Gordon J. Hendry, PhD1,6, Gordon F. Watt, DPodm1, Mhairi Brandon, ProfD2, Lorraine Friel, BSc2, Debbie E. Turner, PhD1, Paula K. Lorgelly, PhD3, Janet Gardner-Medwin, PhD4, Roger D. Sturrock, MD5 and James Woodburn, PhD1
From the 1School of Health & Life Sciences, Institute for Applied Health Research, Glasgow Caledonian University, 2NHS Greater Glasgow and Clyde, Glasgow Royal Infirmary, Glasgow, UK, 3Centre for Health Economics, Monash University, Clayton, Victoria, Australia, 4Department of Child Health, 5Centre for Rheumatic Diseases, University of Glasgow, Glasgow, UK and 6School of Science & Health, University of Western Sydney, Penrith, New South Wales, Australia
OBJECTIVES: To evaluate the effectiveness of multidisciplinary foot-care, and to evaluate the methodological considerations of a trial of multidisciplinary care in juvenile idiopathic arthritis.
DESIGN: Exploratory randomised controlled trial.
Subjects/Patients: Children/adolescents with juvenile idiopathic arthritis and inflammatory joint disease affecting the foot/ankle.
METHODS: Standard medical care was compared with a 12 month program of multidisciplinary foot-care informed by musculoskeletal ultrasound. This program was centred on strict disease control through rigorous examination and interventions delivered by a team comprised of a paediatric rheumatologist, podiatrist, physiotherapist and musculoskeletal ultrasonographer. Patients were assessed on foot impairment and disability scores using the Juvenile Arthritis Foot Disability Index.
RESULTS: Forty-four participants, aged 3–17 years were randomly assigned to receive the experimental (n = 21) or usual care (n = 23) interventions. There was an overall improvement in levels of foot related impairments in both groups over 12 months. Between-group differences in change scores for the Juvenile Arthritis Foot Disability Index were not statistically significant at 6 or 12 month follow-ups.
CONCLUSION: The integrated multidisciplinary foot care interventions described in this trial were safe, but did not improve foot impairment levels relative to usual care. This trial identified several methodological challenges including recruitment/retention, difficulties with outcome tools and potential confounders.
Key words: juvenile idiopathic arthritis; foot; physiotherapy; podiatry.
J Rehabil Med 2013; 45: 467–476
Correspondence address: Gordon J Hendry, School of Health & Life Sciences, Institute for Applied Health Research, Glasgow Caledonian University, Glasgow, G4 0BA, UK. E-mail: gordon.hendry@gcu.ac.uk
Accepted Dec 14, 2012; Epub ahead of print Apr 10, 2013
INTRODUCTION
Juvenile idiopathic arthritis (JIA) is the commonest rheumatic disorder of childhood and a leading cause of paediatric acquired disability with a UK wide prevalence estimated at 0.65–2 per 1,000 children (1). Foot impairments and disability persist in over 60% of children who have JIA despite recent improvements in its medical management (2). Synovitis and peri-articular manifestations such as tenosynovitis and enthesitis have been associated with pain, reduced joint ranges-of-motion, deformity, gait abnormalities, fatigue and poor functional status (3–6). Radiographic imaging studies demonstrate that erosive changes occur early, and there appears to be a small therapeutic ‘window of opportunity’ to prevent permanent damage and poor functional outcomes through pharmacological and rehabilitative interventions (7–8). Moreover, recent studies employing musculoskeletal ultrasound (MSUS) demonstrate that subclinical signs of inflammation are common in the foot and ankle even in those who meet clinical remission criteria (9, 10). Subclinical inflammation has also been associated with continued structural deterioration in adults with rheumatoid arthritis (RA) (11).
Clinical practice guidelines recommend multidisciplinary care for children/adolescents with JIA (12). There is emerging evidence that a multi-disciplinary team (MDT) approach to care may be effective in rheumatology settings (13). However, few studies have evaluated the efficacy of foot specific interventions in the MDT setting (14–17). Image-guided locally administered intra-articular cortico-steroid injections (ICIs) appear to be more efficacious than blindly administered ICIs at eliminating signs of synovial hypertrophy (14–16). However, only one randomised trial has evaluated the efficacy of custom-made foot orthoses (FOs) in JIA and demonstrated improvements in pain and functional ability levels (17).
To provide optimum foot care, it is essential that disease activity, early joint destruction, and functional status be monitored (13). As such a new paradigm of foot care has been proposed (13); taking on board contemporary issues such as early detection, targeted therapy and tight control. This not only includes standard treatments for joint dysfunction such as FOs and exercise programs, but extended scope practice including MSUS assessments, and MSUS-guided ICIs. Accordingly the aims of this study were to 1) evaluate the clinical effectiveness of an integrated multi-disciplinary foot care program for patients with JIA and 2)investigate the key methodological considerations for a two-arm non-pharmacological exploratory parallel trial of an integrated multidisciplinary foot care program versus ‘usual care’.
MATERIALS AND METHODS
Eligibility
Children and adolescents with a definitive diagnosis of JIA according to the International League of Associations for Rheumatology (ILAR) who were being treated at the Royal Hospital for Sick Children. Glasgow, UK between March 2008 and March 2010, were eligible for inclusion (18). Children/adolescents with JIA were included if they satisfied at least one of the following: previously documented arthritis in the foot including small joints derived from medical case notes, previously documented foot arthritis in one or more large joints (tib-talar/subtalar) derived from medical case notes, or current widespread polyarthritis involving large and small foot joints derived from clinical examination by a consultant paediatric rheumatologist. Children/adolescents who were in receipt of podiatric care at the time of enrolment were also eligible for inclusion. Patients with an unconfirmed diagnosis of JIA, and/or only upper limb, jaw, or neck involvement were excluded. The Glasgow West Local Research Ethics Committee granted ethical approval (Ref 06/S0703129). Written informed consent was obtained from participants and parents/guardians in accordance with the Declaration of Helsinki. This trial was registered with the International Standard Randomised Controlled Trial Number (ISRCTN) register (registration number ISRCTN49672274).
Assessments
At baseline all participants were assessed to ensure they satisfied the inclusion criteria. Demographic and disease characteristic data including age, gender, body mass index (BMI), disease sub-type, date of diagnosis, disease duration were recorded. BMI scores were converted to BMI standard deviation scores using standard methods (19). All baseline assessments and outcome measures were recorded prior to randomization. The medical care plan formulated by the paediatric rheumatologist (JGM/RDS) was documented prior to randomisation.
Baseline assessment of disease activity was conducted by the paediatric rheumatologist (JGM/RDS) accompanied by a physiotherapist (MB/LF) using 5 of the 6 American College of Rheumatology (ACR) Core Variables for JIA, a validated group of core clinical outcome variables for disease activity (20). This core set includes active/limited joint counts (0–77), functional ability assessment via the Childhood Health Assessment Questionnaire (CHAQ), and visual analogue scales (VAS) completed by the physician for global assessment of disease activity, and parent/patient for global assessment of well-being (20, 21). Erythrocyte sedimentation rate (ESR) was omitted as blood samples were not routinely collected from each participant.
Participants underwent examinations of their foot and ankle joints to assess for tenderness and swelling by a podiatrist (GJH/JW). Clinical features were recorded as present/absent and summated to derive tender and swollen foot joint counts (tibiotalar, subtalar, calcaneo-cuboid, talo-navicular, 5 metatarsophalangeal, 4 proximal interphalangeal, 5 distal interphalangeal) providing a total score (0–36). Five tendons (tibialis posterior, flexor digitorum longus, flexor hallucis longus, peroneus brevis, and peroneus longus) and 3 miscellaneous soft tissues (Achilles tendon insertion [TA], plantar fascia origin [PF], and retrocalcaneal bursa [RCB]) were assessed for tenderness and/or swelling. Clinical features were recorded as present/absent summated to derive tender and swollen miscellaneous soft tissue site counts (0–16 total score). Tenderness and swelling were determined according to the EULAR handbook of standard methods (22). Foot deformity was assessed according to the Structural Index (SI) to provide summated scores for rearfoot/ankle (0–14) and forefoot (0–24) (23).
MSUS was undertaken by an experience sonographer (DET) using established methodologies for the foot and ankle employing B-mode and colour and Power Doppler (24). These same sites were assessed independently for effusion, synovial hypertrophy (SH), erosion, and power Doppler signal (PD) (joints); tendons for grey scale features of fluid and PD within the tendon sheath; the TA and PF for abnormal thickening, and RCB for effusion. Standardised definitions for ultrasound derived pathology were employed throughout (25). Imaging was conducted using a Siemens Acuson Antares machine (Malvern, USA) with 14L5 (5–13MHz, footprint size 45mm x 9mm) probe, or Esaote Mylab 25 Gold (Genova, Italy) with LA435 (18–16MHz) probe (footprint size 40 mm × 10 mm). US features were recorded as present/absent and summated for a total score for joints (0–28) and soft tissues (0–16).
Outcomes
The primary outcome was assessed at baseline, 6 months and 12 months. The primary outcome employed in this study was the Juvenile Arthritis Foot Disability Index (JAFI), a 27 item questionnaire organised by 3 dimensions related to impairment, activity limitation and participation restriction (26). Each item is scored on a 5-point Likert scale, which describes the frequency of foot-related problems experienced in the previous week (0 = never, 1 = less than once a week, 2 = once a week, 3 = 2 or 3 times a week, 4 = always), and a median score is calculated for each dimension. The JAFI has been shown to be valid and reliable for assessing foot-related disability in JIA (26). Following the recommendations from Andre et al. (26), the JAFI was completed by parents/guardians of children < 10 years and self-completed by adolescents ≥ 10 years of age. Secondary outcomes collected at 12 months from baseline were:
• functional impairment using the CHAQ, a valid and reliable instrument for measuring global functional status in children with JIA (21)
• self- and proxy-reported health-related quality of life (HRQoL) using the EQ-5D-Y (patients) and EQ-5D-3L (parents/guardians) questionnaires, which are both comparable, valid and reliable generic measures of HRQoL in children/adolescents and adults (27, 28)
• disease activity using the ACR core variables for JIA (minus ESR) (20)
• localised foot disease activity using summated clinical examination indices of tenderness and swelling (22)
• foot deformity score using the SI (23)
• localised foot disease activity using summated MSUS examination indices of effusion, SH, erosion, and PD modified from Szkudlarek et al. (24).
Randomization
Treatment allocation was conducted by minimisation, a highly effective method of allocation that is recommended for use in randomised controlled trials (29). Treatment allocation by minimisation depends upon the characteristics of participants already enrolled to ‘minimise’ the influence of confounding factors (30). Minimisation was adopted to achieve balance between groups according to age, gender, and baseline score from the JAFI impairment domain (primary outcome). This method was conducted by the same researcher throughout the study (GJH) using the Minim software package (31). At baseline, the outcome of allocation was concealed from all assessing clinicians until clinical data were documented. Following minimisation, assessing clinician findings remained undisclosed for those participants allocated to the usual care arm. Throughout the trial, usual care arm clinicians were blinded to participants’ inclusion to ensure normal practice remained so. Participants and trial personnel were not blinded to the outcome of allocation.
Proposed interventions
According to the Medical Research Council (MRC) framework for trials of complex interventions, non-pharmacological phase II trials allow for the monitoring and modelling of interventions (32). The intended experimental and ‘usual care’ interventions are described below.
Multidisciplinary foot care intervention
Following clinical assessments and MSUS identification of inflammation, a plan of appropriate clinical care was to be devised by the paediatric rheumatologist (JGM/RDS), the podiatrist (JW/GJH), the sonographer (DET/RDS) and physiotherapist (MB/LF). ICIs prescribed by the rheumatologists (JGM/RDS) were to be conducted under general anaesthesia using MSUS guidance ideally within 4 weeks (13–15). Participants were to receive individualised care packages comprising combinations of FOs and targeted home exercise programs for symptomatic joints including stretching and muscle strengthening (16, 33). The multidisciplinary foot care intervention was delivered exclusively by the trial personnel. Customised FOs were to be manufactured via an external laboratory (Firefly Orthoses, Sligo, Ireland) and distributed within 2 weeks.
Usual care
In the absence of standard practice guidelines for foot care in JIA, usual care was recorded over the course of the trial. Participants randomised to the usual care arm were to receive normal outpatient medical care from their consultant paediatric rheumatologists, who remained blinded to all other clinical assessments. Usual care arm clinicians were not simultaneously involved in the medical care of intervention arm participants. Further referrals to podiatry for care delivered by the usual care arm podiatrist (GFW) were permitted from the usual care paediatric rheumatologists. Clinical care and referrals were monitored and recorded to define the experimental intervention and usual care (see Results).
Adverse events procedure
In the event of any minor adverse reaction or side effects due to any interventions these were to be documented and reported to the trial steering committee and formally reviewed for action at 6-monthly meetings. In the event of any major adverse events experienced by trial participants, it was the chief investigator‘s responsibility to report the major adverse event to the study sponsor and the trial steering committee.
Statistical methods
Based on clinical estimates obtained from a previous pilot study incorporating the JAFI as an outcome measure, sample size was calculated for the analysis to have 90% power of detecting a 1 point reduction in the JAFI impairment domain score over 6 months of intervention at 5% significance (2). A minimum of forty-four patients (22 in each trial arm) were required, and subsequently a sample size of 60 (30 in each arm) was targeted to account for potential loss-to-follow-up (34).
Statistical analyses were conducted via the SPSS 18.0 (SPSS Inc. Chicago, IL, USA). Demographic and disease characteristics data were described using standard descriptive statistics. The primary analysis compared the change in JAFI scores for each dimension between the intervention and control group at 6 months from baseline. A Mann-Whitney U test was used to analyse the primary outcome variable where the distributions of change scores were skewed and therefore not normally distributed (Kolmogorov-Smirnov test, p < 0.05). Two-tailed tests were used for all analyses and α was set at 0.05.
Longitudinal JAFI scores at 0, 6 and 12 months were analysed using repeated measures Friedman’s ANOVA to identify significant differences between time points within each group. Distributions of change scores from baseline to 12 months follow up for all secondary outcomes were not normally distributed and were analysed across treatment groups using the Mann-Whitney U test.
For missing data identified at the end of the study, a sensitivity analysis was performed in order to identify the most appropriate method to address this problem.
RESULTS
One-hundred and twenty-six patients attending the paediatric rheumatology outpatient clinic were identified as potential participants. Forty-one patients refused to participate, and 41 patients did not meet the trial participant inclusion criteria (Fig. 1). Forty-four patients were allocated by minimisation to the experimental intervention arm (n = 21) and the usual care (control) arm (n = 23). In the intervention arm all patients initially received the intervention until 6 months follow up, where one participant declined to participate further due to the burden of multiple appointments. In the control arm all patients received normal standard care for the duration of the trial. Twenty-one participants allocated to receive the intervention completed the primary outcome measure at 6 and 12 months from baseline. Twenty-three participants allocated to the control arm completed the primary outcome measure at 6 months from baseline. Three were lost to final 12 months follow up therefore only 20 patients completed the primary outcome and were included for final analysis.
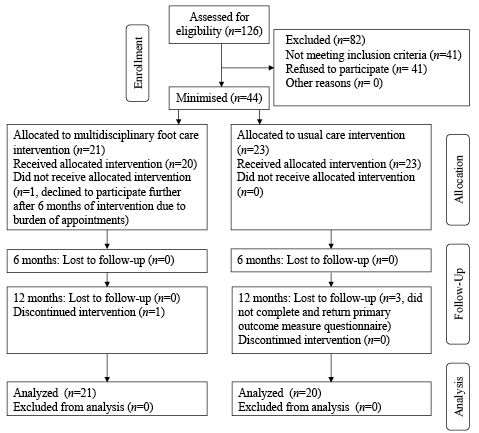
Fig. 1. Trial profile, recruitment/retention, and participant flow.
Baseline data
Baseline results are given in Table I. Both treatment groups were similar in terms of age, gender and BMI. There were no differences between groups for the JAFI impairment domain. No significant differences were observed between groups for JAFI activity limitation and participation restriction domain scores, indices of foot deformity, clinical and US examinations of foot disease activity. Similarly, no significant differences were observed between group medians for disease duration, CHAQ score, VAS pain and general wellbeing, in the direction of worse scores in the intervention group. Indices of joint disease activity and limitation were slightly higher in the control group. Both self- and proxy- reported quality of life outcomes were similar. Differences in baseline characteristics between groups did not reach statistical significance (independent t test/Mann-Whitney U, p > 0.05). Treatment groups were similar in terms of pharmacological management. There were small differences in proportions of JIA disease subtypes.
Actual multidisciplinary foot care interventions delivered over 12 months
Participants had separate consultations with a paediatric rheumatologist, podiatrist, physiotherapist and sonographer at baseline, and 3, 6, 9 and 12 months follow ups. All participants were advised regarding basic foot care, footwear, targeted exercises and basic joint protection. Podiatric intervention was centred on orthotic therapy. Of the 21 participants 17 were prescribed FOs that were designed on a case-by-case basis. All FOs were manufactured from a subtalar-joint-neutral plaster-of-Paris cast using semi-rigid carbon fibre (TL2100 2.2 mm) for the orthotic shell, 3 mm poron/vinyl for cushioning/top cover extended to the toes, and posted to calcaneal vertical position. Additions were made on a case-by-case basis and included: 1 unilateral 14 mm heel raise (for leg length discrepancy), 2 bilateral medial flanges (for severe pes planovalgus foot posture), and 2 bilateral metatarsal bars (for correctable lesser toe deformities). During the manufacture process, 11 of 17 participants received pre-fabricated FOs for the interim period. Four participants received silicone splints for lesser-toe deformities. Thirteen of 21 participants received MSUS-guided ICIs in the joints and/or soft tissues of the foot and ankle. Triamcinolone Hexacetonide (TH) was administered by the same paediatric rheumatologist (JGM) assisted by the same sonographer (DET) throughout. Eight of 17 ICIs took place under general anaesthetic more than 4 weeks after originally planned due to limited theatre availability. Over the 12 months of the trial, 10 patients were started on new medications (4 etanercept, 2 infliximab, 2 oral prednisilone, 1 methotrexate, 1 adalimumab); 6 participants had their dosages escalated (5 methotrexate, 1 etanercept); and 9 of 21 patients received stable medication over the 12 months duration of the trial.
|
Table I. Baseline characteristics of the study participants |
||
|
Characteristic |
Multidisciplinary foot care, n = 21 |
Standard care, n = 23 |
|
Demographics |
||
|
Age, years, mean (SD) |
10.1 (4.22) |
10.0 (3.39) |
|
Male/female, n |
7/14 |
6/17 |
|
Body mass index, mean (SD) |
18.90 (4.38) |
19.34 (3.93) |
|
SDS body mass index percentiles, mean (SD) |
62 (0.3) |
67 (0.3) |
|
Foot impairments and disability |
||
|
JAFI impairment, median (range) |
2 (0–4) |
2 (0–4) |
|
JAFI activity limitation, median (range) |
2 (0–3.5) |
1.5 (0–3) |
|
JAFI participation restriction, median (range) |
1 (0–3.5) |
2 (0–3) |
|
Structural index foot deformity score (0–38), median (range) |
5 (0–17) |
6.5 (0–16) |
|
Structural index rearfoot deformity score (0–14), median (range) |
4 (0–10) |
4.5 (0–10) |
|
Structural index forefoot deformity score (0–24), median (range) |
0 (0–7) |
2 (0–8) |
|
Tender foot joints (0–38), median (range) |
2 (0–23) |
1 (0–17) |
|
Swollen foot joints (0–38), median (range) |
1 (0–19) |
0 (0–17) |
|
Tender misc soft tissues (0–16), median (range) |
0 (0–6) |
0 (0–14) |
|
Swollen misc soft tissues (0–16), median (range) |
0 (0–6) |
0 (0–5) |
|
US effusions (0–28), median (range) |
3 (0–10) |
2 (0–18) |
|
US synovial hypertrophy (0–28), median (range) |
1 (0–6) |
1 (0–8) |
|
US erosions (0–28), median (range) |
0 (0–2) |
0 (0–8) |
|
US power Doppler signal (0–28), median (range) |
0 (0–3) |
0 (0–8) |
|
US tenosynovitis (0–5), median (range) |
0 (0–2) |
0 (0–2) |
|
US power Doppler signal tendon sheaths (0–5), median (range) |
0 (0–2) |
0 (0–2) |
|
Condition |
||
|
Disease duration, years, mean (SD) |
3.74 (2.65) |
4.06 (3.33) |
|
CHAQ (0–3), median (range) |
0.625 (0–1.5) |
0.875 (0–2.125) |
|
VAS pain (0–100), median (range) |
33 (2–81) |
41 (0–92) |
|
VAS gen (0–100), median (range) |
26 (0–57) |
37 (0–72) |
|
Active joints (0–77), median (range) |
3 (0–20) |
0 (0–40) |
|
Limited joints (0–77), median (range) |
3.5 (0–17) |
1 (0–22) |
|
Self EQ-5D utility index, mean (SD) |
0.57 (0.31) |
0.58 (0.35) |
|
Self EQ-5D VAS (0–100), mean (SD) |
69.37 (16.83) |
75.09 (21.71) |
|
Proxy EQ-5D utility index, mean (SD) |
0.69 (0.29) |
0.60 (0.33) |
|
Proxy EQ-5D VAS (0–100), mean (SD) |
76.89 (17.38) |
73.38 (18.64) |
|
Pharmacological management |
||
|
Analgesics, n (%) |
2 (9) |
3 (13) |
|
NSAIDs, n (%) |
2 (9) |
3 (13) |
|
Methotrexate, n (%) |
18 (86) |
16 (70) |
|
Etanercept, n (%) |
7 (33) |
5 (22) |
|
Sulphasalazine, n (%) |
1 (5) |
0 (0) |
|
Rituximab, n (%) |
0 (0) |
1 (4) |
|
Combination methotrexate & etanercept, n (%) |
5 (24) |
5 (22) |
|
Disease subtypes |
||
|
Persistent oligo, n (%) |
7 (33) |
4 (17) |
|
Extended oligo, n (%) |
4 (19) |
5 (22) |
|
Poly–, n (%) |
6 (29) |
10 (43) |
|
Poly+, n (%) |
0 (0) |
2 (9) |
|
PsA, n (%) |
2 (10) |
1 (4) |
|
ERA, n (%) |
2 (10) |
0 (0) |
|
Systemic, n (%) |
0 (0) |
0 (0) |
|
Undifferentiated, n (%) |
0 (0) |
1 (4) |
|
SD: standard deviation; JAFI: Juvenile Arthritis Foot Disability Index; SDS: standardised deviation score British 1990 growth reference (UK 90); NSAID: non-steroidal anti-inflammatory drugs; poly–: polyarthritis rheumatoid factor negative; poly+: polyarthritis rheumatoid factor positive; PsA: psoriatic arthritis; ERA; enthesitis related arthritis. |
||
Actual usual care delivered over 12 months
Of the 23 participants allocated to the usual care arm, 5 received a referral from the paediatric rheumatologist to the standard care arm podiatrist (GFW). Of these participants 3 received FOs on a case-by-case basis. Seven of the 23 participants received blindly administered ICIs (TH) within 4 weeks. Over 12 months 18 of 23 control participants received stable systemic medications. Of those not receiving stable medication, 1 received an escalated dose (methotrexate), and 4 patients were started on new medications (methotrexate, adalimumab, adalimumab + infliximab, infliximab).
Primary outcome data
Descriptive data for the JAFI dimension scores for each group at each time point, as well as change scores between each time point are presented in Table II and Fig. 2. For changes in the JAFI impairment score (Fig. 2) the intervention group did not differ significantly from the control group (median = 0) following 6 months of multidisciplinary foot care (U = 240.0, z = –0.037, p = 0.976, r = –0.006). Similarly, changes in JAFI activity limitation and participation scores in the intervention group did not differ significantly from the control group (U = 224.5, z = –0.418, p = 0.683, r = –0.06 and U = 225.0, z = –0.398, p = 0.698, r = –0.06 respectively). Longitudinal JAFI scores for foot related impairment did not significantly change over the course of the trial in either the intervention group (χ2 (2) = 0.76, p = 0.724) or the control group (χ2 (2) = 1.61, p = 0.462).
|
Table II. Primary outcome measure: differences between groups for changes in JAFI dimension scores following intervention |
|||||
|
Outcome measure |
n |
Intervention Median (IQR) |
n |
Control Median (IQR) |
pa |
|
JAFIimp |
|||||
|
Baseline |
21 |
2 (0 to 2) |
23 |
2 (0 to 3) |
0.807, ns |
|
∆ 6 months |
21 |
0 (–1 to 0.5) |
23 |
0 (–1 to 0) |
0.976, ns |
|
∆ 12 months |
21 |
0 (0 to 0.5) |
19 |
0 (–1 to 0) |
0.358, ns |
|
JAFIal |
|||||
|
Baseline |
21 |
2 (0.25 to 2) |
23 |
1.5 (0 to 2) |
0.480, ns |
|
∆ 6 months |
21 |
0 (–0.5 to 0.5) |
23 |
0 (–0.5 to 0.5) |
0.683, ns |
|
∆ 12 months |
21 |
0 (–0.5 to 1) |
19 |
0 (–0.5 to 1) |
0.607, ns |
|
JAFIpr |
|||||
|
Baseline |
21 |
1 (0 to 2.25) |
23 |
2 (0 to 2.5) |
0.753, ns |
|
∆ 6 months |
21 |
0 (–0.5 to 0.5) |
23 |
0 (–0.5 to 0.5) |
0.698, ns |
|
∆ 12 months |
21 |
0 (–0.5 to 1) |
19 |
0 (–0.5 to 0.5) |
0.995, ns |
|
aMann-Whitney test of change scores intervention arm vs change scores control arm. ∆ differences between 6 months and baseline scores (6 months – baseline scores) and 12 months and baseline scores (12 months score – baseline score), negative scores indicate an improvement, positive scores indicate a deterioration, score of 0 indicates no change. JAFIimp: Juvenile Arthritis Foot Disability Index impairment; JAFIal: JAFI activity limitation; JAFIpr: JAFI participation restriction; IQR: inter-quartile range. |
|||||
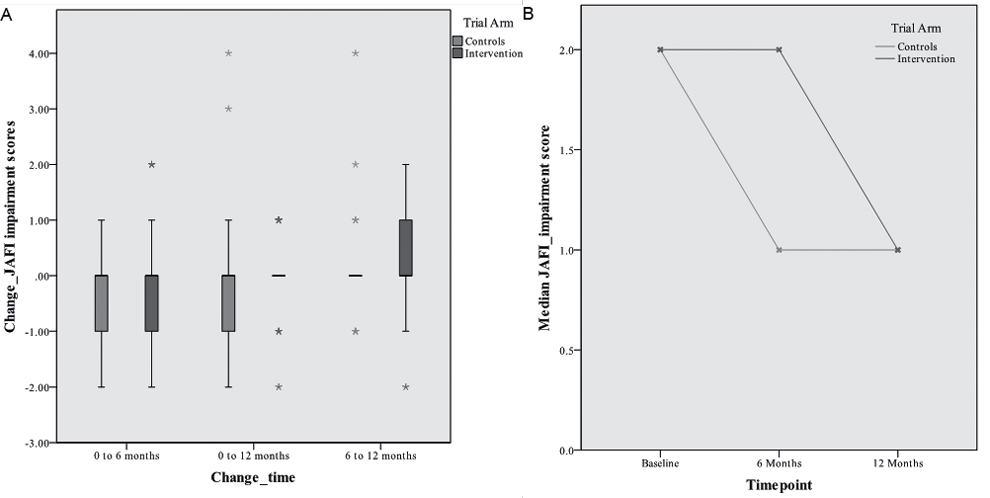
Fig. 2. A; JAFI impairment change scores (0–6 months), and B; longitudinal JAFI impairment scores in each group.
Secondary outcome data: disability, health-related quality of life and local foot impairments
Secondary outcome data with comparisons between groups are presented in Tables III and IV. There were no significant differences between treatment groups for secondary outcomes at final follow up. In terms of adverse events, one participant did not tolerate FOs due to inadequate shoe space.
|
Table III. Secondary outcomes: improvements and differences between study groups for measures |
||||||||
|
Outcome measure |
n |
Intervention Median (IQR) |
n |
Control Median (IQR) |
pa |
U statistic |
Z-score |
Effect size |
|
CHAQ |
||||||||
|
Baseline |
18 |
0.63 (0.22 to 1.03) |
21 |
0.88 (0.5 to 1.5) |
||||
|
∆ 12 months |
21 |
0 (–0.41 to 0.06) |
23 |
0 (–0.06 to 0.06) |
0.558, NS |
169 |
–0.6 |
–0.09 |
|
VAS pain |
||||||||
|
Baseline |
17 |
33 (14.5 to 51) |
21 |
41 (9 to 69) |
||||
|
∆ 12 months |
21 |
0 (–3.5 to 16) |
23 |
0 (0 to 10.5) |
0.959, NS |
176.5 |
–0.06 |
–0.09 |
|
VAS well-being |
||||||||
|
Baseline |
14 |
26 (11.25 to 48.75) |
21 |
37 (13 to 49) |
||||
|
∆ 12 months |
21 |
0 (–17.25 to 8.5) |
23 |
0 (–6 to 7) |
0.522, NS |
128 |
–0.657 |
–0.11 |
|
Active joints |
||||||||
|
Baseline |
18 |
3 (1 to 6.5) |
22 |
0 (0 to 7.5) |
||||
|
∆ 12 months |
21 |
–1.5 (–4.25 to 0) |
23 |
0 (–2.5 to 0) |
0.165, NS |
151 |
–1.4 |
–0.22 |
|
Limited joints |
||||||||
|
Baseline |
18 |
3.5 (1.5 to 8.25) |
23 |
1 (0 to 6) |
||||
|
∆ 12 months |
21 |
–2 (–4.75 to 0) |
23 |
0 (–1.5 to 0.25) |
0.128, NS |
142.5 |
–1.532 |
–0.24 |
|
EQ-5D U selfb |
||||||||
|
Baseline |
19 |
0.62 (0.52 to 0.76) |
21 |
0.66 (0.52 to 0.75) |
||||
|
∆ 12 months |
21 |
0 (–0.1 to 0.01) |
23 |
0 (–0.04 to 0.04) |
0.973, NS |
199 |
–0.014 |
–0.022 |
|
EQ-5D VAS selfb |
||||||||
|
Baseline |
19 |
65 (54 to 85) |
21 |
75 (60 to 96) |
||||
|
∆ 12 months |
21 |
0 (–10 to 20) |
23 |
0 (–7.5 to 0) |
0.342, NS |
165.6 |
–0.966 |
–0.153 |
|
EQ-5D U proxyb |
||||||||
|
Baseline |
19 |
0.69 (0.58 to 1) |
21 |
0.62 (0.55 to 0.82) |
||||
|
∆ 12 months |
21 |
0 (0 to 0.11) |
23 |
0 (0 to 0.1) |
0.539, NS |
176.5 |
–0.627 |
–0.099 |
|
EQ-5D VAS proxyb |
||||||||
|
Baseline |
19 |
80 (60 to 90) |
21 |
70 (55 to 95) |
||||
|
∆ 12 months |
21 |
0 (–1 to 14) |
23 |
0 (0 to 15) |
0.325, NS |
183 |
–0.473 |
–0.074 |
|
aMann–Whitney U test of change scores intervention arm vs change scores control arm. ∆ differences between 12 months and baseline scores (12 months score – baseline score), negative scores indicate an improvement, positive scores indicate a deterioration (unless otherwise state withb). IQR: inter-quartile range; CHAQ: Childhood Health Assessment Questionnaire; VAS: visual analogue scale. Effect sizes: 0.1 = small, 0.3 = medium, 0.5 = large. |
||||||||
|
Table IV. Secondary outcomes: improvements and differences between study groups for measures of foot disease and deformity |
||||||||
|
Outcome measure |
n |
Intervention Median (IQR) |
n |
Control Median (IQR) |
pa |
U-statistic |
Z-score |
Effect size |
|
SI foot |
||||||||
|
Baseline |
21 |
5 (2 to 7) |
22 |
6.5 (3.5 to 12) |
||||
|
∆ 12 months |
21 |
0 (–1 to 2.5) |
23 |
0 (–5.25 to 0) |
0.304, NS |
189.5 |
–1.042 |
–0.16 |
|
SI rearfoot |
||||||||
|
Baseline |
21 |
4 (1 to 6) |
22 |
4.5 (2 to 8) |
||||
|
∆ 12 months |
21 |
0 (–2 to 2) |
23 |
0 (–4 to 0.25) |
0.574, NS |
208 |
–0.575 |
–0.09 |
|
SI forefoot |
||||||||
|
Baseline |
21 |
0 (0 to 3) |
22 |
2 (0 to 5.25) |
||||
|
∆ 12 months |
21 |
0 (0 to 1) |
23 |
0 (–0.5 to 0) |
0.173, NS |
180.5 |
–1.42 |
0.213 |
|
Tender joints |
||||||||
|
Baseline |
21 |
2 (0 to 7.5) |
23 |
1 (0 to 5) |
||||
|
∆ 12 months |
21 |
0 (–1 to 0) |
23 |
0 (0 to 0) |
0.496, NS |
220.5 |
–0.672 |
–0.101 |
|
Swollen joints |
||||||||
|
Baseline |
21 |
0 (0 to 5) |
23 |
0 (0 to 4) |
||||
|
∆ 12 months |
21 |
0 (0 to 0) |
23 |
0 (–1 to 0) |
0.486, NS |
220.5 |
–0.672 |
0.101 |
|
Tender soft tissues |
||||||||
|
Baseline |
21 |
0 (0 to 2) |
23 |
0 (0 to 2) |
||||
|
∆ 12 months |
21 |
0 (0 to 0) |
23 |
0 (0 to 0) |
0.749, NS |
231 |
–0.388 |
0.058 |
|
Swollen soft tissues |
||||||||
|
Baseline |
21 |
0 (0 to 1.5) |
23 |
0 (0 to 1) |
||||
|
∆ 12 months |
21 |
0 (0 to 0) |
23 |
0 (0 to 0) |
0.781, NS |
231 |
–0.448 |
0.073 |
|
US effusions |
||||||||
|
Baseline |
19 |
3 (0 to 6) |
15 |
0 (0 to 4) |
||||
|
∆ 12 months |
19 |
0 (–3 to 3) |
15 |
0 (–3 to 0) |
0.414, NS |
119 |
–0.835 |
–0.143 |
|
US synovitis |
||||||||
|
Baseline |
19 |
1 (0 to 3) |
15 |
0 (0 to 2) |
||||
|
∆ 12 months |
19 |
0 (–2 to 0) |
15 |
0 (–2 to 0) |
0.331, NS |
115 |
–0.992 |
–0.17 |
|
US erosions |
||||||||
|
Baseline |
19 |
0 (0 to 0) |
15 |
0 (0 to 0) |
||||
|
∆ 12 months |
19 |
0 (0 to 0) |
15 |
0 (0 to 0) |
0.944, NS |
142 |
–0.026 |
–0.004 |
|
US PDS |
||||||||
|
Baseline |
19 |
0 (0 to 1) |
15 |
0 (0 to 1) |
||||
|
∆ 12 months |
19 |
0 (0 to 0) |
15 |
0 (–1 to 0) |
0.807, NS |
136 |
–0.28 |
–0.048 |
|
US soft tissues fluid |
||||||||
|
Baseline |
19 |
0 (0 to 0) |
15 |
0 (0 to 0) |
||||
|
∆ 12months |
19 |
0 (0 to 0) |
15 |
0 (0 to 0) |
0.727, NS |
134.5 |
–0.358 |
–0.061 |
|
US soft tissue PDS |
||||||||
|
Baseline |
19 |
0 (0 to 0) |
15 |
0 (0 to 0) |
||||
|
∆ 12 months |
19 |
0 (0 to 0) |
15 |
0 (0 to 0) |
0.220, NS |
114.5 |
–1.462 |
–0.25 |
|
aMann-Whitney U test of change scores intervention arm vs change scores control arm. ∆ differences between 12 months and baseline scores (12 months score – baseline score), negative scores indicate an improvement, positive scores indicate a deterioration. SI: structural index; PDS: Power Doppler Signal; IQR: inter-quartile range. Effect sizes: 0.1 = small, 0.3 = medium, 0.5 = large. |
||||||||
DISCUSSION
This trial was designed to conform to the MRC framework for trials of complex interventions, which recommends that trial research be conducted according to a continuum of evidence based upon the quality of existing preliminary evidence (32). This design permitted the gathering of valuable information regarding feasibility, which could be used to inform future trials (35).
Integrated multidisciplinary foot care did not result in a significant reduction in disease-related foot impairments and disability. Both the treatment groups appeared to improve by one point on the JAFI impairment scale between baseline and 12 months follow up, however, the differences between groups for change scores did not reach statistical significance. Long disease durations, and moderate levels of foot impairment and rearfoot deformity scores were detected at baseline. There is significant evidence which demonstrates that greater functional improvements are more achievable with second-line drugs in patients with early RA as opposed to those with established disease (36). Furthermore, van der Leeden et al. (37) recently demonstrated that earlier intervention with customised foot orthoses was predictive of improved foot pain and disability outcomes in patients with RA. Therefore residual disease characteristics in the present study may have resulted in a decreased likelihood for participants to improve (2, 6).
The experimental intervention was based upon an early disease detection model coupled with tight control (13). This was centred on early detection of signs of new inflammation in the form of synovitis in those already diagnosed with JIA, in order to develop and implement a personalised multidisciplinary care plan. Aggressive systemic pharmacological management involving escalating dosages and/or advancement to anti-tumor necrosis factor or T-cell modulating therapies did not appear to improve levels of foot impairments levels in the intervention arm participants. This was surprising as short- and long-term benefits of such therapies are well documented (38). However, actual medical care delivered in both groups did not appear to differ significantly over 12 months. This suggests that the lack of any significant difference in primary outcome change scores may have been a result of many patients having received second-line systemic pharmacological therapy before and during the trial.
Evidence from systematic reviews suggests that customised foot orthoses are sustainably effective in reducing painful foot symptoms and disability in patients with RA (39). The positive effects of foot orthoses for reducing pain and image-guided ICIs for reducing synovial hypertrophy in JIA were not demonstrated at follow-up in this study (14,17). The longer-term benefits from integrated multi-disciplinary care may not have been detectable within a 12 month period. Previous studies in RA have demonstrated significant and sustainable reductions in foot related disability levels at follow up after 30 months of orthotic therapy (40). Indeed, Powell et al.’s (17) findings were from a study of JIA patients with disease durations of 2 years or less, with final follow up at 3 months from baseline. Furthermore, ICIs are considered to be effective in the short-term but long-term beneficial effects have not been established (14). Participants enrolled in this study typically had established disease (> 3 years disease duration) and therefore did not receive interventions early.
The use of MSUS to inform systemic therapy was a contemporary feature of this trial. Several researchers recommend image guidance for administering ICIs particularly for joints of the rearfoot as a result of several positive responses in recent studies (14–16). An important issue arising from the use of MSUS to inform care in this trial was that US findings appeared to influence clinical decision making as more ICIs were administered in the intervention group. Similar findings in adult rheumatology clinical practice have been reported (41). Further investigation is required to determine the prognostic value of US informed therapeutic decision making.
Several limitations of this study merit further attention. The desired number of study participants outlined a priori was not achieved therefore the analysis was statistically underpowered (34). Therefore the analysis may have been vulnerable to type II error. Recruitment and retention of participants was challenging as many parents of potential participants were reluctant to commit to additional appointments. This resulted in the exclusion of a significant proportion of eligible patients (n = 41, 32% of patients assessed for eligibility). Furthermore, recruitment resulted in a heterogeneous sample. This led to difficulties with standardisation of treatments, a well-recognised problem associated with complex interventions (42). An additional problem experienced during the trial was the prevalence of missing and/or incomplete data, particularly for secondary outcome measures data. Missing data is a common and ever-present problem in randomized controlled trials and several methods have been proposed and implemented to combat this problem (43). The method adopted to address missing data in this trial was the last-observation-carried-forward (LOCF) method, where missing values are imputed by the participant’s last available observations. LOCF is based on the assumption that outcome remains constant at the last observed value after drop-out/loss to follow-up. This is considered to be an unlikely outcome for many clinical trials (44). However, a sensitivity analysis was conducted on the original trial data sets plus several new data sets that were developed according to the use of various missing-data-imputation methods (LOCF, mean value imputation, maximum value imputation, minimum value imputation, and random value imputation). The aim of this analysis was to explore the magnitudes of differences in change scores between groups to determine which would be the most conservative method. LOCF was found to be the most conservative method while being less labour intensive, thus it was subsequently used to impute all missing data at final follow-up.
The trial was vulnerable to bias where participants and care providers were not blinded to the outcome of allocation. The nature of the study dictated that those in the intervention arm would be removed from their regular programs of outpatient care. Therefore elimination of patient and/or care provider’s expectations and/or preferences regarding treatments and outcomes was not possible and so the trial was vulnerable to response bias. Allocation concealment and blinding is acknowledged as being more difficult to achieve in trials of non-pharmacological and complex interventions and often relies upon complex methods (42). This trial may also have been vulnerable to intervention choice bias where the time of intervention delivery, and the complexity of the intervention including many facets, may have led to type II error regarding the clinical effectiveness of the experimental intervention. While the process of minimisation employed would have reduced the vulnerability to selection biases, the exploratory nature of the trial restricted the potential for allocation concealment and blinding amongst trial personnel. Therefore ascertainment bias could have been a potential confounding factor as personnel delivering interventions and recording assessments were aware of participants’ allocations.
The results of this study suggest that sensitivity and responsiveness of the primary outcome measure for detecting change in foot impairments and disability outcomes may be limited. For example 12/44 participants scored 0 for impairment at baseline but only 2/12 had no positive responses. This finding suggests that foot related disability may be underestimated by the JAFI due to a significant floor effect. Similar problems have been observed in RA where the Foot Impact Scale (FIS), a well validated questionnaire with good psychometric qualities, apparently lacks responsiveness to conventional podiatry care (35).
The use of the SI, a tool specifically developed for use in adults with RA has not been validated for use in children with JIA. Since the completion of this trial an alternative measure of foot deformity/posture known as the foot posture index (FPI) has been shown to be highly reliable for use in children (45–46). As yet the FPI has not been used to measure foot posture in children with JIA, but it may be a promising tool for evaluation in future studies. At present there is no normative paediatric data available for the SI, however it has shown good face validity in previous studies in JIA (2). In addition, it may be more suitable for measuring children with JIA compared to the FPI as it also includes a scale for forefoot deformity.
Integrated multi-disciplinary foot care with the use of MSUS is a logical intervention designed to address disease-related foot problems in JIA. The results of this study suggest that this model of care is no more effective than current usual care offered through the National Health Service, which for the most part comprises medical management without image-guidance or podiatric therapies. These results should be carefully considered in the context of the study limitations, the exploratory nature of the trial, and the heterogeneity of the study sample participants. Moreover, this was a single-centre study and results may not be generalisable. This trial successfully identified several useful areas for the development of future trials by highlighting sources of bias, procedural problems, and limitations of the primary outcome measure. Overall the experimental intervention was safe with few reports of intolerance to treatment or adverse events.
ACKNOWLEDGMENTS
We would like to thank all the patients and their parents who participated in this study. Special thanks are also extended to Michael Browne, Dr Joyce Davidson, Dr Paul Galea, Dr Jo Walsh and Dr Mary Cruikshank for assisting with recruitment of study participants, and to all the staff at the Glasgow Clinical Research Facility for accommodating our research. This work, and co-authors GJH and DET were supported through funding from Arthritis Research UK (Ref GJH; 18076, DET; 17832). Customised foot orthoses for patients in the intervention arm of the study were manufactured by and purchased from Firefly Prescription Foot Orthoses, Sligo, Ireland. The authors have no other conflicts of interest to declare.
REFERENCES
