Margreth Grotle, PhD1,2, Mari Klokkerud, MSc1, Ingvild Kjeken, PhD1,3, Ann Bremander, PhD4,5,7, Sofia Hagel, MSc6,7, Britta Strömbeck, PhD7,8, Kim Hørslev-Petersen, PhD9, Jorit Meesters, MSc10,11, Thea P. M. Vliet Vlieland, PhD10,11, Kåre B. Hagen, PhD1,3 and the Scandinavian Team Arthritis Register – European Team Intiative for Care Research (STAR-ETIC) collaboration
From the 1National Resource Centre for Rehabilitation in Rheumatology, Diakonhjemmet Hospital, 2FORMI (Communication Unit for Musculoskeletal Disorders), Oslo University Hospital, Ullevaal, 3Institute of Health and Society, University of Oslo, Oslo, Norway, 4School of Business and Engineering, Department of Exercise Physiology, Biomechanics and Health, Halmstad University, Halmstad, 5Research and Development Centre, Spenshult, Oskarström, 6Department of Clinical Sciences Lund, Section of Rheumatology, Lund University and Skane University Hospital, 7Department of Clinical Sciences in Lund, Orthopaedics, Lund University, Lund, 8Department of Clinical Sciences Malmö, Section of Rheumatology, Malmö University and Skane University Hospital, Malmö, Sweden, 9King Christian Xth Hospital for Rheumatic Diseases, Gråsten, Denmark 10Department of Rheumatology and 10Department of Orthopaedics, Rehabilitation Medicine and Physical Therapy, Leiden University Medical Center, Leiden, The Netherlands
BACKGROUND: In evaluating complex interventions, it is a challenge for researchers to provide transparent reporting of the intervention content with sufficient detail and clarity such that effects can be compared across studies or countries.
OBJECTIVE: To describe and compare the content of current rehabilitation for patients with inflammatory arthritis across 4 northern European countries.
Patients and methods: A total of 731 patients with inflammatory rheumatic diseases participated in a multicentre, longitudinal observational study carried out in Sweden, The Netherlands, Denmark and Norway. Data on context, structure and process were reported by patients and teams at the different participating study sites according to the Scandinavian Team Arthritis Register – European Team Intiative for Care Research (STAR-ETIC) framework.
RESULTS: Although large similarities were found in the context, there were important differences between the Netherlands and the Scandinavian countries. Regarding structure, there were considerable differences in the length of the rehabilitation period across settings and countries. The most evident differences concerned process variables, especially the type and dosage of individual treatment modalities.
CONCLUSION: The variation in important aspects of arthritis rehabilitation found in the present study underline the need for transparent and standardized description of these variables when comparing effects across settings and countries. A standardized description of current practice can be achieved by the STAR-ETIC framework.
Key words: arthritis; team rehabilitation; context; structure; process; outcome.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Margreth Grotle, National Resource Center for Rehabilitation in Rheumatology, Diakonhjemmet Hospital, PO Box 23 0319 Oslo, Norway. E-mail: margreth.grotle@medisin.uio.no
Accepted Nov 21, 2012; Epub ahead of print Feb 28, 2013
INTRODUCTION
Despite improvements in pharmacological and surgical treatment for patients with arthritis, non-pharmacological treatment in terms of arthritis rehabilitation is still often required (1, 2). Arthritis rehabilitation is a complex, multidisciplinary treatment approach defined as “a group of health professionals from various disciplines who work together towards a common goal” (2). This type of healthcare service can take place in various clinical settings; as inpatient and outpatient programmes at rheumatology hospital departments, and at specialized rehabilitation centres, mostly as inpatient programmes. In recent years, there has been increased use of outpatient programmes in arthritis rehabilitation (3, 4).
Both pharmacological and non-pharmacological care, provided by single professions or teams, should be based on evidence from best available research into the healthcare service that is provided. A common challenge for clinicians and researchers who want to evaluate arthritis rehabilitation in clinical trials, routine quality management and/or registry systems, is to provide transparent reporting of the intervention content with sufficient detail and clarity such that the study can be compared with other studies or replicated by others. The difficulties in reporting all necessary details of complex interventions, such as arthritis rehabilitation, is one of the reasons why it is challenging for researchers to synthesize data and systematically review such interventions (5, 6).
The lack of consensus regarding how to describe the content of non-pharmacological interventions in trials and other studies stands in sharp contrast to the last years’ achievement in consensus regarding how healthcare interventions should be evaluated. The Outcome Measures in Rheumatology recommendations (7) on evaluating patients’ health status and the Consolidated Standards of Reporting Trials (CONSORT) recommendations (8, 9) on guiding clinical studies represent large steps forward with respect to international consensus on crucial aspects of healthcare evaluations. The lack of detailed guidance with respect to describing the content of interventions, however, was one of the reasons why the Transparent Reporting of Evaluations with Non-randomized Designs (TREND) statement was developed (10). The TREND guidelines emphasize the report of theories used and descriptions of behavioural and public health interventions. This topic has also been emphasized by research groups within arthritis, for example a framework for reporting health service models for managing rheumatoid arthritis (RA) has been published (11), and a quality measure of the process of care in RA has been developed (12). Another initiative in this area is the Scandinavian Team Arthritis Register – European Team Intiative for Care Research (STAR-ETIC) collaboration. On the basis of a systematic literature search and Delphi consensus rounds within a group of clinicians, researchers and patients in the Netherlands, Sweden, Denmark and Norway, a framework to describe the context, structure, process and outcome of arthritis team rehabilitation have been suggested (13). The overarching component of context describes the national welfare and healthcare systems, whereas structure describes core elements at institutional level, process describes details of the rehabilitation provided at an individual level, and outcome suggests domains for evaluation in arthritis rehabilitation (13).
The objective of this study was to describe and compare the content of arthritis rehabilitation for patients with inflammatory arthritis across 4 northern European countries.
MATERIAL AND METHODS
The STAR-ETIC (www.star-etic.se) is an international collaboration between multidisciplinary researchers, clinicians and patient representatives with expertise in the field of arthritis rehabilitation. The overall aim of the STAR-ETIC project was to study the structure, process and outcome of arthritis rehabilitation of rheumatic diseases, and to create a common database.
The present article presents results on the context, structure and process of arthritis rehabilitation, while the outcomes are presented in the accompanying article. Data were available from two sources: (i) patient-reported data from a multicentre, longitudinal observational study carried out in Sweden, the Netherlands, Denmark and Norway; and (ii) additional data on context, structure and process provided by the teams at the different participating study sites.
Design
This was a multicentre, longitudinal observational study in which rheumatology hospital departments and specialized rehabilitation centres that provided rehabilitation for patients with inflammatory arthritis participated. Eligible patients at the participating centres were consecutively recruited in the period from 2006/2007 to 2009. Data was registered in the STAR-ETIC register, which included 1,449 patients by September 2010.
Each of the teams at the different healthcare sites was asked to complete information in the STAR-ETIC checklist on behalf of the arthritis rehabilitation provided at their site. A research coordinator in each of the countries was responsible for administrating this checklist.
Patients and study sites
Inclusion criteria were patients aged 18 years or more who were scheduled for a rehabilitation period of at least one week duration and with an inflammatory joint disease (RA and spondyloarthritis (SpA); including ankylosing spondylitis, undifferentiated spondylarthritis and psoriatic arthritis). Patients’ diagnosis was confirmed by a rheumatologist at each site. Exclusion criteria were severe psychiatric comorbidity or inability to communicate in written Swedish/Dutch/Danish/Norwegian. In Norway, patients > 75 years were also excluded. The study was approved by the regional committee for medical research ethics in each of the 4 countries.
In Denmark, the King Christian X’s Hospital, University of Southern Denmark participated and included a total of 115 patients with inflammatory joint disease (94 patients with RA and 21 with SpA). Complete data from Denmark for the current study was provided for 91 patients.
In the Netherlands, the Department of Rheumatology, Leiden University Medical Center, participated and included a total of 81 patients with inflammatory joint disease (49 patients with RA and 32 with SpA). Complete data from the Netherlands for the current study was provided for 80 patients.
In Norway, a total of 4 rheumatology hospital departments and 9 rehabilitation centres participated and included eligible patients with various rheumatic diseases referred to a rehabilitation stay during a 3-month recruitment period at the end of 2006. In the current study, including only patients with inflammatory joint disease, the patients were recruited from the 4 rheumatology hospital departments and 6 rehabilitation centres. The hospital departments were the NRRE, Diakonhjemmet Hospital, Oslo, Martina Hansen Hospital, Bærum/Oslo, Lillehammer Rheumatological Hospital, Lillehammer, and Østfold Hospital, Sarpsborg. The rehabilitation centres were Borger Bad Rehabilitation Center, Jeløya Rehabilitation Center, Skogli Rehabilitation Center, Tonsåsen Rehabilitation Center, Valnesfjord Rehabilitation Center, and Vikersund Kurbad. A total of 157 patients had an inflammatory joint disease (73 patients with RA and 84 patients with SpA). Complete data from Norway for the current study was provided for 149 patients.
In Sweden 3 rheumatology departments participated; the Spenshult Hospital for Rheumatic Diseases and the Clinics of Rheumatology in Lund and Malmö, Skane University Hospital. They included a total of 494 with inflammatory joint disease (282 patients with RA and 212 patients with SpA). Complete data from Sweden for the current study was provided for 411 patients.
Measurement of sociodemographic and health characteristics
At admission, physicians examined the patients and provided diagnostic information. The patients completed a comprehensive questionnaire at admission and discharge. The baseline questionnaire included sociodemographic and health status variables. Sociodemographic variables concerned age, gender, marital status (married/co-habitant or living alone), and work status (employed or not).
Health status variables included primary diagnosis (RA, SpA, psoriatic arthritis (PsA)), comorbidity (presence of other diseases based on a list of 12 possible diagnostic groups), use of medication (biologics, disease-modifying anti-rheumatic drug (DMARDS), corticosteroids, and pain medication), and functional status assessed by the Health Assessment Questionnaire) (14).
Arthritis rehabilitation
Checklist for context, structure and process. Based on the STAR-ETIC framework (13) we developed a checklist of information to include when reporting arthritis rehabilitation or other non-pharmacological interventions. The definitions for each of the items within the context, structure and process components of the framework (13) were standardized into a checklist containing a total of 18 items; 1 for the context, 9 for structure, and 8 for process (Table I).
|
Table I. The Scandinavian Team Arthritis Register – The European Team Initiative of Care research (STAR-ETIC) checklist of information to include when reporting studies of arthritis rehabilitation care in arthritis |
|
|
Items |
Description of reporting |
|
Context |
|
|
1. Welfare and healthcare systems |
Description of the national healthcare and welfare systems in which the arthritis rehabilitation finds place |
|
Structure |
|
|
2. Funding |
Description of funding/reimbursement of the rehabilitation service |
|
3. Criteria for admission/discharge |
Description of criteria for admission and discharge |
|
4. Clinical setting |
Description of level of care (primary/secondary/other), type of setting (inpatient/outpatient, rehabilitation centre/general hospital), and primary diagnosis (secondary diagnosis if relevant) |
|
5. Rehabilitation team |
5a. Specify type and number of each professions (medical doctor/specialist, physiotherapist, occupational therapist, nurse, psychologist, social worker, nutritionist, other) in the team |
|
5b. Specify communication form, e.g. informal communication every day and/or frequency of formal team meetings |
|
|
6. Rehabilitation management |
Brief description of the rehabilitation plan provided. Specify if a particular tool was used, e.g. the ICF-based ”Rehab-Cycle” |
|
7. Patient involvement |
Description of how patient was involved in treatment decisions and plan. Provide number of organized meetings between the team and the patient |
|
8. Family involvement |
Description of how patient’s family was involved in treatment decisions and plan |
|
9. Length of team care |
Specify number of active rehabilitation days for the whole team care period |
|
10. Follow-up |
Description of how patient was followed up after discharge from the rehabilitation period (e.g. continuing management in primary care or other follow-up management) NB. Administration of self-reported questionnaires is not considered as a structural procedure for follow-up. |
|
Process |
|
|
11. Goals |
Specify whether treatment goals were defined or not and how they were developed (patient self-report tool, patient interview, organized goal setting meeting with team members etc). If needed, specify goals classified according to the ICF components (frequency of BF, BS, AL, PR) |
|
12. Assessment and evaluations |
Describe the standardized assessment and evaluation at admission/discharge, e.g. according to the ICF components |
|
13. Interventions |
13a. Specify frequency of individual treatment sessions for each health profession in the team (medical doctor/specialist, physiotherapist, occupational therapist, nurse, psychologist, social worker, nutritionist, other), and provide estimates of average duration per session (if available). Based on this information, give the total number of individual treatment sessions for the whole rehabilitation period, mean duration per session |
|
13b. Specify frequency of group education and group exercise sessions for the whole rehabilitation period, and provide estimates of mean duration per session |
|
|
13c. Specify type of individual treatment modalities that are available: 1) Individual patient education/information/counsellinga 2) Individual exercises (e.g. mobility, muscle strength, coordination, aerobic capacity, relaxation, ADL, other individual exercises) 3) Electrotherapy (e.g. ultrasound, TENS, low-level laser therapy, short-wave therapy and pulsed electromagnetic energy, shock wave therapy) 4) Thermotherapy (e.g. heat and/or cold packs) 5) Acupuncture 6) “Hands-on” intervention (e.g. massage, passive joint mobility/manipulation, stretching, acupressure/trigger-point treatment, other soft tissue techniques) 7) Psychological consultation 8) ADL help (e.g. help with activities of daily living from nurse) 9) Other (specify) For each treatment modality that is available specify for how large proportion of the patients this is used in the following categories: 0–25%; 26–50%; 51–75%; 76–100%; all |
|
|
13d. Specify frequency of self-training for the whole rehabilitation period |
|
|
13e. Specify frequency of formal interdisciplinary team meetings |
|
|
aIf necessary, topics can be specified according to the following list: pain, physical activity, coping, weight control/nutrition, joint protection, assistive tools, Activities of Daily Living (ADL), social network, leisure activities, economy, official services, home visit, work/education, individual plan, other consultations, contact family/relatives, contact official services, contact healthcare providers, external collaboration meeting, other external meetings. ICF: International Classification of Functioning, Disability and Health; BF; Body Function; BS: Body Structure; AL: Activity Limitation; PR: Participation Restrictions; TENS: transcutaneous electrical nerve stimulation. |
|
Rehabilitation diary. During the rehabilitation period the patients provided information regarding the type and frequency of various treatment modalities in a rehabilitation diary. The original rehabilitation diary was developed in two expert groups, first in Norway and second in the STAR-ETIC project group (13, 15).
Statistical analysis
Descriptive statistics were calculated for each of the countries and study sites. Continuous variables are presented by means with standard deviations (SDs) and categorical variables are presented by frequencies and percentages. Differences between the countries/sites were analysed using analysis of variance analysis. SPSS version 14.0 was used for the statistical analysis. The level of statistical significance was set at 5%. String/text variables were analysed by a simple qualitative analysis of similarities and differences across the countries/sites.
RESULTS
Patients
A total of 839 patients had a diagnosis of inflammatory joint disease (RA and SpA) and completed a period of arthritis rehabilitation. Of these patients, 93 were excluded due to incomplete data at baseline or discharge, 14 had a second episode of rehabilitation, and 1 had a rehabilitation length of less than 1 week, leaving 731 (86.3%) available for the present study. Analyses of possible differences between the study group (n = 731) and those who could not be included (n = 108) showed that there were no statistical significant differences in any of the sociodemographic and health characteristics except for age and work status. The patients who were not included in the material were significantly older (mean age 62 vs 54 years) and were more likely to be unemployed.
The patients had a mean age of 54.3 years SD 13.5 years) and there were more women (67%) than men (33%). Approximately one-third (35%) were employed. DMARDS (56%) was the most commonly used medication, followed by analgesics (47%), corticosteroids (25%) and biologics (21%). Many patients (82%) reported comorbidities. Table II shows the variation in baseline characteristics of the included patients across the 4 countries.
|
Table II. Sociodemographic and health characteristics of patients (total n = 731) |
||||
|
Denmark n = 91 |
The Netherlands n = 80 |
Norway n = 149 |
Sweden n = 411 |
|
|
Age, years, mean (SD) |
59 (13) |
53 (15) |
53 (12) |
54 (14) |
|
Gender; women, n (%) |
64 (70) |
44 (55) |
102 (69) |
281 (68) |
|
Marital status, living alone, n (%) |
37 (41) |
19 (24) |
43 (29) |
116 (29) |
|
Work status, employed, n (%) |
21 (23) |
33 (41) |
52 (35) |
150 (38) |
|
Primary diagnosis |
||||
|
Rheumatoid arthritis |
74 (81) |
49 (61) |
67 (45) |
427 (58) |
|
Spondyloarthritis |
17 (19) |
31 (39) |
173 (42) |
303 (42) |
|
Biologics at admission, n (%) |
16 (19) |
20 (25) |
23 (15) |
91 (26) |
|
DMARDs at admission, n (%) |
67 (78) |
46 (58) |
45 (30) |
255 (68) |
|
Corticosteroids at admission, n (%) |
23 (27) |
19 (24) |
38 (26) |
103 (29) |
|
Use of analgesics at admission, n (%) |
70 (81) |
53 (66) |
59 (44) |
166 (57) |
|
Functional status (HAQ2), mean (SD) |
1.04 (0.69) |
1.24 (0.67) |
0.71 (0.51) |
0.93 (0.59) |
|
DMARDs: disease-modifying anti-rheumatic drugs; HAQ: Health Assessment Questionnaire, scored from 0 = best possible score to 3 = worst possible; SD: standard deviation. |
||||
Context and structure of arthritis rehabilitation
The context across the 3 Scandinavian countries was rather similar as they all have public welfare and healthcare systems, in which there is a high degree of public responsibility for citizens’ social and economic security (Table III). The context in the Netherlands differed slightly, as the Dutch patients need to be insured to receive the same social benefits.
|
Table III. Context and structure components of arthritis rehabilitation |
||||||||||
|
Study site |
Denmark (n = 91) Site D1 |
The Netherlands (n = 80) Site NL1 |
Norway (n = 149) |
Sweden (n = 411) |
||||||
|
Site N1 |
Site N2 |
Site N3 |
Site N4 |
Site N5 |
Site S1 |
Site S2 |
Site S3 |
|||
|
Context |
||||||||||
|
1. Welfare and healthcare systems |
||||||||||
|
Public healthcare system with equal access to all healthcare services |
× |
– |
× |
× |
× |
× |
× |
× |
× |
× |
|
Insurance system necessary to have access to healthcare services |
– |
× |
– |
– |
– |
– |
– |
– |
– |
– |
|
Structure |
||||||||||
|
2. Funding |
||||||||||
|
Covered by the health and welfare systems |
× |
– |
× |
× |
× |
× |
× |
× |
× |
× |
|
Full coverage by insurance companies |
– |
× |
– |
– |
– |
– |
– |
– |
– |
– |
|
3. Criteria for admission and discharge |
||||||||||
|
Referred from medical doctors within or outside hospital |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Discharge set at admission |
× |
– |
× |
× |
× |
× |
× |
× |
× |
× |
|
Discharge predetermined (fixed programme) |
– |
– |
– |
– |
– |
× |
– |
– |
× |
× |
|
Discharge dependent on goal achievement |
– |
× |
– |
– |
– |
– |
– |
– |
– |
– |
|
4a. Clinical setting (level of care) |
||||||||||
|
Secondary level |
× |
× |
– |
× |
× |
× |
× |
× |
× |
× |
|
Tertiary level |
– |
– |
× |
– |
– |
– |
– |
– |
– |
– |
|
4b. Clinical setting (type of setting) |
||||||||||
|
Hospital inpatient |
× |
– |
× |
× |
× |
– |
– |
× |
– |
– |
|
Hospital outpatient |
– |
× |
– |
– |
– |
× |
– |
– |
× |
× |
|
Rehabilitation centre |
– |
– |
– |
– |
– |
– |
× |
– |
– |
– |
|
4c. Clinical setting (diagnosis) |
||||||||||
|
Primary diagnosis inflammatory arthritis |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Secondary diagnoses |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
5a. Rehabilitation team (type of professions on department level) |
||||||||||
|
Medical doctor (rheumatologist) |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Physiotherapist |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Occupational therapist |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Nurse |
× |
× |
× |
× |
× |
× |
× |
– |
× |
× |
|
Social worker |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Psychologist |
– |
– |
× |
– |
– |
– |
– |
– |
– |
– |
|
Nutritionist |
× |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Other |
× |
– |
× |
× |
× |
– |
× |
– |
× |
× |
|
5b. Rehabilitation team (communication form) |
||||||||||
|
Weekly team meetings |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Other meetings when needed |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
6. Rehabilitation management |
||||||||||
|
Individual rehabilitation plan |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Standardized tool |
– |
× |
– |
– |
– |
– |
– |
– |
– |
– |
|
Electronic-based tool |
– |
× |
– |
– |
– |
– |
– |
– |
– |
– |
|
7. Patient involvement |
||||||||||
|
Patient participation in team meeting at admission/discharge |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Patient participation in all team meetings |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
8. Family involvement |
||||||||||
|
Standard for family involvement |
– |
– |
– |
– |
– |
– |
– |
– |
× |
– |
|
Family involvement based on indication |
× |
× |
× |
× |
× |
× |
× |
× |
– |
× |
|
9. Length of rehabilitation |
||||||||||
|
Median days (min–max) |
14 (4–19) |
21.5 (5–88) |
21 (9–23) |
11 (8–12) |
10.5 (7–27) |
10 (10–11) |
21 (5–30) |
16 (7–41) |
18 (18–18) |
5 (5–5) |
|
10. Follow-up |
||||||||||
|
Standard for follow-up management |
– |
× |
– |
– |
– |
– |
– |
– |
× |
× |
|
The details are presented as present (× = yes, provided) or not (– = no, not provided) or not actual according (na) to study sites in the 4 countries. The detailed results on which this table is based can be provided by contacting the authors of this paper. |
||||||||||

Table III shows large similarities across the 4 countries in structures related to funding, criteria for admission and discharge, clinical setting, rehabilitation team, and patient and family involvement (Items 2, 3, 4, 5, 7, 8 in Table III). For example, in all the countries there were few other criteria for referring patients to arthritis rehabilitation than a referral from a doctor inside or outside a hospital. Criteria for discharge were predetermined and set at admission in all the 3 Scandinavian countries, whereas in the Netherlands this criterion was related to the achievement of goals. Furthermore, the inpatient and outpatient arthritis rehabilitation programmes were provided in rheumatology hospital departments in all the 4 European countries, whereas in Norway arthritis rehabilitation was also provided in rehabilitation centres. Moreover, there were only minor variations in the type of health professions available within the teams, and all teams held interdisciplinary team meetings once a week with informal communication between the team members when needed. All study sites also had standardized structures for patient involvement, whereas only one study cited in Sweden had an explicit standardized structure for family involvement (an information meeting in one of the outpatient clinics).
Differences concerned structures of rehabilitation management, length of team rehabilitation, and follow-up (items 7, 9, 10 in Table III). Only the Dutch team provided a standardized tool for rehabilitation management in addition to an individual rehabilitation plan, which all the study sites included. Furthermore, the outpatient clinics in the Netherlands and Sweden had a structure for routine follow-up management of ordinary patients at their department, whereas the inpatient clinics did not. Finally, there were large differences in the length of arthritis rehabilitation, varying from a 5-day course at one of the outpatient clinics in Sweden up to a median of 21 working days at one of the inpatient clinics and the rehabilitation centres in Norway.
Process of arthritis rehabilitation
Table IV shows that the process of describing goals and providing a standardized assessment at admission and discharge were similar across the 4 countries (items 11 and 12 in Table IV). All the study sites classified clinical findings according to the main components of the International Classification of Functioning, Disability and Health (ICF) (16) as part of their routine practice, but only the Dutch team classified goals. Similar domains of body functions and activity/participation in the ICF were assessed before and after arthritis rehabilitation at all study sites (data not shown).
|
Table IV. The process component of arthritis rehabilitation. The details are presented as present (× = yes, provided) or not (– = no, not provided) or not actual (na) according to study sites in the 4 countries |
||||||||||
|
Study site |
Denmark (n = 91) Site D1 |
The Netherlands (n = 80) Site NL1 |
Norway (n = 149) |
Sweden (n = 411) |
||||||
|
Site N1 |
Site N2 |
Site N3 |
Site N4 |
Site N5 |
Site S1 |
Site S2 |
Site S3 |
|||
|
11. Goals |
||||||||||
|
Individual goals defined |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Developed together with team member(s) |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Goals classified according to the ICF |
– |
× |
– |
– |
– |
– |
– |
– |
– |
– |
|
12. Assessment and evaluation |
||||||||||
|
Standardized assessment at admission |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Standardized assessment at discharge (evaluation) |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
Use of the main components of the ICF |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
13a. Individual treatment by health profession (on individual level) |
||||||||||
|
Medical doctor |
× |
× |
× |
× |
× |
na |
× |
× |
× |
na |
|
Physiotherapist |
× |
× |
× |
× |
× |
na |
× |
× |
× |
na |
|
Occupational therapist |
× |
× |
× |
× |
× |
na |
× |
× |
× |
na |
|
Nurse |
× |
× |
× |
× |
× |
na |
× |
× |
– |
na |
|
Social worker |
× |
× |
× |
× |
× |
na |
× |
× |
× |
na |
|
Psychologist |
– |
– |
× |
– |
– |
na |
– |
– |
– |
na |
|
Nutritionist |
× |
– |
– |
– |
– |
na |
– |
– |
– |
na |
|
Other |
× |
– |
× |
× |
× |
na |
× |
× |
× |
na |
|
13b. Group sessions |
||||||||||
|
Group education |
× |
– |
× |
× |
× |
× |
× |
– |
× |
× |
|
Group exercise |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
13c. Type of individual treatment modalities |
||||||||||
|
Information/counsellinga |
– |
– |
× |
× |
× |
na |
× |
× |
× |
na |
|
Individual exercisesb |
× |
× |
× |
× |
× |
na |
× |
× |
× |
na |
|
Electrotherapyc |
– |
– |
× |
– |
× |
na |
× |
× |
× |
na |
|
Thermotherapyd |
– |
– |
× |
× |
× |
na |
× |
× |
× |
na |
|
Acupuncture |
– |
– |
– |
– |
– |
na |
× |
× |
× |
na |
|
“Hands-on”e |
– |
– |
× |
× |
× |
na |
× |
× |
× |
na |
|
Psychological treatment |
– |
– |
× |
– |
– |
na |
– |
– |
– |
na |
|
ADL help by nursef |
× |
– |
× |
× |
× |
na |
× |
× |
na |
na |
|
13d. Self-training |
× |
– |
× |
× |
× |
× |
× |
× |
× |
na |
|
13e. Interdisciplinary team meetings |
× |
× |
× |
× |
× |
× |
× |
× |
× |
× |
|
aIncludes the following main topic: pain, physical activity, coping, weight control/nutrition, joint protection, assistive tools, ADL at home, social network, leisure activities, economy, official services, home visit, work/education, individual plan, other consultations, contact family/relatives, contact official services, contact healthcare providers, external collaboration meeting, other external meetings. bMobility, muscle strength, coordination, aerobic capacity, relaxation, ADL, other individual exercises. cUltrasound, TENS, low-level laser therapy, short-wave therapy and pulsed electromagnetic energy, shock-wave therapy. dHeat and/or cold packs. eMassage, passive joint mobility/manipulation, stretching, acupressure/trigger point treatment, other soft-tissue techniques. fHelp with ADL. The detailed results on which this table is based can be provided by contacting the authors of this paper. ADL: activities of daily living; TENS: transcutaneous electrical nerve stimulation; ICF: International Classification of Functioning, Disability and Health. |
||||||||||
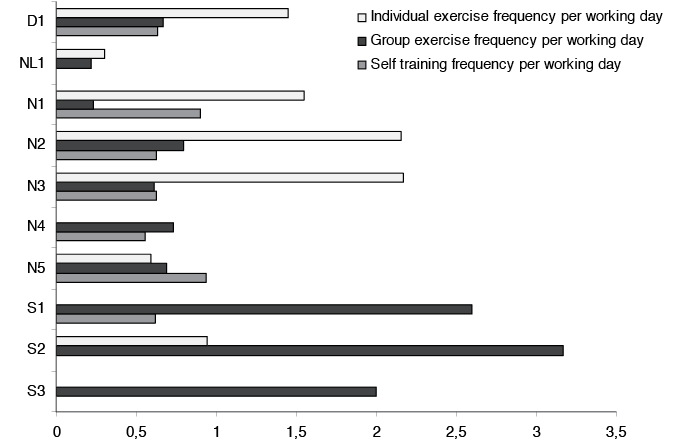
In all the study sites most of the individual treatment sessions were provided by physiotherapists (data not shown). Furthermore, a combination of individual and group sessions was usually delivered, except in two outpatient clinics (one in Sweden and one in Norway) which delivered a standardized group programme (item 13 in Table IV). Although there were large overlaps in the type of health profession that provided individual and group treatment sessions, the number of sessions varied widely between the different study sites (Table IV). For example, the number of sessions with exercises, either in terms of individual and group sessions or as self-training during the rehabilitation period, also showed large variations across the sites after adjusting for rehabilitation length (Fig. 1). The duration per session varied from 15 to 60 min across the study sites. Furthermore, the number of formal interdisciplinary team meetings varied from 1 meeting during the whole period up to a mean of 4.7 meetings. The number of interdisciplinary team meetings was highest in the Dutch team. In general, differences in type and frequency of treatment modalities included in arthritis rehabilitation were more evident across study sites than across countries.
Fig.1. Different modes of exercises in arthritis rehabilitation across the 10 study sites in the four European countries (D1=one site in Denmark, NL1 = one site in the Netherlands, N1–5 = five sites in Norway, S1-3 = three sites in Sweden). All estimates are in mean number of sessions, adjusted for length of rehabilitation (number of working days).
DISCUSSION
This study explored different aspects of arthritis rehabilitation across 4 European countries using the STAR-ETIC framework. The findings revealed large similarities across the countries in the current practice of arthritis rehabilitation with respect to: (i) the lack of a structure for admission and discharge except being referred from medical doctors within or outside hospital criteria; (ii) similar structures for the rehabilitation teams including medical doctor, physiotherapist, occupational therapist, and social worker as the most common team members; (iii) emphasizing patient involvement as all patients participated in at least one team meeting at admission; (iv) a similar process of developing and defining individual goals; (v) a standardized assessment at admission and discharge, in which the main components of the ICF (body function and structure, activity, participation, contextual factors) were used as a tool to guide this process; and (vi) the inclusion of group exercises in the intervention. There were also similarities in the context and structures for funding, clinical setting, rehabilitation management, family involvement, and follow-up management. The finding that all 4 included countries had many similarities with respect to context and several structural and process factors, are promising with respect to comparing aspects of arthritis rehabilitation across our countries and study sites. These findings also underline topics that should be further improved, for example the fact that none of the countries had clear criteria for admission other than referral from a doctor, that most of the teams did not have a standardized structure for rehabilitation management, and that most teams lacked a structure for follow-up management. These findings are of clinical importance as they show a discrepancy between what is emphasized theoretically in the framework and in current practice. These topics deserve more attention from clinicians and researchers in collaboration.
The most evident differences in the content of arthritis rehabilitation concerned the length of the rehabilitation period and the report of type and dosage of treatment modalities involved in arthritis rehabilitation. The differences in length of rehabilitation are important to consider when comparing outcomes of the different arthritis rehabilitation programmes, especially with respect to health economics outcomes (17). Although there was some overlap in many of the available treatment modalities, this study revealed large discrepancies in both type and dosage of in particular individual treatment modalities.
The report of type and dosage of treatment modalities have been a key issue for the CONSORT recommendations (8, 9) in order to enable replication of complex interventions such as arthritis rehabilitation across studies. Even if the CONSORT for non-pharmacological treatments recommends that the interventions in trials should report details of the interventions “sufficient to replication”, the recommendations are very general, thereby leaving it up to the researchers to decide the details of this critical information. A greater specificity of interventions has later been requested by both the CONSORT extension (9) and the TREND statement (10). They ask for full descriptions of treatment with any procedures used described, for example the timing of treatment, including duration and intervals of dosing or sessions, any materials needed (such as patient handouts or devices), and accessibility of any materials or instructions, including overcoming language barriers (5). Due to the multiple health professions involved in arthritis rehabilitation this is a resource-demanding task. The two previous initiatives within the arthritis field, the Canadian framework for reporting health service models for managing RA (11) and the quality measure of the process of care in RA (12), also provide relevant information for describing arthritis rehabilitation. However, they do not provide enough information to allow replication of arthritis interventions; for example, with respect to type and dosage for each of the treatment modalities involved in arthritis rehabilitation. If this critical information is not systematically recorded and reported the variations may be too large to allow for valid research comparisons and/or syntheses. The large variation in the reporting of type and frequency of treatment modalities involved in this study clearly shows that, without a standard and transparent way to report these details, it is difficult to compare arthritis rehabilitation interventions across different units, studies or countries. In addition, replication of the interventions in research studies would be impossible.
Another important topic to consider for both clinicians and researchers is the level of evidence for the delivered treatment modalities in arthritis rehabilitation. Overall, there is much empirical evidence that exercise therapy can decrease pain and improve physical functioning in patients with arthritis. For example, in a Cochrane Review of dynamic exercises for RA, Hurkmans et al. (18) identified a total of 8 randomized, controlled trials with evidence that short-term aerobic capacity and muscle strength training can provide a positive effect of exercises on functional ability and pain. Similarly, when comparing home exercises with no intervention (1 trial/155 patients) Dagfinrud et al. (19) found a significant positive effect of exercise on pain for patients with anky losing spondylitis and a non-significant effect on function. Furthermore, a previous umbrella overview summarized evidence from 28 systematic reviews on the effect of non-pharmacological and non-surgical interventions for RA (20), of which many of the interventions overlap with treatment modalities provided in the present study. High-quality evidence was found for beneficial effects of joint protection and patient education, which was a treatment modality that was delivered frequently in the present study. They also found moderate quality evidence for beneficial effects of low-level laser therapy, which was delivered at approximately half of the present study sites. Importantly, the quality of evidence for the effectiveness of most non-pharmacological therapy in RA was found to be moderate to low, also for treatment modalities that are delivered frequently in arthritis rehabilitation; for example, acupuncture, “hands-on” interventions, such as massage, passive joint mobility/manipulation, stretching, acupressure or trigger-point treatment. This shows the need for more high-quality research on the different treatment modalities within arthritis rehabilitation.
There are limitations to this study. First, the STAR-ETIC framework and checklist need to be tested for validity in general, as well as feasibility in countries other than the 4 European countries involved. A critical aspect of a future validation is to ensure that all the information collected by the framework is representative for the arthritis rehabilitation provided at the study site, and not biased by the contribution or representation of any of the health professionals involved in the arthritis team. Secondly, process data were provided by both health professionals and patients. These do not necessarily always agree. A reliability study of agreement between patients and healthcare providers should therefore be part of a validation study of the checklist. Thirdly, this study concerned patients with different inflammatory joint diagnoses. Even if it is likely that most of the structure and process components are similar across these diagnoses, we cannot rule out minor variations. For example, the delivery of exercises might be more frequent in SA patients compared with RA. Also, one of the sites included only RA patients, and hence, is not representative for patients with SA and PsA. Some of the differences between the countries and sites could also be due the difference in the samples included at each site. Fourthly, another source of variation might be the different professionals who were included at the study sites and the differences in the composition of the team care programme among the study sites. This issue illustrates the complexity of multidisciplinary rehabilitation and must be taken into account when interpreting the results from the current study. Lastly, the administrative routine in data collection (e.g. using a research coordinator) could have been calibrated more strictly across the countries in order to reduce some of the variation in results.
There are also advantages with this study. By using the STAR-ETIC framework and checklist we were able to provide a detailed and transparent report of arthritis rehabilitation, both with respect to context, structural and process components. The checklist was based on the theoretical STAR-ETIC framework for describing different aspects of rehabilitation care, including definitions and conceptualizations of core elements in this health service. Although we realize that improvements and adaptations are needed before the STAR-ETIC framework and checklist are implemented in clinical practice in particular, we suggest the use of this tool for clinicians and researchers who want to explore more systematically the “black box of multidisciplinary care” for patients with arthritis. A thorough and transparent description of the content of various arthritis rehabilitation interventions is also of crucial importance for patients and referring doctors. This can improve the basis for decision-making in clinical practice. A thorough description of arthritis rehabilitation should be provided when there is a particular need for it, either for research or clinical purposes. The variations in report of the content of treatment modalities in the present data underline the need for further work regarding improving the report of type and dosage of various treatment modalities within arthritis rehabilitation.
The content of current practice of arthritis rehabilitation was described and compared across 4 north European countries by using the STAR-ETIC framework and its checklist. The findings of this study suggest that there are large similarities in the content of such practice across the countries in several of the structure and process variables as well as in context. The most evident differences concerned the length of the rehabilitation period and the report of type and dosage of individual treatment modalities involved in arthritis rehabilitation.
ACKNOWLEDGEMENTS
The STAR-ETIC project was supported by the European League Against Rheumatism (EULAR) by EULAR grant CLI022. The Norwegian arm of this study, the SPOR project, was financed by Extra-stiftelsen Helse og Rehabilitering in Norway. The authors would like to thank all of the participating patients and health professionals at all participating rehabilitation sites for their contribution.
The STAR-ETIC project group is funded by Eular, and consists of the following participants: from Sweden: Ingemar Petersson, Ann Bremander, Elisabet Lindqvist, Britta Strömbeck, Ingegerd Wikström and Sofia Hagel; from Denmark: Kim Hørslev-Petersen, Inger-Henriette Stovgaard and Susanne Jürgensen; from the Netherlands: Thea Vliet Vlieland and Jorit Meesters; from Norway: Kåre Birger Hagen, Margreth Grotle, Mari Klokkerud and Ida Løchting/Ingvild Kjeken. From 2009 Ingvild Kjeken has participated in the project group in place of Ida Løchting.
In addition, the following patient representatives from Norway, Denmark and Sweden participated: Gerd Jenny Anerud (Norway), Connie Ziegler (Denmark) and Birgitta Smedeby (Sweden).
REFERENCES
