). In the present study, anxiety at 3 months was identified as the strongest predictor for depressive symptoms at 1 year. Another study found that individuals with co-existing depression and anxiety had longer durations of symptoms than those who were only depressed (4). Other studies have found that depression can co-exist with other psychiatric conditions, such as substance abuse and post-traumatic stress disorder (1, 3, 6, 7, 9).
In this study, repeated measures revealed that neither pain scores nor scores for fatigue (FSS) or somatic complaints (somatization dimension SCL-90-R) exhibited significant increases over time. In this study, fatigue and pain correlated moderately with depressive symptoms at 1 year, reflecting the complex interactions between physical and psychological disturbances in individuals with TBI. The most frequent symptom of depression is fatigue (20). Englander et al. (37) found that patients with TBI taking anti-depressant medications had higher fatigue scores, probably because of medication side-effects or that depression contributed to fatigue. Only a few studies have focused on pain and depression in the TBI population (13, 37, 38). Hibbard et al. (13) noted that pain had a greater impact on chronic depression after TBI, suggesting that pain served as a stimulus or maintainer of depressive symptoms. Another study (38) reported a higher prevalence rate of pain (66.7%) among TBI patients 1 year after injury, and found that depression was strongly associated with poorer pain outcomes. These findings are partly in contrast to those of the current study, which found that pain was not significantly associated with depression in the regression analysis but only on a bivariate level.
An important implication of the current study is that the employment status represents a sensitive indicator of depressive symptoms, in agreement with other studies (15, 17, 18). On the one hand, depression may delay or hinder recovery from TBI and may complicate the process of returning to work, school and social life (4, 13). On the other hand, an individual’s decreased ability to function at work and at home due to biological, interpersonal and social disruptions may cause emotional distress that may further lead to the development of mood disorders (13, 23).
Disturbances in psychosocial function affecting employment situation and rehabilitation are often described in the TBI literature (13). As noted above, psychosocial stressors were not frequent in individuals without depressive symptoms and perhaps in some cases these stressors were normal psychological reactions. This study did not determine the type of stressors involved in depressive symptoms related to family (e.g. marital, economic problems) or psychological (e.g. substance abuse, previous psychiatric illness). At 5 years, 25% of individuals reported substance abuse (CAGE), i.e. approximately at the same level as pre-injury, but substance abuse was not found to correlate with depressive symptoms or general distress.
This study has a number of limitations. First, because of the small size of the sample, which was representative of the population (aged 16–55 years) in eastern Norway, caution should be exercised in when generalizing results to other populations. The sample attrition rate over time was systematic, not random; the participants that tended to stay in the study had moderate-to-severe TBI, thus biasing the long-term data toward more severe cases. Secondly, the exclusion of previous severe substance abuse or known previous psychiatric disorders (n = 21) may have resulted in the underestimation of pre-existing psychosocial stressors and psychiatric problems. Thirdly, the present study used two subscales of depression to estimate the prevalence of depressive symptoms. It is not known if these levels of depressive symptoms are sufficient to warrant a diagnosis of major depressive disorder. Recently, the HADS has been recognized to have a high rate of false negatives and to exhibit inconsistency in differentiating anxiety and depression (39). In this study, the HADS-Depression score was highly correlated with the GSI (SCL-90-R), a result that may support the finding that depressive items on the HADS consist of a non-specific component of general emotional reactions. The SCL-90-R has been divided into the “Brain Injury Scale” with 14 items and the remaining 76 SCL-90-R items. A previous study did not support this distinction (40) as the ratings for both scales were equally related to affective reactions, cognitive performance and behavioural disturbances related to brain injury. The findings of Hoofien et al. (40) indicate that the SCL-90-R is a valid measure of psychological distress for individuals with TBI.
The findings of this study indicate that psychosocial stressors and employment status contributed to depressive symptoms and psychological distress at 1 year after injury, whereas injury severity did not have any predictive value. The prevalence of depressive symptoms remained stable over time, emphasizing the importance of recognizing and treating depressive symptoms early after injury.
ACKNOWLEDGEMENTS
The authors are grateful to all the persons for their participation. The study was supported by grants from the South-Eastern Norway Regional Health Authority. Thanks to Morten Hestnes and Nils Oddvar Skaga from the Oslo University Hospital’s Trauma Registry, for the extraction of trauma scores, and Tone Jerstad, neuroradiologist, for the CT and MRI assessments.
REFERENCES
