Sang Seok Yeo, MS and Sung Ho Jang, MD
From the Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Taegu, Republic of Korea
OBJECTIVE: We report on a patient who appeared to demonstrate neural reorganization after head trauma resulting in bilateral injury of the fornix crus.
CASE REPORT: A 58-year-old male patient and 8 control subjects were recruited. The patient had undergone head trauma as the result of a car accident and had lost consciousness for 30 min. Brain magnetic resonance imaging, performed 3 years after the head trauma, showed no evidence of abnormality.
RESULTS: Discontinuation of both crus in the proximal region was observed on diffusion tensor tractography of the fornix. In the right fornix, an abnormal neural tract originating from the right crus passed through the splenium of the corpus callosum to connect with the right inferior longitudinal fasciculus. By contrast, in the left fornix, another abnormal neural tract originating from the left column passed through the left inferior longitudinal fasciculus and the splenium of the corpus callosum. None of these abnormal neural tracts was observed in normal subjects.
CONCLUSION: We presume that the abnormal neural tracts of the fornix observed in this patient were the result of neural reorganization triggered by bilateral injury of the fornix crus. The results of this study suggest a mechanism for recovery of the injured fornix following head trauma.
Key words: head trauma; fornix; memory; diffusion tensor imaging; brain plasticity.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Sung Ho Jang, Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 317-1, Daemyungdong, Namku, Taegu, 705-717, Republic of Korea. E-mail: strokerehab@hanmail.net
Accepted Dec 21, 2012; Epub ahead of print Apr 12, 2013
Introduction
Plasticity of the brain enables neuronal reorganization after injury. Elucidation of mechanisms of recovery following brain injury is important because such information provides a scientific basis for neurorehabilitation. With development of brain mapping techniques, such as functional magnetic resonance imaging, active investigation of the mechanisms of recovery of motor and somatosensory function following brain injury has been conducted (1–4). However, because most neural structures related to memory are probably located deep within the brain, little is known about the mechanisms for recovery of memory.
The fornix transfers information from episodic memory between the medial temporal lobe and the medial diencephalon (5). Diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), allows for 3D visualization and estimation of the fornix (6). Several studies using DTI have reported on injury of the fornix following traumatic brain injury (TBI) (7–14). However, little is known about the mechanisms for recovery of an injured fornix after TBI.
In this study, we report on a patient who appeared to demonstrate neural reorganization after bilateral injury of the fornix crus resulting from head trauma.
Case report
One patient and 8 age-matched control subjects (3 male; mean age 54.0 years, age range 48–62 years) with no history of neurological disease were recruited for this study. All subjects provided written informed consent, and our institutional review board approved the study protocol.
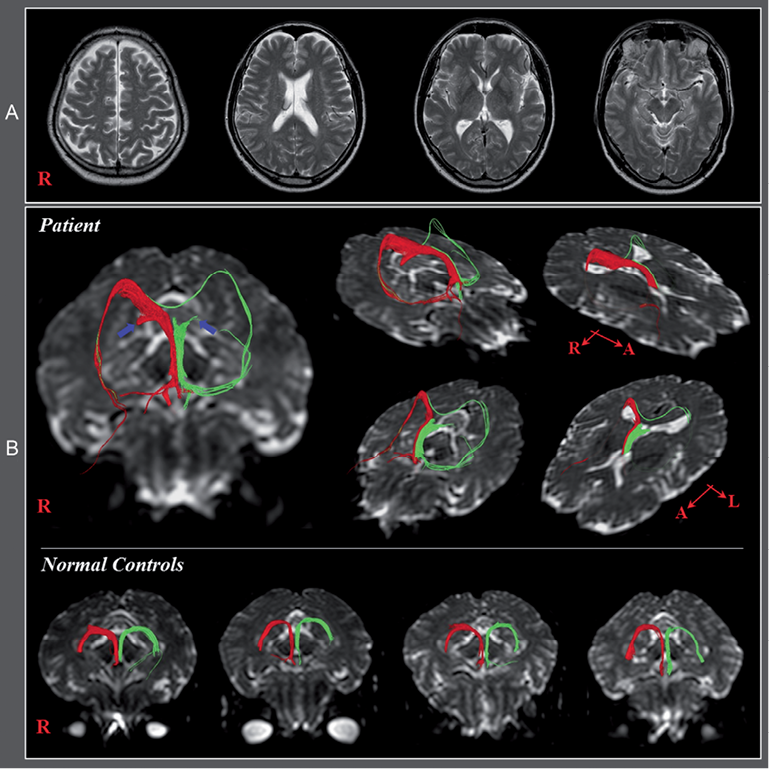
The patient was a 58-year-old right-handed male who had undergone head trauma as the result of a car accident. He had subsequently received approximately 80 stitches in the forehead at a local hospital. The patient lost consciousness for approximately 30 min from the time of the accident. Despite reports of memory impairment, he had not undergone any evaluation or rehabilitation over the subsequent 3 years except for brain computed tomography (CT), which was scanned at onset. Brain CT and T2-weighted brain images performed onset and 3 years post-TBI respectively showed no abnormality (Fig. 1A).
Fig. 1. (A) Brain computed tomography and T2-weighted magnetic resonance images taken at onset and 3 years post-traumatic brain injury, respectively, show no specific lesion. (B) Diffusion tensor tractography of the fornix showing discontinuations (blue arrows) in both crus. Abnormal neural tracts were observed in both hemispheres, compared with normal controls. In the right fornix, an abnormal neural tract originating from the right crus proximal to the discontinuous crus connected to the right inferior longitudinal fasciculus (ILF) via the splenium of the corpus callosum. By contrast, in the left fornix, an abnormal neural tract originating from the left column passed through the left ILF and joined with the right ILF via the splenium of corpus callosum. None of these abnormal neural tracts was observed in normal control subjects.
Clinical evaluation
Three scales were used for evaluation of cognitive function at the time of DTI scanning; the Mini-Mental State Examination (MMSE) (15), the Wechsler Intelligence Scale (WAIS) (16), and the Memory Assessment Scale (MAS) (17). MMSE evaluates the subject’s orientation, memory, attention, calculation, and visual memory, and language abilities and WAIS is an intelligence test battery (15, 18). MAS is a comprehensive standardized memory assessment battery that consists of 4 memory subsets: total memory, short-term memory, verbal memory, and visual memory (17). The reliability and validity of MMSE, WAIS, and MAS are well-established (15–18).
The patient showed memory impairment on the MAS (global memory: 70 (2%ile), short term memory: 92 (30%ile), verbal memory: 58 (< 1%ile), and visual memory: 95 (37%ile)), although total intelligence on the MMSE and the WAS were within the normal range (MMSE: 28, total IQ: 109).
Diffusion tensor imaging
Three years post-TBI, acquisition of DTI was performed using a 1.5-T Philips Gyroscan Intera system (Philips, Best, The Netherlands) equipped with a Synergy-L Sensitivity Encoding (SENSE) head coil using a single-shot, spin-echo planar imaging pulse sequence. For each of the 32 non-collinear and non-coplanar diffusion sensitizing gradients, we acquired 60 contiguous slices parallel to the anterior commissure-posterior commissure line. Imaging data were acquired from the area between the cortex and the middle of the second cervical vertebral body. Imaging parameters were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192 matrix, field of view = 240 × 240 mm2, TR = 10,726 ms, TE = 76 ms, parallel imaging reduction factor (SENSE factor) = 2, EPI factor = 49, b = 1000 s/mm2, NEX = 1, and slice thickness 2.5 mm (acquired isotropic voxel size 2.5 × 2.5 × 2.5 mm3). The fibre assignment continuous tracking (FACT) algorithm implemented within the DTI task card software (Philips Extended MR Work Space 2.6.3) was used in performance of fibre tracking (19). For analysis of fornix, we placed a seed region of interest (ROI) at the junction between the body and column of each fornix on a coronal image of the colour map. The target ROIs were placed on the crus of the right and left fornix on a coronal image of the colour map respectively. The termination criteria applied were a fractional anisotropy of < 0.15 and an angle change of > 60º.
On DTT images, we observed discontinuation of both crus in the proximal portion anterior to the splenium of the corpus callosum. In the right fornix, an abnormal neural tract originating from the right crus proximal to the discontinuous crus passed through the splenium of the corpus callosum to connect with the right inferior longitudinal fasciculus (ILF). By contrast, in the left fornix, another abnormal neural tract originating from the left column passed through the left ILF, the splenium of the corpus callosum to join with the right ILF. None of these abnormal neural tracts was observed in normal control subjects (Fig 1B).
In the current study, we investigated DTT findings of the fornix in a patient who had undergone head trauma. Findings on DTT showed discontinuation of the bilateral fornix crus and abnormal neural tracts that passed through the splenium and ILF, which was not observed in normal control subjects. Connection of the fornix crus to the medial temporal lobe and connection of the occipital and temporal lobes by the ILF in the normal human brain have been reported (20–22). However, in our patient, the bilateral discontinuous fornix appeared to communicate with the temporal lobe through an abnormal neural tract that passed through the splenium of the corpus callosum and the ILF in the right hemisphere, and through the fornix column and the ILF in the left hemisphere. In addition, connection of these two abnormal neural tracts through the splenium of the corpus callosum was also observed. It has been reported that the right medial temporal lobe is specialized for visual memory and the left medial temporal lobe for verbal memory (13, 23, 24). This patient showed relatively good visual memory (95; 37%ile) compared with poor verbal memory (58; < 1%ile). Therefore, we assume that these contrasting memory results might be related with DTT findings that the injured right fornix was connected through the splenium to the right medial temporal lobe and the injured left fornix was not connected to the left medial temporal lobe. As a result, we believe that these abnormal changes of the fornix are the result of neural reorganization following bilateral injury of the fornix crus. Unfortunately, because DTI was not available at the time of the incident, we were unable to perform direct comparisons.
In conclusion, we report here on a patient who appeared to demonstrate development of compensatory neural tracts following bilateral injury of the fornix crus resulting from head trauma; findings suggest a mechanism for recovery of the injured fornix following head trauma. However, because it is based on a single case report, this study is limited; thus, we suggest conduct of larger-scale complementary studies. In addition, lack of the clinical data for cognition and DTI data at the early stage of TBI is also a significant limitation of this study. Another limitation is that we could not consider the other neural tracts that have been known to be involved in memory function, including the mammillothalamic tract and the thalamocortical tract between anterior thalamus and cingulate cortex. In addition, several limitations of DTI should be kept in mind (25–28). First, the fibre tracking technique is operator-dependent. Secondly, DTI may underestimate fibre tracts. DTI is a powerful anatomic imaging tool with a capability for demonstration of gross fibre architecture; however, it cannot visualize functional or synaptic connections. Thirdly, regions of fibre complexity and crossing can obscure the underlying fibre architecture.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A4A01001873).
References
