Manon Fens, MSc1,2, Tom P. Vluggen, MSc2,3, Jolanda C. van Haastregt, PhD2,3, Jeanine A. Verbunt, MD, PhD2,4, George H. Beusmans, MD, PhD2,5 and Caroline M. van Heugten, PhD6,7
From the 1Department of Patient & Care, Maastricht University Medical Centre, 2CAPHRI School for Public Health and Primary Care, 3Department of Health Services Research, 4Department of Rehabilitation, Maastricht University, 5Department of General Practice, 6Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University Medical Centre and 7Department of Neuropsychology and Psychopharmacology, Faculty of Psychology and Neurosciences, Maastricht University, Maastricht, The Netherlands
OBJECTIVE: A systematic review of randomized controlled trials was performed to evaluate the effectiveness of multidisciplinary care for stroke patients living in the community.
DATA SOURCES: Databases PubMed, EMBASE, CINAHL and the Cochrane Library from January 1980 until July 2012.
STUDY SELECTION: Randomized controlled trials focused on multidisciplinary interventions for stroke patients living at home after hospitalization or inpatient rehabilitation were selected. The outcome domains were activities of daily living, social participation and quality of life. A total of 14 studies were included.
DATA EXTRACTION: Two authors independently extracted the data and independently assessed the quality of reporting of the included studies using the Consolidated Standards of Reporting Trials (CONSORT) statement 2010.
DATA SYNTHESIS: None of the studies showed favourable effects of the intervention on activities of daily living and none assessed social participation. Furthermore, two studies reported favourable effects of the intervention in terms of quality of life. These concerned an intervention combining assessment with follow-up care and a rehabilitation intervention.
CONCLUSION: There is little evidence for the effectiveness of multidisciplinary care for stroke patients being discharged home. Additional research should provide more insight into potentially effective multidisciplinary care for community-living stroke patients.
Key words: review; stroke; ambulatory care; long-term care; quality of life; randomized controlled trial.
J Rehabil Med 2013; 45: 321–330
Correspondence address: Caroline van Heugten, Maastricht University, Department of Psychiatry and Neuropsychology, PO Box 616, 6200 MD Maastricht, The Netherlands. E-mail: c.vanheugten@maastrichtuniversity.nl
Accepted Nov 21, 2012; Epub ahead of print Mar 15, 2013

*This paper was presented as a poster at the annual School for Public Health and Primary Care research day, 19 May 2011, Maastricht, The Netherlands.
Introduction
Stroke is one of the major causes of mortality, loss of independence, and decreased quality of life (1, 2). Care for stroke patients is concentrated largely in the acute and clinical phase, probably because most recovery occurs within this first period (3). However, there is a considerable group of patients with persistent disabilities, even many years after stroke (4–6). These disabilities can be physical limitations, such as paralysis or fatigue (7–9), but also psychological and cognitive problems, such as depression and memory deficits (10–12). Many stroke survivors return to their former living environment, where they can be confronted with various difficulties in managing their daily activities and resuming their former social roles (13, 14). Patients have to learn how to deal with these difficulties for the rest of their lives and learn how to reintegrate socially in the community. Although there seems to be a clear need for long-term care after being discharged home, adequate care is often lacking in this period (15).
Previous research has indicated that organized inpatient care (stroke unit) is the healthcare model of choice within a hospital (16). However, nowadays there is still a lack of insight into how other components of stroke care should be provided (17). In particular, it is unclear how care should be organized after discharge from hospital or inpatient rehabilitation (18–22). In the last 10 years there have been several reviews of the effects of stroke care after discharge home, but these are dated (18, 21), included cross-sectional studies and (non)-randomized trials (20), focused on a single discipline (22), or focused on more than 1 year post-stroke (19). A recent review by Hillier & Inglis-Jassiem (23) examined the effectiveness of stroke rehabilitation delivered at home or in an outpatient clinic for community-dwelling patients. This review showed that outpatient rehabilitation is more effective when it is provided in the patient’s home. This study, however, concerned a specific comparison (i.e. home-based vs clinic-based care), and therefore there is still a need for additional insight into the effectiveness of other care programmes for stroke patients after discharge.
The present review aims to assess the effectiveness of different forms of multidisciplinary care delivered to stroke patients living in the community after discharge from hospital or inpatient rehabilitation. We reviewed the effectiveness of the interventions in terms of activities of daily living, social participation and quality of life, which we consider to be highly relevant outcome measures for stroke patients living in the community after discharge home.
Methods
A systematic literature review was performed using the following databases: PubMed, EMBASE, CINAHL and the Cochrane Library from January 1980 until July 2012. The search strategy, developed to identify the appropriate studies, comprised 4 categories: diagnosis; type of intervention; outcome; and setting (see Appendix I). The following inclusion criteria were used for the identified studies: randomized controlled trial; patients with a diagnosis of stroke; 18 years or older; community living after hospitalization or inpatient rehabilitation; multidisciplinary intervention; and outcome measures in the domains of activities of daily living, social participation and/or quality of life. We considered care to be multidisciplinary when it was provided by two or more different care professionals working together as, or supported by, a team. Studies were excluded if the language was not English, Dutch or German. Furthermore, studies were excluded if the primary aim of the intervention was to reduce the length of stay in hospital (i.e. early supported discharge).
Studies were independently selected by two reviewers (MF and TV) based on title and abstract, and the selected articles were subsequently reviewed based on full text. Additional articles were tracked by hand search from the references of selected articles. In case of disagreement during the selection process, a third author (CvH) made the final decision. After the final selection, the two reviewers (MF and TV) extracted data independently and assessed the quality of reporting of the studies, using the CONSORT statement 2010 (24). The quality of the studies was indicated by the percentage of items of the CONSORT statement reported in the articles. Given the considerable heterogeneity of the interventions we decided not to statistically pool the data of the studies.
Results
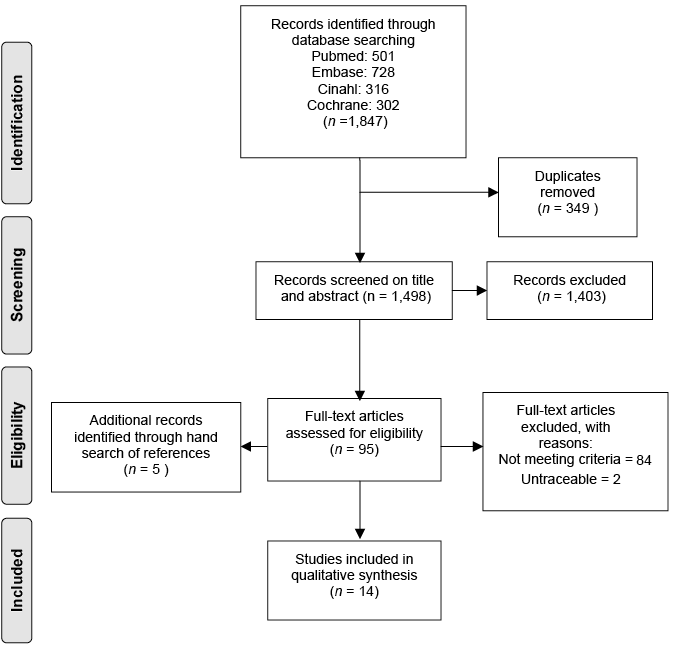
Fig. 1 shows the results of the selection process. Out of 1,498 articles that were screened based on title and abstract, the two reviewers agreed on 1,425 articles, and 73 articles were presented to the third reviewer. A total of 95 articles and 5 additional articles found by hand search of references were read in full. The two reviewers reached consensus on 89 articles, 9 articles were presented to the third reviewer and two were untraceable. Fourteen articles were selected for the review (25–38) and 84 articles did not meet the inclusion criteria; no randomized clinical trial (n = 54), no stroke patients or community living patients (n = 12), no multidisciplinary intervention (n = 14), other outcome domains (n = 3) and no English, Dutch or German (n = 1). The selected 14 articles were published in English. Table I presents the characteristics of the included studies and Table II presents the characteristic of the interventions assessed in these studies.
Fig. 1. Selection process of the systematic review. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi: 10.1371/journal.pmed1000097. Fore more information, visit www.prisma-statement.org.
Study design
Table I shows that 13 studies compared an intervention with usual care and 1 study compared intensive with non-intensive home-based rehabilitation (37). The content of the interventions will be discussed in more detail below. The definition of usual care differed considerably between studies, such as outpatient rehabilitation at a day clinic, inpatient case management, care from a general practitioner, home care services with non-professional support or a service information pack. In 12 studies patients were included immediately after discharge home from hospital, in 1 trial patients were included ≥ 18 months post-stroke (33) and in another trial patients were included after discharge from a rehabilitation centre (35). The period between stroke occurrence and discharge was described by only 3 out of 14 studies (29, 35, 37), varying from a mean of 45 days (37) to 2.5 years (33).
|
Table I. Study characteristics of studies included in the systematic review |
|||||||
|
Study and country |
Sample size (E/C) |
Mean age and Male (n/%) |
Type and severity of stroke |
Intervention |
Follow-up outcome |
Outcome measures of interest and effects Score, SD or IQRa |
Effect for experimental groupb |
|
Allen et al., 2002 (25) USA |
E:47 C:46 |
70.5 years 43 (46%) |
Ischaemic stroke and TIA, Rankin scale ≤ 3 |
Post-discharge care management vs regular care physician |
Post-intervention |
BI: E:95 (SD –), C:95 (SD –) (NS) SA-SIP30: E: 0.8 (SD –), C:0.71 (SD –) (S) |
0 + |
|
Allen et al., 2009 (26) USA |
E:190 C:190 |
68.5 years 190 (50%) |
Ischaemic stroke, NIHSS ≥ 1 |
Post-discharge care management vs regular care physician |
Post-intervention |
SSQoL: E:196 (SD –), C:199 (SD –) (NS) |
0 |
|
Bjorkdahl et al., 2006 (27) Sweden |
E:30 C:29 |
53 years median 44 (75%) |
Ischaemic stroke or haemorrhage, Not described |
Individual tailored-training by PT and OT |
Post- intervention, 2 and 11 months after intervention |
Post-intervention: AMPS motor: E:1.71 (SD 0.91), C:1.52 (SD 0.71) (NS) AMPS process: E:1.26 (SD 0.75), C:1.37 (SD 0.53) (NS) FIM motor E: 2.83 (SD 2.05), C: 2.38 (SD 1.7) (NS) FIM social E: 2.62 (SD 1.85), C: 2.94 (SD 1.57) (NS) IAM E: 0.29 (SD 1.35), C: 0.08 (SD 0.99) (NS) 2 months: AMPS motor E: 2.02 (SD 1.08), C: 1.88 (SD 0.78) (NS) AMPS process E: 1.23 (SD 0.64), C: 1.54 (SD 0.53) (NS) FIM motor E: 3.22 (SD 2.12), C: 2.86 (SD 1.9) (NS) FIM social E: 2.65 (SD 1.7), C: 3.04 (SD 1.48) (NS) IAM E: 0.54 (SD 1.47), C: 0.59 (SD 1.2) (NS) 11 months: AMPS motor E: 2.18 (SD 1.04), C: 2.28 (SD 0.94) (NS) AMPS process E: 1.55 (SD 0.76), C: 1.59 (SD 0.68) (NS) FIM motor E: 3.14 (SD 2.07), C: 2.99 (SD 1.76) (NS), FIM social E: 2.68 (SD 1.67), C: 3.29 (SD 1.5) (NS) IAM E: 0.7 (SD 1.63), C: 1.05 (SD 1.76) (NS) |
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 |
|
Burton et al., 2005 (28) UK |
E:87 C:89 |
75.2 years 92 (52%) |
Not described |
Nurse follow-up vs usual care |
1 and 10 months post-intervention |
1 months : BI: E:15 (IQ 11), C:14 (IQ 8.5) (NS) FAI: E:7 (IQ 14), C:7 (IQ 15) (NS) 10 months : BI: E:17 (IQ 10), C:13.5 (IQ 7.25) (NS) FAI: E:14 (IQ 16.3), C:12 (IQ 19.5) (NS) |
0 0 0 0 |
|
Forster et al., 2009 (29) UK |
E:132 C:133 |
77/79 years median 121 (46%) |
Ischaemic stroke or haemorrhage, Not described |
Structured assessment system vs existing care |
6–7 months post-intervention |
FAI: E: 6 (IQR 2–18), C: 4 (IQR 1–14) (NS) BI: E: 16 (IQR 12–18), C: 16 (IQR 11–18) (NS) |
0 0 |
|
Gladman et al., 1993 (30) UK |
E:162 C:165 |
70 years 173 (53%) |
Not described |
Domiciliary service vs usual practice |
Post-intervention |
BI: E: 17 (IQR 14–19), C: 18 (IQR 15–20) (NS) Extended ADL: E: 8.5 (IQR 4–14), C: 8 (IQR 4–14) (NS) |
0 0 |
|
Gladman et al., 1994 (31) UK |
Not described |
Post-intervention and 6 months post-intervention |
6 months: BI: E: 17 (SD –), C: 18 (SD –) (NS) Extended ADL: E: 8 (SD –), C: 10 (SD –) (NS) |
0 0 |
|||
|
Lincoln et al., 2004 (32) UK |
E:189 C:232 |
72 years 222 (53%) |
Not described |
Community-stroke team vs routine care |
Post-intervention |
BI: E: 16 (IQR 12–18), C: 16 (IQR 12–19) (NS) Extended ADL: E: 24 (IQR 13–38), C: 25.5 (IQR 11–39) (NS) EQ-5D: E: 52 (IQR 41–78), C: 55 (IQR 40–72) (NS) |
0 0 0 |
|
Markle- Reid et al., 2011 (33) Canada |
E:43 C:39 |
73 years 45 (37%) |
Not described |
Specialized interprofessional team vs usual home care services |
Post-intervention |
SF-36 physical functioning: E: 28.84 (SD 30.68), C: 28.85 (SD 28.48) (NS) SF-36 social functioning: E: 66.57 (SD 34.69), C: 59.29 (SD 30.71) (NS) |
0 0 |

|
Table I. Contd. |
|||||||
|
Study and country |
Sample size (E/C) |
Mean age and Male (n/%) |
Type and severity of stroke |
Intervention |
Follow-up outcome |
Outcome measures of interest and effects Score, SD or IQRa |
Effect for experimental groupb |
|
Mayo et al., 2008 (34) Canada |
E:96 C:94 |
71 years 116 (61%) |
Not described, CNS < 6 |
Case-management vs usual care |
Post-intervention and 4.5 months after intervention |
Post-intervention: PCS: E: 40 (SD 1.3), C: 38.4 (SD 1.4) (NS) MCS: E: 6.4 (SD 1.4), C: 45.6 (SD 1.4) (NS) EQ-5D: E: 0.63 (SD 0.02), C: 0.62 (SD 0.02) (NS) BI: E: 91.4 (SD 2.1), C: 90.4 (SD 1.7) (NS) 4,5 months: PCS: E: 43.4 (SD 1.4), C: 40.1 (SD 1.5) (NS) MCS: E: 50.6 (SD 1.3), C: 48.2 (SD 1.5) (NS) EQ-5D: E: 0.69 (SD 0.02), C: 0.64 (SD 0.03) (NS) BI: E: 92.7 (SD 2.0), C: 89.9 (SD 2.2) (NS) |
0 0 0 0 0 0 0 0 |
|
Mulders et al., 1989 (35) The Netherlands |
E:38 C:18 |
56.8 years 30 (53.6%) |
Not described |
Rehabilitation programme vs usual care |
6 months post-intervention |
SIP: E: 22.7 (SD –), C: 17.5 (SD –) (NS) |
0 |
|
Roderick et al., 2001 (36) UK |
E:66 C:74 |
79 years 65 (46%) |
Not described, BI < 10 |
Domiciliary stroke team vs multi-disciplinary team |
6 months post-discharge |
BI: E: 17 (IQR 10.8–19), C: 15.5 (IQR 9–18) (NS) FAI: E: 12 (IQR 3–25.3), C: 7.5 (IQR 3–16.5) (NS) SF-36 physical functioning, E: 35.2 (IQR 26.5–43.7), C: 32.7 (IQR 26.8–39.2) (NS) SF-36 mental functioning: E: 57.4 (IQR 49.9–62.9), C: 57.1 (IQR 50.6–63) (NS) |
0 0 0 0 |
|
Ryan et al., 2006 (37) UK |
E:45 C:44 |
76.8 years Not described |
Not described, BI |
Intensive vs non-intensive home-based rehabilitation |
Post-intervention |
BI: E: 19 (IQR 17–20), C: 18,5 (IQR 17–20) (NS) EQ-5D: E: 0.71 (IQR 0.59–0.81, C: 0.54 (IQR 0.26–0.73) (S) FAI: E: 14 (IQR 6–26), C: 18 (IQR 6–24) (NS) |
0 + 0 |
|
Ytterberg et al., 2000 (38) Sweden |
E:56 C:55 |
73.5 years 57 (51%) |
Not described |
Follow-up-visits vs usual care |
2 months post-intervention |
Katz ADL E: 100% (SD –), C: 98% (SD –) (NS) |
0 |
|
aThe outcome measure is presented, followed by the mean score of the experimental group(s), the mean score of the control group and the difference between groups in terms of (non)significant. b0: no differences between groups; +: positive effect for experimental group; –: negative effect for experimental group. E: experimental group; C: control group; n: number of patients; IQR: interquartile range; TIA: transient ischaemic attack; BI: Barthel Index; SA-SIP30: Stroke Adapted-Sickness Impact Profile 30; NIHSS: National Institutes of Health Stroke Scale; SSQoL: Stroke Specific Quality of Life Scale; PT: physiotherapist; OT: occupational therapist; AMPS: Assessment of Motor and Process Skills; FIM: Functional Independence Measure; IAM: Instrumental Activity Measure; FAI: Frenchay Activities Index; extended ADL: extended Activities of Daily Living; EQ-5D: EuroQol-5D; SF-36: Short-Form 36; CNS: Canadian Neurological Scale; PCS: Physical Component Summary; MCS: Mental Component Summary; SIP: Sickness Impact Profile; Katz ADL index: Katz Activities of Daily Living index; NS: not significant; S: significant; SD: standard deviation. |
|||||||
Patient characteristics
The number of stroke patients in the intervention groups varied from 30 (27) to 190 (26). The mean age of patients was under 70 years in 3 studies (26, 27, 35) and over 70 years in 11 studies. In general, men and women were equally represented in each of the studies; however, in 1 study there were considerably more men (75%) in the study group (27).
Description of intervention
Table II shows that the 14 interventions differed in terms of organization, disciplines involved, duration and intensity. Four main types of intervention could be identified: assessment (n = 2); assessment combined with follow-up care (n = 8); rehabilitation (n = 3); and education (n = 1).
|
Table II. Intervention characteristics of studies included in the systematic review |
|||||
|
Study and country |
Aim of intervention |
Intervention |
Disciplines involved |
Start intervention and duration |
Control |
|
Allen et al., 2002 (25) USA Allen et al., 2009 (26) USA |
Not described |
Post-discharge care management in which nurses perform home assessment, consult with interdisciplinary team and follow-up visits if necessary |
Nurse, internist, physiotherapist and geriatric |
After discharge 3 months After discharge 6 months |
Usual care from physician |
|
Bjorkdahl et al., 2006 (27) Sweden |
To give support, info and training at home to transfer skills achieved in hospital into the home environment |
Individually tailored training based on patient’s needs in home setting |
Physiotherapist and occupational therapist |
After discharge 9 h/week for 3 weeks |
Ordinary outpatient rehabilitation – a multiprofessional team offered training of deficits and functioning at a day clinic |
|
Burton et al., 2005 (28) UK |
To promote coping and adaptation to the consequences of stroke |
Usual follow-up care (liaison with general practitioner, outpatient follow-up and access to multi-professional rehabilitation services) + inpatient nurse assessment of recovery and follow-up visits of nurse at home |
Nurse cooperating with physiotherapist, occupational therapist and community psychiatric nurse |
After discharge 3 times in 2 months |
Usual follow care + standard care – inpatient case management by stroke nurse |
|
Forster et al., 2009 (29) UK |
Not described |
Existing care supplemented with structured assessment at 5–6 months post stroke onset by a nurse and multidisciplinary team or only nurse |
Nurse and multidisciplinary team (team members are not described) |
After discharge At 5–6 months |
Existing care arrangement and a service information pack |
|
Gladman et al., 1993 (30) UK Gladman et al., 1994 (31) UK |
Domiciliary service improves functional independence |
Domiciliary service with assessment and adequate help |
Physiotherapist and occupational therapist |
After discharge 6 months post-discharge |
Usual practice – day hospital or outpatient physiotherapy or occupational therapy |
|
Lincoln et al., 2004 (32) UK |
Rehabilitation by specialist multi-professional team improves functional abilities, mood, QoL and satisfaction with care |
Community-stroke team with assessment, discussion and therapy |
Occupational therapist, physiotherapist, speech therapist and nurse |
Not described One visit and then as long as needed |
Routine care – day hospitals, outpatients departments and social services occupational therapy |
|
Markle-Reid et al., 2011 (33) Canada |
To improve health related quality of life, physical functioning, perceived social support, depressive and anxiety symptoms, number of strokes, cognitive function and the level of community reintegration |
Interprofessional rehabilitation programme of 12 months with home visits and an individualized care plan |
Care coordinator, nurse, physiotherapist, occupational therapist, speech language pathologist, dietician, social worker and personal support worker |
≤ 18 months post-stroke 12 months |
Usual home care services – routine follow-up by care coordinator in collaboration with multidisciplinary team and non-professional support services |
|
Mayo et al., 2008 (34) Canada |
To improve the health-related quality of life and decrease ER visits and non elective hospitalizations |
Case-management by nurses home visits and interventions |
Nurse (and personal physician) |
After discharge 6 weeks |
Usual care – appointment with their physician or local community health centre |

|
Table II. Contd. |
|||||
|
Study and country |
Aim of intervention |
Intervention |
Disciplines involved |
Start intervention and duration |
Control |
|
Mulders et al., 1989 (35) Netherlands |
To positively influence active recreation and pastime and stimulate social contacts after clinical rehabilitation |
Rehabilitation programme of exercises, discussion and information-education |
Physiotherapist and occupational therapist |
Not described 22 meetings of 2.5 h in 1 year |
Usual care |
|
Roderick et al., 2001 (36) UK |
Not described |
Domiciliary stroke team planning activities using goal-setting approach |
Physiotherapist, occupational therapist and geriatrician |
Not described Until maximum recovery |
Usual day hospital rehabilitation - individual of group care by a multi-disciplinary team |
|
Ryan et al., 2006 (37) UK |
Not described |
Intensive home-based rehabilitation of 6 or more contacts per week with a local multidisciplinary team |
Physiotherapist, occupational therapist, speech therapist & therapy assistant |
After discharge Maximum 12 weeks |
Non-intensive home-based rehabilitation of 3 or less contacts per week with a local multidisciplinary team |
|
Ytterberg et al., 2000 (38) Sweden |
Preventing a negative course of events by means of follow-up visits |
One time all-day follow-up visit after discharge |
Counsellor, physiotherapist, occupational therapist and nurse, doctor |
After discharge 1 month |
Usual care from general practitioner |
The first type of intervention (assessment) consisted of a single visit at home or at a clinic, which aimed to prevent a negative course of events (29, 38). The assessments were performed by a multidisciplinary team (38), consisting of a physiotherapist, occupational therapist, counsellor and doctor, or a nurse with a consultant multidisciplinary team or with links to social services (29). The assessments were performed at 1 month (38) or 5–6 months after discharge home (29).
The second type of intervention (assessment combined with follow-up care) could be subdivided into assessment with either subsequent follow-up visits (n = 5) (25, 26, 28, 33, 34) or assessment with subsequent rehabilitation (n = 3) (30–32). The 5 assessments with follow-up visits were aimed at coping with the consequences of stroke (28) and improving the quality of life (33, 34). Nurses performed the assessment and follow-up visits and consulted with the patient’s physician (34) or a multidisciplinary team (25, 26, 28, 33). There was considerable variation in the duration of assessment and follow-up visits, varying from 6 weeks (34) to 12 months (26, 33) The 3 assessments performed with subsequent rehabilitation were focused on improving functional abilities of stroke patients (30–32). The interventions were provided by a physiotherapist and an occupational therapist (30, 31), who could work together with a speech therapist and a nurse (32). They provided therapy for a period of 6 months (30, 31) or as long as needed (32).
The third type of intervention (rehabilitation) aimed to improve functional outcome and skills and involved disciplines such as physiotherapists, occupational therapists, physicians and speech therapists (27, 35, 36). The duration of the programme varied between 3 weeks (27) to as long as needed (35), as well as varying in intensity. All interventions were performed at the patient’s home.
The fourth type of intervention (education) aimed to stimulate social contacts and active recreation (34). Patients participated in group discussions about current events and in outdoor activities, such as dining and going to the theatre. The intervention was performed by physiotherapists and occupational therapists, providing education and information 22 times in 1 year.
Ten interventions started directly after discharge home from hospital and 1 intervention started within 18 months post-stroke (33). For the other 3 interventions it was unclear when the interventions started (32, 35, 36).
Outcome measures and effects
Eleven studies assessed activities of daily living using the Barthel Index (n = 9), Frenchay Activities Index (n = 4), extended Activities of Daily Activities (n = 3), Functional Independence Measure (n = 1), Instrumental Activity Measure (n = 1), Assessment of Motor and Process Skills (n = 1), Mental Component Summary/Physical Component Summary (n = 1), and Katz Index (n = 1) (25, 27–32, 34, 36–38). None of these studies found an effect of the intervention on daily activities. Social participation was assessed in none of the studies. Eight studies assessed quality of life, using the Euroqol-5D (n = 3), Stroke Adapted-Sickness Impact Profile 30 (n = 1), Short-Form 36 (n = 2), Stroke Specific Quality of Life Scale (n = 1) and/or Sickness Impact Profile (n = 1) (25, 26, 32–37). Out of these 8 studies, the studies of Allen et al. (25) and Ryan et al. (37) reported favourable effects of the intervention on quality of life.
In the study of Allen et al. (25) (assessment with follow-up care), an advanced practice nurse care manager performed a telephone assessment 3–7 days after discharge home and provided some education. A month later the advanced nurse visited patients at home for a standardized biopsychosocial assessment for stroke-specific problems. The findings of this assessment were discussed by the post-stroke consultation team to develop an individual care plan. Three months after discharge home, patients receiving the intervention reported an increased quality of life, using a stroke adapted outcome measure (SA-SIP30). In the study of Ryan et al. (37), (rehabilitation) a multidisciplinary team provided 6 or more face-to-face contacts a week. During these contacts, patients received therapy for a maximum period of 12 weeks, which was compared with a control group receiving 3 or less face-to-face contacts a week. None of the patients needed 12 weeks of therapy. The patients receiving 6 or more face-to-face contacts a week reported a better quality of life than patients who received 3 or less face-to-face contacts a week.
Quality of reporting of the study
The percentage of the CONSORT items reported in the included studies ranged from 35% to 73%, with a mean of 55% (Table III). The study of Markle-Reid et al. (33) had the highest quality (73%), while the study of Ytterberg (38) had the lowest quality (35%). The CONSORT statement can be divided into 7 categories: “title/abstract”; “introduction”; “methods-trial”; “methods-randomization”; “results”; “discussion”; and “other information”. In 4 of these categories (“methods-trial”, “methods-randomization”, “results” and “other information”) ≤ 50% of the items was reported on average.
|
Table III. Quality assessment of included studies using the CONSORT statement 2010 |
|||||||||||||||
|
Allen et al., 2002 (25) |
Allen et al., 2009 (26) |
Bjorkdahl et al., 2006 (27) |
Burton et al., 2005 (28) |
Forster et al., 2009 (29) |
Gladman et al., 1993 (30) |
Gladman et al., 1994 (31) |
Lincoln et al., 2004 (32) |
Markle-Reid et al., 2011 (33) |
Mayo et al., 2008 (34) |
Mulders et al., 1989 (35) |
Roderick et al., 2001 (36) |
Ryan et al., 2006 (37) |
Ytterberg et al., 2000 (38) |
Total category (%) |
|
|
Title, abstract |
|||||||||||||||
|
RCT in title |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
No |
No |
No |
Yes |
Yes |
No |
|
|
Structured summary |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
|
|
(maximum 2) |
2 |
2 |
2 |
1 |
2 |
2 |
2 |
2 |
1 |
1 |
1 |
2 |
2 |
0 |
22 (79%) |
|
Introduction |
|||||||||||||||
|
Background scientific |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Hypothesis |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
(maximum 2) |
1 |
1 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
26 (93%) |
|
Methods-trial |
|||||||||||||||
|
Description trial design |
No |
No |
No |
Yes |
No |
No |
Yes |
No |
Yes |
Yes |
Yes |
No |
No |
No |
|
|
Changes trial design |
No |
No |
No |
No |
No |
No |
No |
Yes |
No |
No |
No |
No |
No |
No |
|
|
Eligibility criteria |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Setting and location |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
No |
No |
No |
|
|
Description intervention |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
|
|
Description outcomes |
No |
No |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Changes outcome |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
Sample size |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
No |
Yes |
No |
No |
Yes |
Yes |
No |
|
|
Interim analysis/stopping guidelines |
No |
No |
No |
Yes |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
(maximum 9) |
4 |
4 |
5 |
6 |
4 |
5 |
5 |
5 |
6 |
5 |
3 |
4 |
4 |
2 |
62 (49%) |
|
Method-randomization |
|||||||||||||||
|
Method randomization |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
No |
|
|
Type of randomization |
Yes |
Yes |
No |
No |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
|
|
Allocation concealment |
Yes |
Yes |
No |
No |
No |
Yes |
No |
No |
Yes |
Yes |
No |
No |
Yes |
No |
|
|
Implementation |
No |
No |
No |
No |
No |
No |
No |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
No |
|
|
Blinding |
No |
Yes |
Yes |
Yes |
No |
No |
No |
Yes |
Yes |
Yes |
No |
No |
Yes |
No |
|
|
Similarity intervention |
No |
No |
Yes |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
Statistics group measures |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Additional statistics |
Yes |
No |
Yes |
No |
Yes |
No |
No |
No |
Yes |
Yes |
No |
No |
No |
No |
|
|
(maximum 8) |
5 |
5 |
5 |
3 |
2 |
3 |
2 |
4 |
7 |
6 |
3 |
4 |
6 |
1 |
56 (50%) |
|
Results |
|||||||||||||||
|
Participation flow |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Losses and exclusion |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Period recruitment and follow-up |
No |
Yes |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
Reason ending trial |
Yes |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
Baseline data |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Numbers analysed |
No |
Yes |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Outcomes |
Yes |
Yes |
No |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
|
|
Binary outcomes |
NA |
No |
NA |
NA |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
Ancillary analysis |
Yes |
Yes |
Yes |
No |
Yes |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
Harms |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
No |
|
|
(maximum 10) |
4 |
7 |
4 |
4 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
4 |
68 (49%) |

|
Table III. Contd. |
|||||||||||||||
|
Allen et al., 2002 (25) |
Allen et al., 2009 (26) |
Bjorkdahl et al., 2006 (27) |
Burton et al., 2005 (28) |
Forster et al., 2009 (29) |
Gladman et al., 1993 (30) |
Gladman et al., 1994 (31) |
Lincoln et al., 2004 (32) |
Markle-Reid et al., 2011 (33) |
Mayo et al., 2008 (34) |
Mulders et al., 1989 (35) |
Roderick et al., 2001 (36) |
Ryan et al., 2006 (37) |
Ytterberg et al., 2000 (38) |
Total category (%) |
|
|
Discussion |
|||||||||||||||
|
Limitations |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
Generalizability |
No |
No |
Yes |
No |
No |
Yes |
No |
No |
Yes |
No |
No |
Yes |
No |
No |
|
|
Interpretation |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
(maximum 3) |
1 |
2 |
3 |
1 |
2 |
3 |
2 |
2 |
3 |
2 |
2 |
3 |
2 |
2 |
30 (71%) |
|
Other information |
|||||||||||||||
|
Registration |
No |
No |
No |
No |
No |
No |
No |
No |
Yes |
No |
No |
No |
No |
No |
|
|
Protocol |
No |
No |
No |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
|
|
Funding |
No |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
(maximum 3) |
0 |
1 |
0 |
1 |
1 |
2 |
2 |
1 |
3 |
2 |
1 |
2 |
2 |
2 |
20 (48%) |
|
Total study (%) |
17 (46%) |
22 (59%) |
21 (57%) |
18 (49%) |
18 (49%) |
22 (59%) |
20 (54%) |
21 (57%) |
27 (73%) |
23 (62%) |
17 (46%) |
22 (59%) |
23 (62%) |
13 (35%) |
|
|
CONSORT: Consolidated Standards of Reporting Trials. |
|||||||||||||||
Discussion
This systematic review, evaluating the effectiveness of multidisciplinary care for stroke patients living in the community after being discharged home, showed that none of the 11 studies that assessed daily activities reported a favourable effect of the intervention on this outcome. In addition, the review showed that none of the included studies assessed the effects of the intervention on social participation. Furthermore, with regard to quality of life, our review showed that, of the 8 studies that assessed the effects of the intervention on quality of life, only two showed a favourable effect on this outcome domain. These two interventions were an assessment combined with follow-up visits (25) and a rehabilitation intervention (37). These interventions differed considerably in organization, disciplines involved, duration and intensity, which makes the comparison and identification of essential care elements of effective multidisciplinary care impossible.
Previous reviews, which assessed the effects of home-based interventions provided by multidisciplinary teams, physiotherapists or occupational therapists, showed a statistically significant favourable effect of these interventions on daily activities (18, 21). This appears to be in contrast with the findings of the present review. However, our review focused only on multidisciplinary interventions and reported only significant results. The results of the multidisciplinary studies included in previous reviews are in favour of the treatment on daily activities, but their results are non-significant (18, 21), which is in line with our findings. In addition, a previous review, which focused on the effects of therapy-based rehabilitation 1 year or more after stroke, found inconclusive evidence for the effectiveness of therapy-based interventions and reported that interventions were different in design, type of intervention and outcome, which is consistent with the findings of this review (19).
The methodological quality of the 14 studies differed considerably and ranged between 35% and 73%, indicating that substantial quality improvements can be made in future research. For example, description of trial design, implementation procedure and period of recruitment could be reported more accurately. Furthermore, the generalization of the results should be reported because it can provide valuable information for clinical use. However, we have to consider that some items (such as blinding, serious harms and interim analysis) are less applicable for studies evaluating non-pharmacological interventions, which also decrease the percentage of reported items and thus the quality. Furthermore, with regard to research in the field of stroke, we consider it very important to report the time between stroke and start of the intervention to facilitate a proper comparison of the effects of the different types of interventions and to gain insight into the phase in which these patients were at time of the intervention (rehabilitation or long-term care).
We conclude that there is only limited evidence for the effectiveness of multidisciplinary care programmes for community living stroke patients after being discharged home. There may be several explanations for the lack of effectiveness of these interventions. First, it is possible that the time between stroke and the start of the intervention was, in general, too long, which may make it more difficult to achieve significant favourable effects (39). This assumption is supported by the fact that two recent studies that evaluated interventions, that started in the acute phase and continued in the home setting (early supported discharge), showed favourable effects on functional outcome, even after 5 years (40, 41). A second explanation might be found in the design of the studies. The experimental intervention was, in almost all included studies, compared with care as usual, which is, in general, poorly described in the studies. It is therefore unclear whether the contrast between the experimental care and care as usual was big enough to raise a substantial effect. A third explanation might be found in the fact that, for most interventions, it was not described whether the intervention was based on a specific theoretical framework and/or evidence of previous research. Furthermore, most studies did not present a clear description of the intensity and contents of the programme. It is therefore possible that the quality of the interventions was simply too low, because the interventions were insufficiently based on theoretical frameworks and/or evidence from previous research.
A major strength of our review is the inclusion of 8 studies that had not been evaluated by previous reviews. A limitation of this review may be the selection of appropriate search terms for the interventions, because multidisciplinary care can be described by many different terms. Therefore, it is possible that we have missed relevant studies. Another limitation may be the fact that we focused in our review on 3 outcome measures: daily activity, quality of life and social participation. Although we have only found two effective interventions regarding quality of life, the included interventions may have had favourable effects on other outcomes, such as depression, cost reduction or care satisfaction, which we did not consider in this review.
Our systematic review showed that only 2 of the 8 multidisciplinary interventions that assessed quality of life reported favourable effects on quality of life in stroke patients discharged home after hospitalization or inpatient rehabilitation. Furthermore, none of the studies showed favourable effects on daily activity. Therefore, there is still a great need for additional high-quality studies assessing the effectiveness of different types of multidisciplinary care for stroke patients after being discharged home. It seems important that future intervention programmes are based on theoretical frameworks and/or results of previous research, in order to increase the (potential) quality of the programmes. In addition, future research into the effects of multidisciplinary care among stroke patients discharged home should also evaluate the effects on social participation, as this important outcome has not been included in previous research.
AcknowledgementS
This study was funded by Foundation Annadal, Foundation Elisabeth Strouven, health insurance company VGZ and CZ and the Netherlands Organization for Health Research and Development (ZonMw, grant number 313070301).
References
Appendix I. Search terms |
Stroke: |
- stroke |
- cerebral infarction |
- cerebrovascular accident |
- brain infarction |
- cerebrovascular diseases |
Type of intervention: |
- follow-up care/follow-up service |
- ambulatory care/ambulatory care nursing |
- outpatient service |
- aftercare |
- long term care |
- home care services/home care/home health care |
- community-based rehabilitation/community services/community care |
- home health care/community health care |
- home rehabilitation |
Outcome: |
- quality of life |
- activities of daily living/daily life activities/ADL |
- social participation |
Setting: |
- after discharge |
- living in the community/community living/community dwelling |
- patient discharge |
- hospital discharge |
- community residing/home residing |
