Roy G. Elbers, MSc1,2, Erwin E. H. van Wegen, PhD2, John Verhoef, PhD1 and Gert Kwakkel, PhD2
From the 1University of Applied Sciences Leiden, Department of Physiotherapy, Leiden and 2VU University Medical Center, Department of Rehabilitation Medicine, MOVE Research Institute Amsterdam, Amsterdam, The Netherlands
OBJECTIVE: To investigate the predictive value of gait speed for community walking in Parkinson’s disease and to develop a multivariate prediction model for community walking.
DESIGN: Data from baseline assessments in a randomized clinical trial were used.
SUBJECTS: A total of 153 patients with Parkinson’s disease were included.
METHODS: Community walking was evaluated using the mobility domain of the Nottingham Extended Activities of Daily Living Index (NEAI). Patients who scored 3 points on item 1 (“Did you walk around outside?”) and item 5 (“Did you cross roads?”) were considered community walkers. Gait speed was measured with the 6-m or 10-m timed walking test. Age, gender, marital status, disease duration, disease severity, motor impairment, balance, freezing of gait, fear of falling, previous falls, cognitive function, executive function, fatigue, anxiety and depression were investigated for their contribution to the multivariate model.
RESULTS: Seventy patients (46%) were classified as community walkers. A gait speed of 0.88 m/s correctly predicted 70% of patients as community walkers. The multivariate model, including gait speed and fear of falling, correctly predicted 78% of patients as community walkers.
CONCLUSION: Timed walking tests are valid measurements to predict community walking in Parkinson’s disease. However, evaluation of community walking should include an assessment of fear of falling.
Key words: Parkinson’s disease; gait; assessment; diagnostic tests.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Roy G. Elbers, University of Applied Sciences Leiden, Department of Physiotherapy, Zernikedreef 11, PO Box 382, 2300 AJ Leiden, The Netherlands. E-mail: elbers.r@hsleiden.nl
Accepted Nov 13, 2012; Epub ahead of print Feb 28, 2013
Introduction
Community walking is an important enabler to participation in community activities and a range of societal, work and leisure roles (1). Unfortunately, impaired walking is common in patients with Parkinson’s disease (PD) and is associated with a loss of independence (2). Gait disorders, such as decreased speed, reduced step length and increased step frequency, vary with the nature and the complexity of concurrent tasks (3, 4) and environment (5). The physical, social and attitudinal environments are generally more variable and less predictable in the community than in patients’ own home settings (1), which may comprise community walking in patients with PD.
There is no uniform definition of community walking in the literature, and little is known about the dimensions of the activity and the specific attributes required for its safe and independent execution. Community walking has been defined as independent mobility outside the home, which includes the ability to confidently to negotiate uneven terrain, private venues, shopping centres and other public venues (6). Patla & Shumway-Cook (7) developed an operational definition that consisted of 8 dimensions (i.e. ambient conditions, terrain characteristics, external physical load, attentional demands, postural transition, traffic density, time constraints, and walking distance) that reflect environmental demands on community walking. One qualitative study (1) confirmed that ambient conditions, terrain characteristics, attentional demands, crowded and cluttered environments and temporal demands negatively influenced community walking in patients with PD. With that, one may hypothesize that geographical and cultural differences exist in patients’ ability to walk in their own community. Furthermore, community walking was complicated by fluctuation of impairments due to varying effects of medication, anxiety and fatigue (1).
Gait speed, measured using the 10-m timed walking test (10MTW), has been used as a proxy measure for community walking in patients with stroke (6, 8–10). Thresholds varying from 0.66 m/s (8) to 1.32 m/s (10) were proposed to discriminate between community walkers and non-community walkers. In addition, a recent meta-analysis (11) found a statistically significant association between gait speed and activity limitations in patients with PD. The 10MTW, performed in a patient’s home setting, is a reliable (intraclass correlation coefficient = 0.81) and responsive (smallest detectable change = 0.19 m/s) test for measuring gait speed in patients with PD (12) and may be a simple test to discriminate between community walkers and non-community walkers in PD. However, temporal factors are only one aspect of community walking and therefore gait speed needs to be considered alongside other measures that reflect the broader dimensions of community walking, including geographical and cultural differences (13).
The aim of the present study was to investigate whether comfortable gait speed was a valid measure to predict community walking in patients with PD. First, a clear cut-off value for gait speed was determined to discriminate between community walkers and non-community walkers. Subsequently, the discriminative ability of walking speed for community walking was tested for its geographical and cultural differences. Finally, a multivariate logistic model was derived for predicting community walking on the basis of the dichotomized cut-off value for gait speed, age, gender, marital status, disease duration, disease severity, motor impairment, balance, freezing of gait, fear of falling, previous falls, cognitive function, executive function, fatigue, anxiety and depression.
Methods
Population and design
This study was part of a randomized clinical trial (the “Rescue” trial (Rehabilitation in Parkinson’s Disease: Strategies for Cueing) QLK-CT-2001-00120) about the effect of cueing training on gait and gait-related activity in patients with PD (14). In this study, 153 patients with PD were recruited from 3 centres: Northumbria University, Newcastle upon Tyne (UK); Katholieke Universiteit Leuven, Leuven (Belgium), and the VU University Medical Center, Amsterdam (The Netherlands). The study was approved by the ethics committee of each centre (ethics committee approval number: 2002/42 (UK); 2001/08/27 (Belgium) and 2001/152 (The Netherlands). All patients gave written informed consent. Further details about design and outcomes of the study have been published previously (14).
Subjects
Patients were recruited according to the following criteria: (i) age 18–80 years; (ii) diagnosis of PD, defined by the UK Brain Bank Criteria (15); (iii) Hoehn and Yahr (H&Y) stage II–IV (16); (iv) stable drug usage; and (v) mild to severe gait disturbance (score > 1 on the Unified Parkinson’s Disease Rating Scale (UPDRS) item 29) (17). Patients were excluded if they had: (i) undergone deep brain stimulation or other stereotactic neurosurgery; (ii) cognitive impairment (Mini Mental State Examination (MMSE) < 24) (18); (iii) disorders interfering with participation in cueing training, including neurological (stroke, multiple sclerosis, brain tumour), cardiopulmonary (chronic obstructive disorders, angina pectoris) and orthopaedic (osteoarthritis, rheumatoid arthritis and back pain) conditions; (iv) unpredictable and long lasting “off” periods (score 1 on item 37 and score > 2 on item 39 of the UPDRS) (17); or (v) had participated in a physiotherapy programme 2 months before starting the trial.
Measuring community walking
Community walking was evaluated using the mobility domain of the Nottingham Extended Activities of Daily Living Index (NEAI) (19). The NEAI was developed to measure change in independence in performing activities in daily living and consists of 22 self-report items in 4 domains (i.e. mobility, in the kitchen, domestic tasks and leisure activities). Items are scored on a 4-point Likert scale ranging from 0 (not at all) to 3 (on your own). Patients who scored 3 points on item 1 (“Did you walk around outside?”) and item 5 (“Did you cross roads?”) were considered as community walkers. Patients who scored 2 points or less were considered non-community walkers. No explicit instructions regarding the use of assistive walking devices were provided.
Measuring gait speed
Gait speed was measured with the 10MTW. If necessary, the measured distance was adapted to deal with difficulties inherent to testing in the patients’ home. To standardize measurement as much as possible, gait speed was measured over a minimum distance of 6 m. Patients started from stance and were asked to walk at their preferred (comfortable) speed. If necessary, the use of a walking aid was permitted, as well as in the subsequent assessments. The investigator used “1, 2, 3, start” as the start sequence. At the same time as pronouncing the “start” signal, the stopwatch was put in action. After the patient had put 1 foot past the finish line, timing was ended. No restrictions regarding footwear were given, but footwear was standardized between assessments. The time (s) was converted to gait speed (m/s). The test was repeated 3 times, and the mean of the 3 trials was calculated. The 10MTW is a reliable and responsive test to measure walking speed in patients with PD (12).
Other factors considered for community walking
To identify relevant factors for the prediction of community walking, demographic characteristics (i.e. age, gender and marital status) and clinical variables (i.e. disease duration, disease severity, motor impairment, balance, freezing of gait, fear of falling, history of falls, cognitive and executive function, fatigue, anxiety and depression) were measured. Disease severity was assessed with the H&Y scale (16), motor impairment with the UPDRS part III (17) and balance with the Functional Reach test (FR) (20). The Freezing of Gait Questionnaire (FOGQ) (21) was used to evaluate freezing and the adapted 13-item version of the Falls Efficacy Scale (FES) (22) was used to evaluate fear of falling. Previous falls were evaluated (i.e. no falls, near-falls or falls in the previous 3 months) (23). Cognitive and executive function was assessed with the Brixton test (24) and the MMSE (17). The Multidimensional Fatigue Inventory (MFI) (25) was used to assess physical (i.e. MFI general and physical fatigue subscale combined (GF/PF) (26) and MFI reduced activity) and mental aspects (i.e. MFI metal fatigue and MFI reduced motivation) of fatigue and the Hospital Anxiety and Depression Scale (HADS) (27) was used to evaluate anxiety and depression.
Procedure
Patients completed all assessments at baseline during visits from a trained observer not involved in data analysis. All assessments were performed in the patients’ homes in the on phase, approximately 1 h after medication intake.
Statistical analysis
Discrimination of gait speed for predicting community walking. A receiver operating characteristic (ROC) curve was constructed to investigate the diagnostic accuracy of gait speed in discriminating between community walkers and non-community walkers. An optimal cut-off point was determined. Sensitivity, specificity and the area under the curve (AUC) were calculated. Positive and negative predictive values (PPV and NPV, respectively) were calculated to determine the proportion of patients with a walking speed above the cut-off score who were community walkers (PPV) and the proportion of patients with a walking speed below the cut-off score who were non-community walkers (NPV). In addition, ROC curves per country were constructed to investigate whether geographical and cultural differences (i.e. UK, Belgium or The Netherlands) influenced the cut-off point for gait speed. Differences between country-specific ROC curves were tested using Venkatraman’s test for comparing unpaired ROC curves (28).
Multivariate logistic regression model for predicting community walking. To evaluate the added value of demographic and clinical covariates in predicting community walking, a multivariate logistic regression analysis was performed. First, bivariate logistic regression analysis was conducted with community walking as dependent variable and the candidate determinants (including the dichotomized cut-off point for gait speed) as independent variables. Secondly, candidate determinants with a liberal significance level of p < 0.2 were selected for multivariate regression analysis. To avoid collinearity between included determinants, candidate determinants were removed if Pearson’s or Spearman’s correlation coefficients (r) were ≥ 0.70. A stepwise backward approach was applied to derive the multivariate regression model. The performance of the model was investigated in terms of explained variance (Nagelkerke R2), calibration (Hosmer-Lemeshow test, Cooks distances and Leverage values for influential cases (residuals > 2 standard deviation (SD)) and discrimination. A ROC curve was constructed and an optimal cut-off point was determined. Sensitivity, specificity, the AUC, PPV and NPV were calculated.
Subsequently, the AUCs of the multivariate model and gait speed were compared using the bootstrap percentile method based on the method originally described by Hanley & McNeil (29, 30).
Data were analysed using SPSS statistical package (SPSS Statistics version 19.0, IBM Corp., New York, USA). The pROC package (27) and the R environment for statistical computing (R64 version 2.15, R Foundation for Statistical Computing, Vienna, Austria) were used to construct and analyse the ROC curves. Depending on distribution by visual plot, parametric or non-parametric analyses were applied. A two-tailed significance level of 0.05 was used for all tests.
Results
Table I presents the characteristics of the included patients. A total of 153 patients with a mean age of 67.06 years (SD 7.54) were included in the study. Most patients had mild-to-moderate disease severity as 46% (n = 71) of patients were classified in H&Y stage II, 42% (n = 64) in stage III and 12% (n = 18) in stage IV. Seventy (46%) out of 153 patients were classified as community walkers. Gait speed data were available for 150 patients. Mean gait speed was 0.84 m/s (SD 0.20).
|
Table I. Patient characteristics (n = 153) |
|
|
Mean (SD) |
|
|
Demography |
|
|
Age, years |
67.06 (7.54) |
|
Male/femalea |
88/65 |
|
Partnereda |
123 |
|
UK/Belgium/The Netherlandsa |
48/51/54 |
|
PD characteristics |
|
|
Disease duration, years |
8.25 (5.09) |
|
H&Y (on), (min (best) = 1; max (worst) = 5 |
2.78 (0.60) |
|
Clinical data |
|
|
Community walkera |
70 |
|
Gait speed (m/s) |
0.84 (0.20) |
|
UPDRS-total (on), min (best) = 0; max (worst) = 199 |
56.03 (16.01) |
|
UPDRS I (on), min (best) = 0; max (worst) = 16 |
3.30 (1.72) |
|
UPDRS II (on), min (best) = 0; max (worst) = 52 |
16.42 (6.03) |
|
UPDRS III (on), min (best) = 0; max (worst) = 108 |
33.05 (11.28) |
|
UPDRS IV (on), min (best) = 0; max (worst) = 23 |
3.34 (3.26) |
|
FR |
25.55 (7.94) |
|
FOGQ, min (best) = 0; max (worst) = 24 |
8.73 (5.29) |
|
Freezera,b |
63 |
|
FES, min (worst) = 0; max (best) = 130 |
81.59 (27.91) |
|
Faller, no falls/near-falls/fallsa |
74/18/61 |
|
Brixton R, min (best) = 0; max (worst) = 55 |
22.17 (10.12) |
|
Brixton S, min (worst) = 1; max (best) = 10 |
3.99 (2.22) |
|
MMSE, min (worst) = 0; max (best) = 30 |
28.17 (1.82) |
|
MFI-total, min (best) = 20; max (worst) = 100 |
62.74 (17.94) |
|
MFI GF/PF, min (best) = 8; max (worst) = 40 |
27.76 (8.18) |
|
MFI reduced activity, min (best) = 4; max (worst) = 20 |
13.45 (4.98) |
|
MFI mental fatigue, min (best) = 4; max (worst) = 20 |
10.36 (4.68) |
|
MFI reduced motivation, min (best) = 4; max (worst) = 20 |
11.16 (4.30) |
|
HADS anxiety, min (best) = 0; max (worst) = 21 |
6.90 (3.91) |
|
HADS depression, min (best) = 0; max (worst) = 21 |
7.20 (3.50) |
|
aExpressed as number of patients. bFreezing of Gait Questionnaire, item 3: score ≥ 2. Brixton R: Brixton Test Raw score; Brixton S: Brixton Test Scaled score; FES: Falls Efficacy Scale; FOGQ: Freezing of Gait Questionnaire; FR: Functional Reach test; GF/PF: general and physical fatigue subscale combined; HADS anxiety: Hospital Anxiety and Depression Scale anxiety subscale; HADS depression: Hospital Anxiety and Depression Scale depression subscale; H&Y (on): Hoehn and Yahr stages during on; MFI: Multidimensional Fatigue Inventory; Max: Maximal obtainable score; Min: minimal obtainable score; MMSE: Mini Mental State Examination; PD: Parkinson’s disease; SD: standard deviation; UPDRS-total, I, II, III, IV (on): Unified Parkinson’s Disease Rating Scale total score, part I, II, III and IV during on. |
|
Discrimination of gait speed for predicting community walking
ROC analysis showed good diagnostic accuracy for gait speed in predicting capability for community walking with an AUC of 0.78 (95% confidence interval (CI) 0.70–0.85) (Fig. 1). A cut-off point of 0.88 m/s (sensitivity 0.67, specificity 0.75) correctly predicted 70% of the patients as community walkers (PPV) and 72% were correctly classified as non-community walkers (NPV). Venkatraman’s test showed no statistically significant differences between the different locations (p = 0.21 (UK vs Belgium), p = 0.75 (UK vs The Netherlands) and p = 0.56 (Belgium vs The Netherlands) (Fig. 2).
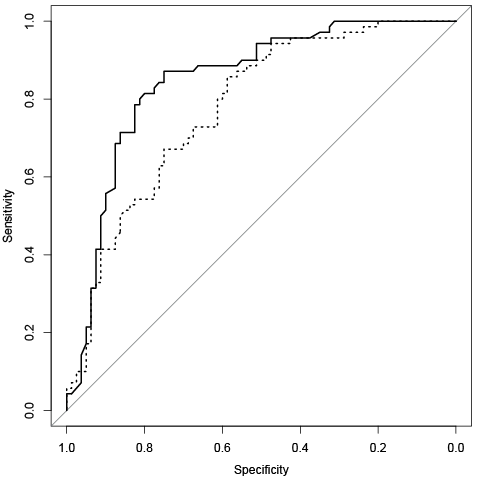
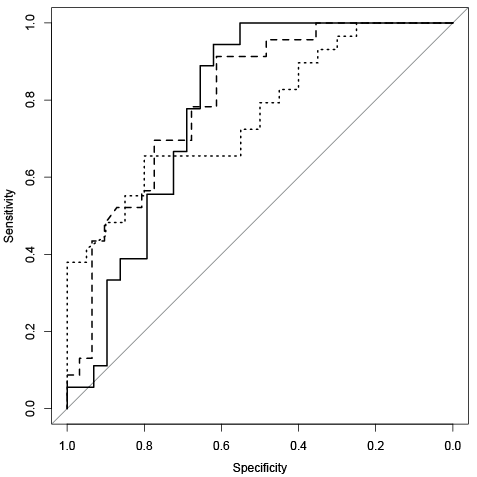
Fig. 2. Receiver operating characteristic curves of gait speed per country. Bold line represents UK (n = 47) (area under the curve (AUC) = 0.78 (95% confidence interval (CI) = 0.65–0.91)). Dotted line represents Belgium (n = 49) (AUC = 0.76 (95% CI = 0.63–0.90)). Dashed line represents The Netherlands (n = 54) (AUC = 0.80 (95% CI = 0.69–0.92)).
Bivariate association between community walking and candidate determinants
Table II shows the associations of candidate determinants with community walking. Sixteen variables (i.e. age, gender, H&Y, gait speed (dichotomized), UPDRS III, FR, FOGQ, FES, falls, MFI-total, MFI GF/PF, MFI reduced activity, MFI mental fatigue, MFI reduced motivation, HADS anxiety and HADS depression) were associated with community walking (p < 0.2) and were considered for multivariate logistic regression analysis. The total score of the MFI (MFI-total) showed collinearity with other dimensions of fatigue (Pearson r ranged from 0.78–0.88 with MFI GF/PF, MFI reduced activity and MFI reduced motivation) and was therefore not included in the multivariate logistic regression model. The remaining 15 variables were entered into the multivariate logistic regression model.
|
Table II. Bivariate association between community walking and determinants (n=153) |
|||||
|
Determinant |
β value |
SE |
OR (95% CI) |
Nagelkerke R2 |
Wald-test p-value |
|
Demography |
|||||
|
Age |
–0.04 |
0.02 |
0.96 (0.92–1.00) |
0.03 |
0.05a |
|
Gender |
0.63 |
0.33 |
1.87 (0.97–3.60) |
0.03 |
0.06a |
|
Marital status |
0.12 |
0.41 |
1.13 (0.51–2.53) |
0.01 |
0.77 |
|
PD characteristics |
|||||
|
Disease duration |
–0.01 |
0.03 |
0.99 (0.93–1.05) |
0.00 |
0.73 |
|
H&Y (on) |
–1.35 |
0.34 |
0.26 (0.13–0.51) |
0.16 |
0.00a |
|
Clinical data |
|||||
|
Gait speed (dichotomized) |
1.81 |
0.36 |
6.13 (3.01–12.48) |
0.23 |
0.00a |
|
UPDRS III |
–0.06 |
0.02 |
0.94 (0.91–0.98) |
0.12 |
0.00a |
|
FR |
0.08 |
0.02 |
1.08 (1.03–1.14) |
0.11 |
0.00a |
|
FOGQ |
–0.09 |
0.03 |
0.92 (0.86–0.98) |
0.06 |
0.01a |
|
FES |
0.05 |
0.01 |
1.05 (1.04–1.07) |
0.38 |
0.00a |
|
Falls (no falls/near-falls/falls) |
–0.31 |
0.18 |
0.74 (0.52–1.04) |
0.03 |
0.08a |
|
Brixton R |
–0.02 |
0.02 |
0.98 (0.95–1.01) |
0.01 |
0.26 |
|
Brixton S |
0.08 |
0.08 |
1.08 (0.93–1.25) |
0.01 |
0.30 |
|
MMSE |
0.03 |
0.09 |
1.03 (0.86–1.22) |
0.00 |
0.78 |
|
MFI-total |
–0.05 |
0.01 |
0.95 (0.93–0.97) |
0.19 |
0.00a |
|
MFI GF/PF |
–0.11 |
0.02 |
0.89 (0.85–0.94) |
0.21 |
0.00a |
|
MFI reduced activity |
–0.11 |
0.04 |
0.90 (0.84–0.96) |
0.09 |
0.00a |
|
MFI mental fatigue |
–0.09 |
0.04 |
0.91 (0.85–0.98) |
0.06 |
0.01a |
|
MFI reduced motivation |
–0.15 |
0.04 |
0.86 (0.79–0.93) |
0.12 |
0.00a |
|
HADS anxiety |
–0.07 |
0.04 |
0.93 (0.86–1.02) |
0.02 |
0.11a |
|
HADS depression |
–0.10 |
0.05 |
0.91 (0.83–1.00) |
0.04 |
0.04a |
|
ap < 0.2. Brixton R: Brixton Test Raw score; Brixton S: Brixton Test Scaled score; CI: confidence interval; FES: Falls Efficacy Scale; FOGQ: Freezing of Gait Questionnaire; FR: Functional Reach test; GF/PF: general and physical fatigue subscale combined; HADS anxiety: Hospital Anxiety and Depression Scale anxiety subscale; HADS depression: Hospital Anxiety and Depression Scale depression subscale; H&Y (on): Hoehn and Yahr stages during on; MFI: Multidimensional Fatigue Inventory; MMSE: Mini Mental State Examination; OR: odds ratio; PD: Parkinson’s disease; SE: standard error; UPDRS III (on): Unified Parkinson’s Disease Rating Scale part III during on. |
|||||
Multivariate logistic regression model for predicting community walking
A multivariate logistic regression model was derived (Table III) containing gait speed (dichotomized) (β = 1.46, standard error (SE) 0.41, p = 0.00, odds ratio (OR) 4.32) and FES (β = 0.05, SE 0.01, p = 0.00, OR 1.05). The explained variance was 45% and the Hosmer-Lemeshow test for goodness of fit was not statistically significant (p = 0.26). Cooks distances (mean 0.02) and Leverage values (mean 0.02) did not show influential cases. ROC analysis showed good diagnostic accuracy for the multivariate model in predicting capability for community walking with an AUC of 0.85 (95% CI 0.78–0.91) (Fig. 1). A cut-off point of 0.45 for predicted probability (sensitivity 0.81, specificity 0.80) correctly predicted 78% of the patients as community walkers (PPV) and 83% were correctly classified as non-community walkers (NPV). Compared with gait speed alone, the AUC of the multivariate model was significantly larger (p = 0.03).
|
Table III. Multivariate logistic regression model for predicting community walking (n=150) |
||||
|
Variables in the modela |
β value |
SE |
Wald-test p-value |
OR (95% CI) |
|
Constant |
–4.70 |
0.82 |
0.00 |
0.01 |
|
Gait speed (dichotomized) |
1.46 |
0.41 |
0.00 |
4.32 (1.93–9.69) |
|
FES |
0.05 |
0.01 |
0.00 |
1.05 (1.03–1.07) |
|
aVariables excluded after stepwise backward regression: age, gender, Hoehn and Yahr Stages, Unified Parkinson’s Disease Rating Scale part III, Functional Reach test, Freezing of Gait Questionnaire, falls, Multidimensional Fatigue Inventory (MFI) general and physical subscale combined, MFI reduced activity, MFI mental fatigue, MFI reduced motivation, Hospital Anxiety and Depression Scale (HADS) anxiety and HADS depression. CI: confidence interval; FES: Falls Efficacy Scale; OR: odds ratio; SE: standard error. Nagelkerke R2 = 0.45. |
||||
Discussion
The present study shows that a cut-off value of 0.88 m/s for gait speed correctly predicted 70% of the patients as community walkers. In addition, the discriminative ability of walking speed was not affected by the country where patients were tested (i.e. UK, Belgium or The Netherlands). The multivariate logistic regression model indicates that fear of falling has an added value in predicting community walking and suggests that patients who experience less fear of falling are more likely to be community walkers.
The determined cut-off point for gait speed in our study is partly in line with previous findings. Studies in patients with stroke (6, 8–10) found thresholds varying from 0.66 m/s (8) to 1.32 m/s (10) to be predictive for community walking. However, it is difficult to directly compare results between studies. Gait disorders in patients with PD are likely to fluctuate in time (on or off phase), whereas gait disorders in patients with stroke tend to be more stable in time. Furthermore, different definitions for community walking were used to classify patients as community walkers or non-community walkers. For example, the cut-off point of 0.88 m/s to classify community walking in our study (i.e. patients who walked around outside and crossed roads on their own) may be too low to predict community walking as defined by Lord and colleagues (6) (i.e. independent mobility outside the home, which includes the ability to confidently negotiate uneven terrain, private venues, shopping centres and other public venues). Lord et al. (6) considered a gait speed ranging from 0.82–1.14 m/s to classify patients as “limited” community walkers (i.e. able to walk in the immediate outside environment without physical assistance or supervision).
Although temporal factors are one aspect of community walking, ambient conditions, terrain characteristics and crowded and cluttered environments have been reported to negatively influence community walking in patients with PD (1). These factors may differ between countries and cultures and may influence the cut-off point for gait speed in predicting community walking. However, the cut-off value of 0.88 m/s in our study was robust across the 3 different countries, suggesting that geographical and cultural factors did not differ significantly between the UK, Belgium and The Netherlands.
One qualitative study (1) suggested that anxiety (quoted by patients as “feeling hurried, examined, stigmatized or judged”) and fatigue negatively influenced community walking. In our study, anxiety assessed with the HADS anxiety subscale, was not significantly associated with community walking; however, fear of falling was associated with community walking and significantly contributed to the multivariate prediction model. Although physical and mental aspects of fatigue had statistically significant bivariate associations with community walking, none of these dimensions contributed significantly to the multivariate prediction model. These findings are partly in line with previous research that suggested an inverse relationship between fatigue and physical activity (31); however, the total amount of explained variance of physical activity by fatigue in that study was small, suggesting that fatigue was only a minor factor in the complex of behavioural aspects that determine physical activity in patients with PD.
There are some study limitations that should be acknowledged. First, this study was part of a randomized clinical trial. Patients were excluded if they had cognitive impairments or comorbid conditions that interfered with participation in cueing training. Therefore, the external validity of the multivariate logistic regression model is limited and requires external validation in other samples. Secondly, some candidate factors that may be predictive for community walking; for example, walking endurance, were not investigated in our study. However, a previous study indicated that walking endurance might be less important to community walking than other factors (32). Thirdly, through lack of a uniform definition for community walking, we used item 1 (“Did you walk around outside?”) and item 5 (“Did you cross roads?”) of the NEAI to classify patients as community walker or non-community walker; however, these items may not reflect all relevant aspects of community walking such as negotiating shopping centres and other public venues. This may have resulted in an overestimation of sensitivity and PPVs for both gait speed and the multivariate prediction model. Furthermore, this may explain why freezing of gait did not significantly contribute to the multivariate prediction model as turning and negotiating obstacles, known triggers for freezing (33), are probably more problematic in cluttered and crowded environments, such as shopping centres. Fourthly, the medical management of this sample was aimed at optimizing dopamine levels to maintain smooth motor output and all measurements were performed in the on phase. Therefore, we were not able to investigate the impact of fluctuations in the efficacy of medication on community walking. Finally, all tests were conducted in the patients’ home, which may not reflect all relevant environmental aspects for community walking. The development of tests that take into account the context in which assessments take place, may be helpful in identifying patients who have difficulty generalizing the capacity of gait in a simple environment to performance in a complex environment (13).
In conclusion, the present study shows that gait speed is a valid and geographically independent measurement to predict community walking in patients with PD. However, it is recommended that the evaluation of community walking includes an assessment of fear of falling. A rehabilitation programme targeting gait speed and fear of falling may facilitate community walking in patients with PD. Although our multivariate prediction model showed good fit, research incorporating environmental aspects of community walking is needed to refine and further validate the prediction model.
AcknowledgEments
This research project was supported by a grant from the European Commission (QLK6-CT-2001-00120; Rehabilitation in Parkinson’s Disease: Strategies for Cueing).
The authors would like to thank the project members of the “Rescue” trial for their contribution.
References
