Jing Wang, MD1,2,3, Claire Fritzsch1,4, Johannes Bernarding, MD, PhD5, Susanne Holtze, PhD4, Karl-Heinz Mauritz, MD1,3, Maddalena Brunetti, Dipl-Psych1,4 and Christian Dohle, MD, MPhil1,4,6
From the 1MEDIAN Klinik Berlin-Kladow, Berlin, Germany, 2Centre of Rehabilitation, The Second Affiliated Hospital of Jiaxing University, Jiaxing, China, 3Department of Neurology, Charité, 4Centre for Stroke Research Berlin, Charité – University Medicine Berlin, Berlin, 5Medical Faculty, Institute for Biometry and Medical Informatics, University
Magdeburg, Magdeburg and 6Centre of Rehabilitation Science, University of Potsdam, Postdam, Germany
OBJECTIVE: To compare lateralized cerebral activations elicited during self-initiated movement mirroring and observation of movements.
SUBJECTS: A total of 15 right-handed healthy subjects, age range 22–56 years.
METHODS: Functional imaging study comparing movement mirroring with movement observation, in both hands, in an otherwise identical setting. Imaging data were analysed using statistical parametric mapping software, with significance threshold set at p < 0.01 (false discovery rate) and a minimum cluster size of 20 voxels.
RESULTS: Movement mirroring induced additional activation in primary and higher-order visual areas strictly contralateral to the limb seen by the subject. There was no significant difference of brain activity when comparing movement observation of somebody else’s right hand with left hand.
CONCLUSION: Lateralized cerebral activations are elicited by inversion of visual feedback (movement mirroring), but not by movement observation.
Key words: fMRI; mirror; movement; observation; precuneus.
J Rehabil Med 2013; 45: 410–413
Correspondence address: Christian Dohle, MEDIAN Klinik Berlin-Kladow, Kladower Damm 223, 14089 Berlin, Germany. E-mail: christian.dohle@median-kliniken.de
Accepted Nov 21, 2012; Epub ahead of print Mar 8, 2013
INTRODUCTION
For rehabilitation of deficits after stroke or pain syndromes, therapeutic strategies based on visual stimulation have been developed in recent years. These include mirror therapy (MT) and movement observation therapy (MOT, also called video therapy). In MT, a mirror is positioned such that movements of the non-affected limb appear to the patient as if they were movements of the affected limb. MT was originally shown by Ramachandran et al. (1) to relieve phantom limb pain and has since been shown to have beneficial effects in other conditions, such as hemiparesis after stroke (2, 3) and complex regional pain syndrome (4). In MOT, patients observe and then imitate the movements of another person. Positive effects of MOT on motor dysfunction have been shown in patients with stroke (5) and Parkinson’s disease (6).
There is evidence for positive effects of both of these therapies, but their neural mechanisms are poorly understood, especially with regard to the contribution of both hemispheres. Imaging studies have shown shared motor representations for both movement execution and observation, but the degree of lateralization for these processes is less clear (7). The effect of visual feedback and actual motor performance can be separated by visual inversion, i.e. movement mirroring. Using this approach, the precuneus and “lower” visual areas have been shown to be activated strictly contralateral to the perceived limb (8), demonstrating a clear lateralization during self-initiated movements. In contrast, pure observation, especially of meaningful actions, has been shown to activate the so-called mirror neurone system (MNS), which was first observed in primates and subsequently confirmed in humans (9, 10). Imaging studies in humans do not suggest a strict lateralization (11). However, the two conditions have not been directly compared.
The aim of this study was to test the hypothesis that lateralized cerebral activation occurs only during self-initiated movements, including movement mirroring, but not during movement observation. If this is the case, then it implies different working mechanisms for MT and MOT. An imaging study was thus performed in human subjects to directly compare movement mirroring and movement observation in an otherwise identical setting.
MATERIAL AND METHODS
Subjects
A total of 18 healthy right-handed subjects, as assessed by the German version of the Edinburgh Handedness Inventory (12), were investigated. Three subjects were excluded from the analysis due to excessive artefacts, leaving 15 subjects in the final analysis (6 females; age range 22–56 years; mean age 33.7 years). All participants were screened for use of eyeglasses, corrective lenses in the goggles were used if necessary. The study was approved by the ethics committee of the Charité – University Medicine Berlin. All subjects gave informed consent prior to inclusion in the study.
Experimental design
The experimental paradigm was based on that of a previous study (8). Subjects were positioned in a functional magnetic resonance imaging (fMRI) scanner, measuring brain activity while carrying out movement performance and movement observation tasks as described below. The basic task was an opposition movement sequence of the index finger and thumb, of either the right hand (RH) or left hand (LH), with the hand held above waist level. The hand was videoed (Leutron Vision, Leutron Vision AG, Glattbrugg, Switzerland) from outside the scanner and projected online on LCD goggles (VisuaStim Digital, Resonance Technology, Inc.) worn by the subjects. Subjects could not observe their hand directly, but viewed movements of the hand via the goggles. Using a software package (Leutron Vision Software, Version 1.91, Leutron Vision AG), the image of the hand could be inverted horizontally, thus creating an image of a normal (NOR) or mirrored (MIR) moving hand. For example, in condition RH, the MIR subjects moved their right hand, but this appeared to them as though the left hand was moving (Fig. 1). In addition to this replication of the previous study, a second protocol was introduced requiring observation of movement without actual movement performance. For both protocols, the length of the sequence of finger movements, as well as the corresponding rest conditions with static images, was set to 20 s (10 scans).
Using this set-up, the following protocols were performed (Table I):
• movement execution: subjects either held the relevant hand static or performed an opposition movement sequence of the index finger and thumb. In 50% of the trials, the visual feedback was inverted, producing an image of a NOR or MIR hand. Thus, there was a total of 4 conditions for each hand (NOR static, NOR moved, MIR static, MIR moved). For each hand, 7 sequences of 20 s length for each of the 4 conditions were arranged in a pseudo-randomized protocol.
• movement observation: subjects viewed 7 video-clips, each 20-s long, of opposition movement sequences of somebody else’s index finger and thumb in an identical position to that of subject’s hand (OBS). Subjects then performed the movement for 20 s, followed by a 20 s pause, during which they watched an video image of a static hand.
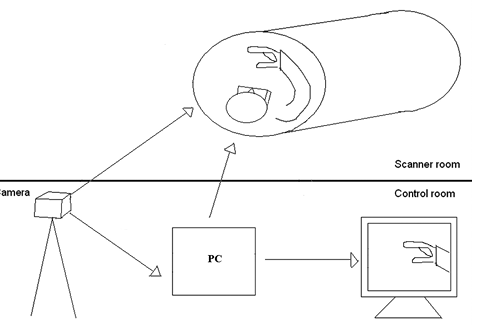
Fig. 1. Experimental design. The subject’s hand is videoed from outside the scanner. The image is processed by software on a PC and projected online on LCD goggles worn by the subject.
In all protocols, 1 scan (2-s long) was inserted between each sequence for verbal command and immediate reaction to it, which was not included into the analysis. Both protocols were performed separately for RH and LH, resulting in a total of 4 protocol blocks. The order of execution and observation protocols and the order of hands were pseudo-randomized across subjects. These protocols were followed by a motor imagery protocol and a rest condition, which were acquired for other purposes, but not included in the present analysis.
In order to test our hypothesis, only those 6 conditions with the visual image of an active hand movement were analysed, thus forming a 2 × 3 factorial design (Table I). In condition LH (RH) NOR, subjects moved their left (right) hand, which appeared as the same, i.e. as a left (right) hand. During condition LH (RH) MIR, subjects moved their left (right) hand, which appeared as a right (left) hand. During condition LH (RH) OBS, subjects watched the movements of somebody else’s left (right) hand in order to imitate them afterwards. Each condition consisted of 7 segments of 20 s, making a total of 140 s for each condition.
|
Table I. Number of opposition sequences (median (IQR)) of the finger and thumb in the different experimental conditions |
|||
|
Motor Activity |
Visual perception |
||
|
Left hand |
Right hand |
||
|
LH |
LH NOR: 27 (25–33) |
LH MIR: 27 (25–32) |
|
|
RH |
RH MIR: 28 (25–32) |
RH NOR: 28 (24–30) |
|
|
OBS |
LH OBS: 26 (25–32) |
RH OBS: 26 (25–32) |
|
|
IQR: interquartile range; LH: left hand; MIR: mirrored; NOR: normal; OBS: pure observation; RH: right hand. |
|||
Behavioural data and analyses
The opposition movement sequences of the index finger and thumb viewed or performed during each condition were videoed and counted. As Kolmogorov-Smirnov test with Lilliefors significance correction (D test) revealed a non-normal distribution, Friedman test was applied to test for significant differences between the conditions. The significance threshold was set at p < 0.05.
Scanning procedure
fMRI measurements were made with a 3 Tesla Scanner with a 12-channel head matrix coil (Magnetom Tim Trio, Siemens, Erlangen, Germany). For the functional images, a fast-gradient echo planar imaging (EPI) sequence was used (Repetition time (TR) = 2000 ms, Echo time (TE) = 25 ms, flip angle 90º, slice thickness 3 mm, 3 × 3 × 3 mm3 voxels, no gap) and each protocol block was preceded by a 20-s dummy sequence in order to achieve haemodynamic stability. For anatomic normalization, a high-resolution 3-dimensional T1-weighted gradient-echo sequence (magnetization-prepared rapid gradient echo (MP-RAGE) , TR = 2000 ms, TE = 2.52 ms, flip angle 9º, slice thickness 1 mm, 1 × 1 × 1 mm3 voxels, no gap) was acquired.
Imaging analyses
The functional images were analysed using statistical parametric mapping software (SPM-8, Wellcome Department of Cognitive Neurology, University College London, London, UK). Images were realigned to remove movement artefacts, co-registered with the corresponding anatomic (T1-weighted) images, and spatially normalized for multi-subject comparison. The normalized images were spatially smoothed with a Gaussian filter (full width at half maximum = 8 mm). T-contrasts were calculated between those conditions only differing in the visual feedback, i.e. between MIR and NOR with either hand, and between movement observation of both hands (RH OBS vs LH OBS and vice versa). p < 0.01 (false discovery rate; FDR) with a minimum cluster size of 20 voxels were considered statistically significant. The SPM anatomy toolbox was used to label the observed activations. As direct comparison between movement execution and movement observation was confounded by motor activity, an additional effect of interest analysis at both precunei was performed, based on a 3 × 2 analysis of variance (ANOVA) with the factors “motor activity” and “visual perception”, as stated in Table I.
RESULTS
Behavioural data
The numbers of finger-thumb movements under the different experimental conditions are shown in Table I. The Friedman test revealed no significant differences between the different conditions (p = 0.41).
Imaging analyses
Comparing movement execution with rest revealed activation of the bilateral motor network, predominantly in the hemisphere contralateral to the moving hand (Table SI; available from: URL: http://www.medicaljournals.se/jrm/content/?doi=10.2340/16501977-1127). Movement mirroring (i.e. MIR > NOR) induced additional activation in the primary and higher-order visual areas (including the precuneus) strictly contralateral to the limb seen by the subject (Table II, Fig. 2). This pattern was stronger for movement mirroring of the right hand. The reverse comparison (i.e. NOR > MIR) showed a relative increase in the precuneus of the left hemisphere for movements of the right hand only.
|
Table II. Activation foci |
|||||||
|
Contrast |
Centre of activation focus in MNI coordinates |
t-value |
Anatomical structure |
Functional structure |
|||
|
Hemisphere |
X |
Y |
Z |
||||
|
RH MIR > NOR |
R |
18 |
–78 |
2 |
7.98 |
Lingual gyrus |
V1 |
|
R |
12 |
–86 |
16 |
8.12 |
Cuneus |
V2 |
|
|
R |
52 |
–72 |
6 |
8.23 |
Middle occipital gyrus |
V5 |
|
|
R |
16 |
–84 |
38 |
7.18 |
Precuneus |
V6 |
|
|
R |
24 |
–40 |
–12 |
6.49 |
Fusiform gyrus |
||
|
RH NOR > MIR |
L |
–8 |
–92 |
28 |
10.22 |
Precuneus |
V6 |
|
LH MIR > NOR |
L |
–18 |
–86 |
34 |
8.89 |
Precuneus |
V6 |
|
LH NOR > MIR |
No significant difference |
||||||
|
OBS RH > LH |
No significant difference |
||||||
|
OBS LH > RH |
No significant difference |
||||||
|
Activations are tresholded at p < 0.01 (false discovery rate; FDR) with a minimum cluster size of 20 voxels. Cluster size is not reported due to confluent activations. MNI: Montreal Neurological Institute; LH: left hand; MIR: mirrored; NOR: normal; OBS: pure observation; RH: right hand. |
|||||||
In contrast, comparison of brain activity during movement observation of a right or left hand (RH OBS > LH OBS and vice versa) revealed no significant difference.
This lack of difference was confirmed by an effects of interest analysis at the precunei in both hemispheres, additionally demonstrating that significant lateralization was present only during movement execution, and not during movement observation (Fig. 2).
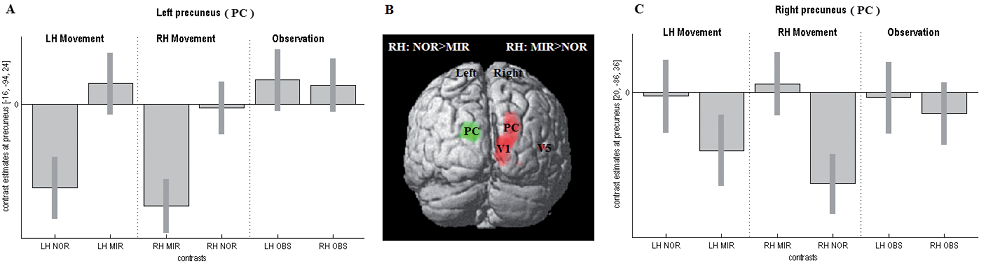
Fig. 2. Activation pattern and strength. (B) Activation differences during movements of the right hand (RH) plotted on a standard 3-dimensial image of the brain, viewed from behind. Red: MIR > NOR, green: NOR > MIR. (A, C) Effects of interests (mean standardized effect sizes and 90% confidence intervals (CI)) at both precunei in all 6 conditions. Note that locations (left: [–16, –94, –24], right: [20, –86, 36]) are slightly different from those in Table II, resulting from the different approach (analysis of variance). NOR: normal moving hand; MIR: mirrored moving hand; PC: precuneus; LH: left hand; OBS: pure observation.
DISCUSSION
This study demonstrates that lateralized cerebral activation of the parieto-occipital cortex opposite to the visually perceived hand is elicited by mirroring of own active movement performance, but not by passive movement observation. The asymmetry of the reverse comparison (i.e. NOR vs MIR) and the lack of difference during the observation tasks excludes the possibility that the activation pattern observed during movement mirroring is due to a pure visual hemifield stimulation.
Additional activation of the contralateral hemisphere by movement mirroring has been reported in studies using a real mirror (13) and video feedback (8). In principle, our findings match those of the previous study with a related set-up (8). However, in that study, both static and moving trials were analysed together. In contrast, in the present study, movement trials only were analysed, in order to allow comparison with the movement observation task. Lateralized activations were no longer found to be symmetrical for both hands, but more pronounced for movements of the right hand. The reverse comparison (i.e. NOR vs MIR) for the right hand showed additional activation to that noted in the previous study with a smaller number of subjects. For all comparisons, the strongest effect was found in the precuneus of either hemisphere, which has been reported to process upper limb configuration (14, 15).
To our best knowledge, comparison of movement observation of a right or left hand with central fixation has not previously been performed. One study reported a significant difference at the pars opercularis as part of the MNS, but hand laterality and visual hemifield were changed simultaneously (16). Cabinio et al. (11) compared the degree of lateralization in right-handers and left-handers, and showed a left lateralized activation pattern in the former and a bilateral activation pattern in the latter. This pattern is modulated with a changing sense of agency (17). Importantly, these binding processes seem to be strictly lateralized (18).
The MNS has been proposed as an underlying neural mechanism of MT (19). However, in our study, no additional activation was elicited in the MNS during movement mirroring. We assume that the parieto-occipital cortex is crucial for the processing of the visually perceived limb configuration of the contralateral side of the body, thus mediating the effects of MT. However, based on our data, it cannot be excluded that the MNS is activated in a uniform fashion during all movement observation conditions.
Finally, these results may lead to further speculation about the effect of MT and MOT on inter-hemispheric rivalry. For stroke patients, it is now well-established that the unaffected hemisphere can further inhibit the affected hemisphere by a transcallosal inhibition mechanism, resulting in deterioration in motor performance (20). One might speculate that only MT, but not MOT could selectively activate the affected hemisphere and beneficially influence this inter-hemispheric balance, which may further explain the positive effect of MT, especially in severe hemiparesis (21).
ACKNOWLEDGEMENTS
The authors are grateful to T. Krause, C. Kunze, J. Fiebach and L. dos Santos for assistance with implementation of the set-up at the Stroke MRI at the Charité – University Medicine Berlin, Campus Benjamin Franklin.
This study was supported by Zhejiang Provincial National Science Foundation of China (LY12H17004) to J.W., grants from the Centre of Stroke Research Berlin (Flex Funds, CS-2009-10) to C.F., S.H. and M.B, and funding from the Gesellschaft zur Förderung der Neurologischen Rehabilitation (GFNR).
REFERENCES
