Raffaele Molino-Lova, MD1, Guido Pasquini, BSc, PT1, Federica Vannetti, Eng, PhD1, Renato Zipoli, MD1, Lorenzo Razzolini, MD1, Valentina Fabbri, MD1, Roberta Frandi, MD1, Francesca Cecchi, MD1, Francesco Gigliotti, MD2 and Claudio Macchi, MD1,3
From the 1Cardiac Rehabilitation Unit, 2Respiratory Rehabilitation Unit, Don Gnocchi Foundation and 3Department Medical and Surgical Critical Area, University of Florence, Florence, Italy
BACKGROUND: Although the six-minute walk test (6MWT) is widely used in cardiac rehabilitation, little is known about the ventilatory strategies adopted by older patients who have recently undergone median sternotomy, in order to meet the increased metabolic demand in the 6MWT.
METHODS: Using a portable gas-analyser we assessed the breathing patterns in the 6MWT before and after a 3-week rehabilitation programme in 84 older patients, 58 men and 26 women, mean age 71 years (standard deviation (SD) 6 years), who had undergone median sternotomy.
RESULTS: After rehabilitation, patients increased end-test ventilation (33.1 l (SD 9.8) vs 30.9 l (SD 8.4), p < 0.001) by increasing tidal volume (1.158 l (SD 0.298) vs 1.065 l (SD 0.255), p < 0.001), while breathing frequency remained unchanged (29.9 bpm (SD 5.4) vs 30.2 bpm (SD 5.8), p = 0.621). As a consequence, the ventilatory equivalent for CO2, was significantly improved (39.9 (SD 5.3) vs 43.5 (SD 7.4), p < 0.001). Furthermore, the improvement in ventilatory efficiency was significantly (p < 0.001) correlated with the improvement in the distance walked on the 6MWT.
CONCLUSION: Older patients who have undergone median sternotomy meet the increased metabolic demand on the 6MWT after cardiac rehabilitation by increasing tidal volume. Accordingly, we should consider including as a routine specific exercises for inspiratory muscle training in current rehabilitation programmes to reduce inspiratory muscle effort and further improve ventilatory efficiency.
Key words: cardiopulmonary exercise testing; six-minute walk test; breathing pattern; cardiac rehabilitation; elderly.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Raffaele Molino-Lova, Cardiac Rehabilitation Unit, Don Gnocchi Foundation, Via di Scandicci snc, 50143 Florence, Italy. E-mail: rmolino@dongnocchi.it; raffmoli@tin.it
Accepted Nov 13, 2012; Epub ahead of print Mar 6, 2013
INTRODUCTION
The ventilatory responses to exercise are characterized by an increase in minute ventilation (VE) as a function of exercise intensity (1). Throughout incremental exercises the relationship between VE and tidal volume (VT) can be characterized by 3 phases (1, 2). In the first phase there is a linear relationship between VE and VT. In the second phase the increase in VE is mainly related to the increase in breathing frequency (BF), with progressive slowing of the rate of increase of VT. In the third phase the increase in VE is due only to the increase in BF, without any further increase in VT, which in fact may also decrease by the end of this phase (2, 3). Interestingly, although the relationship between VE and VT during incremental exercise tests can be modelled equally well with both cycling and treadmill exercise, the increase in VT is steeper with cycling exercise (2). Thus, comparable levels of VE are achieved with higher VT and lower BF during cycling exercise, and, conversely, with higher BF and lower VT during treadmill exercise (1, 2). Independent of the mechanisms responsible for these different ventilatory strategies, which still need to be further clarified, the two breathing patterns have relevant implications. In cycling exercise the greater effort of inspiratory muscles to increase VT (1) results in a more severe perception of breathlessness (4). On the other hand, during treadmill exercise the relatively rapid shallow breathing (1) reduces inspiratory muscle effort (5), but increases the fraction of VT going to dead space, thus resulting in an impairment in ventilatory efficiency (6).
The ventilatory responses to exercise have traditionally been investigated in well-trained subjects or in younger patients receiving cardiac rehabilitation by using cycling or treadmill incremental exercise tests. However, the real-world picture of current in-hospital cardiac rehabilitation shows a high prevalence of older patients who have recently experienced an acute event (mostly a cardiac operation through median sternotomy) and who, along with a slower and more complicated recovery, also exhibit low fitness levels and age-related chronic comorbidities (7–9). As most of these patients are not able to perform cycling or treadmill incremental exercise tests (10), the 6-minute walk test (6MWT), which simply consists of inviting patients to walk overground along a corridor for 6 min at their self-selected maximal speed (11), is used instead (10, 12). In terms of exercise physiology, the 6MWT is a constant work rate exercise (13) whose workload depends on patient’s body mass and self-selected speed (10).
Although the 6MWT is the most frequently used test to assess physical performance in older patients receiving cardiac rehabilitation (10, 12), little is known on the ventilatory strategies adopted by older patients who have recently undergone median sternotomy to meet the increased metabolic demand on the 6MWT performed after the rehabilitation, when patients self-select a higher walking speed and, as a consequence, increase their exercise workload.
Since walking is critical to maintain independence in activities of daily living, to enjoy social relationships and to retain good emotional vitality, all of which are main determinants of quality of life, particularly in older persons (14), understanding how these patients modify their breathing pattern to cope with increased workloads during an exercise test that shows such close similarities to the activities of daily living is a relevant issue.
In this paper we report the results of a study conducted on a selected sample of older patients who had recently undergone cardiac surgery through median sternotomy, in whom we assessed the breathing patterns in the 6MWT before and after rehabilitation.
METHODS
Study sample
Participants were enrolled among 213 consecutive patients admitted to our rehabilitation centre for a 3-week in-hospital cardiac rehabilitation programme. Inclusion criteria were: age 65 years or more and having undergone elective cardiac surgery through median sternotomy in the previous 7–8 days. Patients with cognitive deterioration (corrected Mini Mental State Examination score < 21) or relevant functional impairment due to previous stroke, peripheral artery disease, severe osteoarthritis of weight-bearing joints or other chronic diseases able per se to remarkably limit physical activity, such as chronic heart failure or chronic obstructive pulmonary disease, were excluded from the study. As recommended by the manufacturer, patients with a pacemaker or implantable defibrillator were also excluded, due to possible interferences of the portable gas-analyser with cardiac devices. Finally, patients with postoperative sequelae, such as chest wall or diaphragm mobility impairment, bronchial atelectasis and posture or walking impairment that were not resolved by intensive individual physiotherapy within the first week after admission, were also excluded to obtain a group of patients fit enough to ensure at least two weeks of light physical training in the gymnasium.
The study sample was represented by 88 patients, 61 men and 27 women, all of whom signed their informed consent form to be included in the study. The Institutional Review Board approved the study protocol.
Rehabilitation programme
A detailed description of the rehabilitation programme performed in our centre has been reported elsewhere (8, 9, 15). The programme included optimal medication adjustment, educational and psychological counselling, and physical training. Owing to the advanced age of participants, the programme was conceived not only to improve aerobic capacity, muscle strength and flexibility, but also to optimize balance and coordination in order to prevent falls and mobility disabilities.
Specifically, physical training was based on two 1-h sessions per day that included:
Cardiopulmonary exercise testing
Cardiopulmonary exercise testing was performed using a portable gas-analyser (Oxycon Mobile, Jaeger, Germany), calibrated before each test according to manufacturer’s instructions. Patients performed the 6MWT as recommended by the American Thoracic Society (11), before and after the rehabilitation. The gas-analyser was applied on patients a few minutes before the 6MWT, to allow patients’ adjustment to the mask. During the test ventilatory parameters were continuously monitored, along with the electrocardiogram and peripheral blood O2 saturation.
Oxygen uptake (VO2, ml/min), carbon dioxide output (VCO2, ml/min), minute ventilation (VE, l/min), expiratory tidal volume (VT, l), breathing frequency (BF, breath/min), total respiratory cycle duration (Ttot, s), inspiratory duration (Ti, s), inspiratory duty cycle (Ti/Ttot, %), mean inspiratory flow (VT/Ti, l/s) and the ventilatory equivalent for CO2 (VE/VCO2) were recorded continuously throughout the 6MWT. Occasional errant breaths, due to swallowing, coughing or talking, were marked by the physiotherapist during the test and removed from the data-set before the analysis.
Both before and after rehabilitation, baseline data were calculated as the mean values of the last minute of rest in the sitting position before starting the 6MWT and end-test data were calculated as the mean values of the last minute of the test.
Other variables
Family history of cardiovascular diseases was ascertained based upon the report of death or morbidity for angina, myocardial infarction, stroke or peripheral artery disease in one or both parents, or in 1 or more siblings, at an age of less than 65 years. Modifiable cardiovascular risk factors, such as smoking habit, hypertension, diabetes and dyslipidaemia were considered categorical variables and ascertained using standard criteria (16). The level of regular physical activity performed by patients in the year preceding the operation was assessed by using a questionnaire modelled on the Harvard Alumni Questionnaire and adapted for Italian people that we have described elsewhere (15).
All patients underwent echocardiography, using a MyLab30 apparatus (ESAOTE, Genoa, Italy) equipped with a 2.5 MHz imaging transducer and left ventricular ejection fraction was assessed using standardized criteria (17). Information on medications was gathered from medical records, and the use of cardiovascular drugs was considered as a categorical variable.
Statistics
Statistical analysis was performed using the STATA 7.0 software (Stata Corporation, College Station, TX, USA). Continuous variables are presented as means (standard deviation (SDs)). Categorical variables are presented as absolute value with percentage in brackets. The course of VE,VT and BF over time were fitted using linear and non-linear models by a commercially available mathematical software (MatLab, from MathWorks, Natick, MA, USA). The relationship of VT, VT/Ti, Ti/Ttot and VE/VCO2 with VE were also similarly fitted. The “goodness of fit” for models was assessed by the coefficient of determination (R2) and fitting procedures were considered satisfactory if R2 was ≥ 0.85. Differences in breathing pattern, ventilatory efficiency and model parameters before and after the rehabilitation were tested using the paired Student’s t-test. Type 1 error was set at the two-sided 0.05 level.
RESULTS
Of the 88 enrolled patients, one patient interrupted the rehabilitation for a few days due to fever and was excluded from the study. The remaining 87 patients completed the scheduled two-week physical training in the gymnasium and no relevant adverse event occurred. A further 3 patients were excluded from the study due to poor quality records of either breathing profile or gas exchanges, either before or after the rehabilitation. The final study sample was represented by 84 patients, 58 men and 26 women, mean age 71 years (SD 6, range 65–84, median 69, inter-quartile 66–78), whose general characteristics are shown in Table I.
|
Table I. General characteristics of the study sample (n=84) |
|
|
Demographics |
|
|
Age, years, mean (SD) |
71.2 (6.0) |
|
Female sex, n (%) |
26 (31) |
|
Operation, n (%) |
|
|
Coronary artery by-pass graft |
34 (40) |
|
Valve repair/replacement |
32 (38) |
|
Combined procedures |
18 (21) |
|
Cardiovascular risk factors |
|
|
Family history of cardiovascular diseases, n (%) |
30 (36) |
|
Current smokers, n (%) |
8 (10) |
|
Hypertension, n (%) |
52 (62) |
|
Diabetes, n (%) |
24 (29) |
|
Dyslipidaemia, n (%) |
40 (48) |
|
Body mass index, kg/m2, mean (SD) |
24.6 (3.4) |
|
Reported physical activity in the year before surgery, n (%) |
|
|
Sedentary lifestyle or low-intensity physical activity |
52 (62) |
|
Moderate-intensity physical activity |
32 (38) |
|
Left ventricular ejection fraction, %, mean (SD) |
53.6 (10.2) |
|
Cardiovascular medication intake, n (%) |
|
|
β-blockers |
40 (48) |
|
Ca-antagonists |
23 (27) |
|
ACE-inhibitors |
27 (32) |
|
Diuretics |
37 (44) |
|
SD: standard deviation; ACE: angiotensin-converting-enzyme. |
|
The course of both VE and VT over time fitted satisfactorily a mono-exponential model (Figs 1 and 2) according to the formula:
y(t) = y baseline + (y steady-state – y baseline) (1 – e – t/τ)
where the term (y steady-state – y baseline) is the amplitude of the curve and τ (tau) the time constant, i.e. the time needed to achieve 63% of the asymptotic response. For VE the amplitude was 16.6 l/min (SD 6.0) and 19.8 l/min (SD 7.7) (p < 0.001), while the time constant was 81 s (SD 49) and 84 s (SD 44) (p = 0.691), before and after the rehabilitation, respectively. For VT the amplitude was 0.486 l (SD 0.206) and 0.572 l (SD 0.228) (p = 0.007), while the time constant was 67 s (SD 34) and 79 s (SD 40) (p = 0.052), before and after the rehabilitation, respectively.
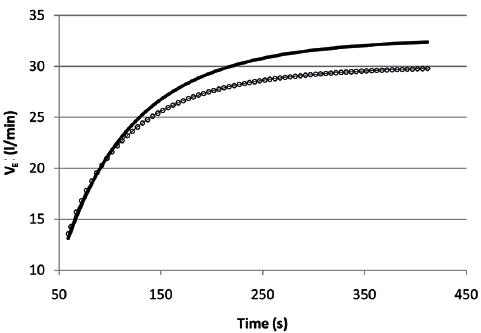
Fig. 1. Course of minute ventilation (VE) over time during the six-minute walk test before (circles) and after (continuous line) the rehabilitation, based on the mean parameters of individual responses.
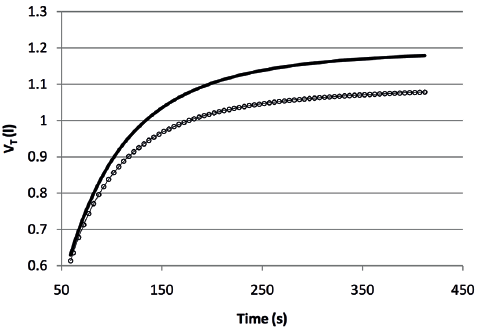
Fig. 2. Course of tidal volume (VT) over time during the six-minute walk test before (circles) and after (continuous line) the rehabilitation, based on the mean parameters of individual responses.
The course of BF over time before and after the rehabilitation showed a poor fitting with both linear and non-linear models. After a sudden increase in the first 15–20 s of the test, BF showed a horizontal trend.
The relationship between VT and VE fitted satisfactorily a linear model (Fig. 3) according to the formula:
y = α + bx
where α is the constant (or intercept) of the line and β is the coefficient (or slope). The coefficient was 0.028 (SD 0.008) and 0.031 (SD 0.006) (p = 0.269), while the constant was 0.232 (SD 0.169) and 0.230 (SD 0.192) (p = 0.947), before and after the rehabilitation, respectively.
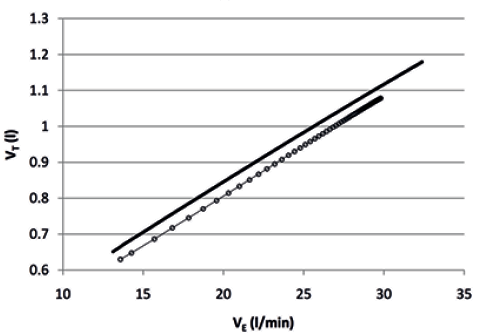
Fig. 3. Course of tidal volume (VT) over minute ventilation (VE) during the six-minute walk test before (circles) and after (continuous line) the rehabilitation, based on the mean parameters of individual responses.
With regard to the drive (VT/Ti) and timing (Ti/Ttot) components of VE (18), while the relationships of VT/Ti with VE fitted satisfactorily a linear model (coefficient 0.038 (SD 0.004) and 0.036 (SD 0.004), p = 0.386; constant 0.031 (SD 0.145) and 0.057 (SD 0.130), p = 0.272, before and after the rehabilitation, respectively), the relationship of Ti/Ttot with VE, showed a poor fitting with both linear and non-linear models before and after the rehabilitation.
Finally, the relationship of VE/VCO2 with VE fitted satisfactorily a mono-exponential model (Fig. 4) according to the formula:
y(VE) = y steady-state + (y baseline – y steady-state) (e – VE/ϕ)
where the term (y baseline– y steady-state) is the amplitude of the curve and ϕ (phi) the flow constant, i.e. the flow (VE) needed to achieve the 63% of the asymptotic response. The amplitude was 12.9 (SD 4.9) and 15.0 (SD 6.7) (p = 0.229), while the flow constant was 5.8 l/min (SD 2.5) and 6.0 (SD 2.8) (p = 0.483), before and after the rehabilitation, respectively.
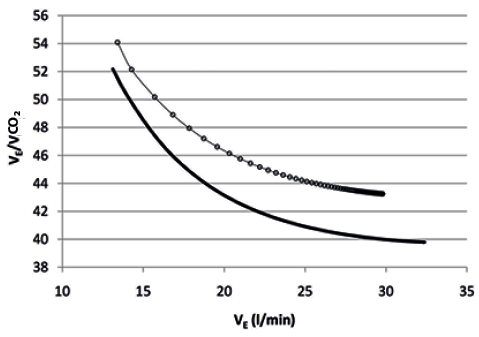
Fig. 4. Course of minute ventilation (VE)/carbon dioxide output (VCO2) over VE during the six-minute walk test before (circles) and after (continuous line) the rehabilitation, based on the mean parameters of individual responses.
Table II shows baseline (left side) and end-test (right side) breathing patterns before and after the rehabilitation. At baseline comparisons, after the rehabilitation patients significantly reduced VE and BF without significant changes in VT. VT/Ti was also significantly reduced due to the increase in Ti. Ti/Ttot remained unchanged. At the end of the rehabilitation programme, as expected, patients significantly increased the distance walked, O2 uptake and CO2 output. At end-test comparisons, after the rehabilitation patients significantly increased VE and VT without significant changes in BF. VT/Ti was also significantly increased due to the increase in VT. Ti/Ttot remained unchanged. After the rehabilitation, both at baseline and at the end of the 6MWT patients showed a significant reduction in VE/VCO2. There was a significant (p < 0.001) correlation between changes in VE/VCO2 (the measure of ventilatory efficiency) and changes in walking distance (Model: Obs = 84; F = 48.61; Prob > F < 0.000; Adjusted R2=0.408). Cardiovascular medication intake and patients’ demographic and anthropometric characteristics were not significantly related to changes in ventilatory efficiency. On the other hand, changes in VE/VCO2 were inversely related (p = 0.019) to left ventricular ejection fraction.
|
Table II. Breathing profile on the six-minute walk test (6MWT) before and after the rehabilitation: baseline (left side) and steady-state data (right side) (n=84) |
|||||||||||
|
Baseline data |
Steady-state data |
||||||||||
|
Before the rehabilitation Mean (SD) |
After the rehabilitation Mean (SD) |
pa |
Before the rehabilitation Mean (SD) |
After the rehabilitation Mean (SD) |
pa |
||||||
|
Distance walked on the 6MWT, m |
NA |
NA |
NA |
331 (93) |
390 (79) |
< 0.001 |
|||||
|
Heart rate, bpm |
79.8 (10.7) |
78.1 (11.9) |
0.156 |
98.9 (15.7) |
101.2 (16.7) |
0.222 |
|||||
|
O2 uptake (VO2), ml/min |
294 (76) |
277 (62) |
0.391 |
763 (187) |
852 (207) |
< 0.001 |
|||||
|
CO2 output (VCO2), ml/min |
266 (72) |
257 (63) |
0.320 |
739 (202) |
840 (239) |
< 0.001 |
|||||
|
Ventilation (VE), l/min |
13.9 (3.5) |
12.7 (2.6) |
< 0.001 |
30.9 (8.4) |
33.1 (9.8) |
< 0.001 |
|||||
|
Tidal volume (VT), l |
0.616 (0.150) |
0.609 (0.135) |
0.293 |
1.065 (0.255) |
1.158 (0.298) |
< 0.001 |
|||||
|
Breathing frequency (BF), breaths/min |
23.6 (4.2) |
21.8 (3.3) |
< 0.001 |
30.2 (5.8) |
29.9 (5.4) |
0.621 |
|||||
|
Total respiratory cycle duration (Ttot), s |
2.64 (0.47) |
2.84 (0.45) |
< 0.001 |
2.09 (0.43) |
2.10 (0.42) |
0.748 |
|||||
|
Inspiratory duration (Ti), s |
1.09 (0.20) |
1.16 (0.22) |
0.002 |
0.94 (0.20) |
0.95 (0.20) |
0.465 |
|||||
|
Inspiratory duty cycle (Ti/Ttot), % |
41.4 (3.7) |
41.0 (3.9) |
0.345 |
45.0 (3.4) |
45.0 (3.2) |
0.445 |
|||||
|
Mean inspiratory flow (VT/Ti), l/s |
0.574 (0.133) |
0.518 (0.089) |
< 0.001 |
1.163 (0.313) |
1.261 (0.369) |
< 0.001 |
|||||
|
Ventilatory equivalent for CO2 (VE/VCO2) |
54.1 (7.4) |
51.2 (7.7) |
0.002 |
43.5 (7.4) |
39.9 (5.3) |
< 0.001 |
|||||
|
aFrom paired Student’s t-test. O2: oxygen; CO2: carbon dioxid; SD: standard deviation. |
|||||||||||
DISCUSSION
The aim of this study was to investigate how older patients who have recently undergone cardiac surgery through median sternotomy modify their breathing pattern to meet the increased metabolic demand on the 6MWT performed after rehabilitation, when patients self-select an increased exercise workload, and we found that the increase in end-test VE resulted from an increase in VT, while BF remained unchanged.
To the best of our knowledge, no study has yet investigated the breathing pattern on the 6MWT in these patients. Thus, direct comparisons of our findings with the existing literature are not feasible: however, a few comments are warranted.
The mono-exponential course of VE and VT over time is not surprising, as it follows the mono-exponential course of gas exchanges kinetics during submaximal constant work rate exercises (13, 19). The relationship between VT and VE fitted a linear model, as in the first phase of incremental exercises, and showed comparable intercepts and slopes before and after the rehabilitation, confirming the negligible contribution of BF to the increase in VE, at least within the low levels of VE observed in our patients during the 6MWT. Similarly, the relationship between inspiratory drive (VT/Ti) and VE also fitted a linear model and showed comparable constants and coefficients before and after the rehabilitation, suggesting that, although VT/Ti and VE were significantly increased at the end of the test after the rehabilitation, physical training is not associated with changes in central inspiratory drive at any given level of VE, at least within the low levels of VE observed in our patients during the 6MWT. Furthermore, the relationship between VE/VCO2 and VE fitted a mono-exponential model and showed comparable amplitudes and flow constants before and after the rehabilitation. However, the curve was significantly shifted downward after the rehabilitation, confirming the established notion that whenever patients increase VE by increasing VT and, consequently, the ratio of dead space to VT is reduced (6), ventilatory efficiency definitely improves. Interestingly, the reduction in VE/VCO2 and the increase in VT showed the same magnitude (8%). Moreover, there was a significant (p < 0.001) correlation between changes in VE/VCO2 and changes in walking distance. Finally, confirming our previously published data (8), changes in VE/VCO2 were inversely related (p = 0.019) with left ventricular ejection fraction, suggesting that patients with relatively poorer left ventricular ejection fraction are most likely to respond more favourably to physical training.
In this study we investigated the ventilatory pattern in patients freely walking overground along a corridor at their self-selected maximal speed, which is a natural task for older persons and shows close similarities to the activities of daily living. This represents the strength of the study. However, some inherent limitations that might restrict the extension of our results to all patients receiving cardiac rehabilitation need to be considered. First, to obtain a group of patients fit enough to ensure at least two weeks of light physical training in the gymnasium we introduced a wide series of exclusion criteria, so that our patients represent a selected sample of older patients who receive rehabilitation after cardiac surgery through median sternotomy. Secondly, patients with chronic heart failure were excluded from the study because, at least for some of them, the 6MWT might be a maximal instead of a submaximal exercise test (20) and the contribution of BF to the increase in VE might be substantial due to the increased ventilatory drive (6). Analogous considerations apply for patients with associated chronic obstructive pulmonary disease, which were excluded based upon the routine clinical and instrumental functional assessment performed by a specialist in respiratory medicine before cardiac surgery. Thirdly, patients enrolled in this study were all older patients receiving in-hospital post-acute rehabilitation after cardiac surgery through median sternotomy, so that our results cannot be directly extended to younger patients, to patients attending outpatient cardiac rehabilitation, and to patients receiving cardiac rehabilitation for clinical conditions other than cardiac surgery. Furthermore, two potential methodological limitations also need to be considered. First, in this study there is no control group, so that we are not allowed claiming with certainty a cause-effect relationship between the rehabilitation programme and the observed changes in breathing pattern. In fact, the control group should be represented by elderly patients who have undergone cardiac surgery through median sternotomy and who do not receive rehabilitation. Since cardiac rehabilitation is strongly recommended for these patients, ethical reasons prevented us from proposing a study project in which randomly selected patients did not receive a recommended treatment and the Institutional Review Board would never approve such a study protocol. However, this study was aimed at understanding how these patients modify their breathing pattern to cope with increased workloads during an exercise test that shows such close similarities to the activities of daily living, which was largely unknown. A second potential methodological limitation of this study is that the cycling exercises included in our rehabilitation programme might have affected the breathing pattern shown by patients on the 6MWT. However, the relationship between VE and VT during the 6MWT showed comparable constants and coefficients before and after rehabilitation, which makes the hypothetical effect of the type of aerobic exercise used for physical training on breathing pattern quite unlikely.
In conclusion, current rehabilitation programmes for older patients who have recently undergone cardiac surgery through median sternotomy are based upon a comprehensive geriatric approach that includes, along with low-intensity aerobic training (which represents the core of cardiac rehabilitation) gentle callisthenic exercises, passive stretching and specific exercises for balance and coordination to prevent mobility disability and falls (8, 9, 15). Only patients with postoperative sequelae, such as chest-wall or diaphragm mobility impairment and bronchial atelectasis, receive chest physiotherapy, generally for few days. As our findings show that the increase in VE during the 6MWT performed after the rehabilitation results from an increase in VT rather than from an increase in BF, although in this study data on inspiratory muscle strength were not available, we should consider routinely including specific exercises for inspiratory muscle training in current rehabilitation programmes in order to reduce inspiratory muscle effort and improve ventilatory efficiency.
Future studies should confirm our findings on a broader scale and test the above hypothesis through an appropriate study design.
References
