Birgit Juul-Kristensen, PhD1, Brian Clausen, Msc1, Inge Ris, MR1, Rikke Vikær Jensen, BSc1, Rasmus Fischer Steffensen, BSc1, Shadi Samir Chreiteh, MSc1, Marie Birk Jørgensen, PhD2 and Karen Søgaard, PhD1
From the 1Research Unit for Musculoskeletal Function and Physiotherapy, Institute of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense and 2National Research Centre for the Working Environment, Copenhagen, Denmark
OBJECTIVE: To investigate neck muscle activity and postural control in patients with whiplash-associated disorder compared with healthy controls.
DESIGN: Cross-sectional study with convenience sampling.
SUBJECTS: Ten females with whiplash-associated disorder (age 37.7 years (21–58), neck pain > 2 years and Neck Disability Index (NDI) > 10) and 10 healthy female controls (age 35.9 years (21–53), NDI < 6).
METHODS: Surface electromyography measured muscle activity of the anterior scalene, sternocleidomastoid, neck extensors and upper trapezius muscles, expressed as mean relative activity related to maximum voluntary electromyography (%MVE). On a force plate, 3 balance tasks (Romberg stance with open and closed eyes, 1-legged stance) and a perturbation task with sudden unloading, were performed. The total area, areas from slow and fast components, and range of displacements were calculated from decomposed centre of pressure anterior-posterior and medial-lateral signals.
RESULTS: During balance tasks with closed eyes and one-legged stance, the relative mean activity of all 4 muscles was significantly increased in whiplash-associated disorder compared with healthy controls. Postural sway was also significantly increased.
CONCLUSION: Increased neck muscle activity and increased postural sway during simple balance tasks indicate disturbed sensory feedback patterns in people with whiplash-associated disorder, which may have negative consequences when performing daily activities.
Key words: whiplash; balance; neck muscle activity; postural sway; force plate.
J Rehab Med 2013; 45: 00–00
Guarantor’s address: Birgit Juul-Kristensen, Research Unit for Musculoskeletal Function and Physiotherapy, Institute of Sports Science and Clinical Biomechanics, University of Southern Denmark, Campusvej 55, DK-5230 Odense M, Denmark. E-mail: bjuul-kristensen@health.sdu.dk
Accepted November 6, 2012, Epub ahead of print Mar 6, 2013
Introduction
Whiplash trauma is one of the most common injuries in motor vehicle collisions. However, it is also one of the most challenging syndromes to diagnose, due to the lack of “gold standard” clinical tests and diagnostic tools (1). In similar industrial countries as Denmark the annual incidence of acute whiplash injuries varies from 0.8 to 4.2 per 1,000 inhabitants, depending on the population studied, type of accident, and inclusion and exclusion criteria (2). Furthermore, the total number of subjects seeking medical attention for whiplash-associated disorder (WAD) in Denmark has increased over the past 30 years, with associated large individual and societal costs (3). The aetiology of WAD is often based on a somatic approach, but recovery seems to be multifactorial (4).
Subjects with acute and chronic WAD are described as having cervical spine problems, such as pain, changes in muscle function/activity, abnormal cervical spine range of motion, in addition to alterations in postural control/balance (5–7). Recent studies of postural control during static balance found an increased area of the slow sway component (rambling area) in subjects with WAD compared with controls (8). An increased area of the slow sway components may reflect disturbance in sensory feedback and processing (8, 9). However, the area of the slow sway components has not been studied in subjects with WAD during one-legged stance, a posture performed during functional activities such as reaching, dressing and walking.
In subjects with WAD with chronic neck pain (defined as a minimum duration of 3 months) increased muscle activity was found in the superficial cervical flexor muscles, in addition to reduced activity of the deep cervical muscles during neck-arm tasks (10–13). Furthermore, reduced ability to relax during rest after a repetitive arm task (11), and delayed activity onset of the deep and superficial cervical flexor muscles were seen during a sudden arm lift and lowering perturbation task (14). Recent studies have shown some positive association between pain intensity and muscle activity of the superficial and deep cervical neck flexors during neck flexion (15, 16). It has been anticipated that the increased muscle activity observed in WAD may be a compensation strategy to minimize activity in the painful and possibly inhibited/weak deep cervical muscles, but with unknown long-term consequences. Previous studies address measures of muscle activity during voluntary neck-arm tasks. However, many daily activities commonly involve activities with low muscle force or sudden perturbations. Thus, it remains to be studied whether the increased muscle activity is evident during functional activities, such as normal balance tasks (open or closed eyes, one-legged stance) or during arm perturbation.
The aim of this study was to investigate neck muscle activity and postural sway simultaneously in a patient group with WAD compared with a healthy control group during static balance and arm perturbation.
Methods
Design
In this cross-sectional study with convenience sampling comparing WAD and age-matched control subjects, data were obtained during two sessions: (i) the screening session; and (ii) the testing session, conducted on the same day for controls. For subjects with WAD these sessions were conducted on two separate days (day 1 screening session of clinical tests and completing questionnaires, day 2 testing session of EMG and postural sway). The screening session consisted of a standardized physical examination, performed by the same trained physiotherapists, and after this the patients completed a questionnaire (approximately 30 min). On the second day the patients underwent the testing session (approximately 1.5 h). Analysts were blinded to the health status of the subject, being WAD or controls.
Procedures
Three balance tasks (Romberg stance with open and closed eyes, and a one-legged stance) and an arm perturbation task (sudden unloading) were performed. The procedure was standardized and rehearsed, and the same examiner instructed the subjects in an undisturbed environment. For each of the postural sway tests the subject was placed in the centre of the room, facing a dot on the wall at eye-height at a distance of approximately 2.5 m away (Appendix I).
Since the Romberg stance with open eyes was anticipated to be very easy for the subjects, this test was performed once for 30 s to familiarize with the test situation as also used previously and for comparison with these studies (17, 18). The other two balance tests, Romberg stance tests with closed eyes, and the one-legged stance, lasting 30 s each, were repeated in 3 sets with the tests in the same order. Subsequently, 3 repetitions of the perturbation test were performed. Custom-made equipment, consisting of a rod with an electromagnetic-attached water-filled weight, corresponding to 3% of the subject’s body weight, was used. The rod was gripped with two hands at shoulder height and width, and held with fully extended arms in the horizontal position. The electromagnet on the rod automatically dropped the weight, without warning, within 5–20 s after the test start. A trigger was synchronized to initiate data collection from the test start, including 5 s of post-drop recording, which was assumed to be enough time for body repositioning (19). In all bipedal stance tests, the subject was standing with feet together (heel-to-heel, toe-to-toe). The test was terminated if 3 failures occurred during the same test (i.e. if the subject talked, fell, or moved from the initial position).
Study population
Subjects with WAD were recruited through pamphlets and posters from local physiotherapy and chiropractor clinics, in addition to personal networks, while control subjects were recruited from amongst the university staff, students and personal networks, age-matched to those with WAD.
Inclusion criteria for WAD. Females between 18 and 60 years of age, a history of chronic neck pain of a minimum of 2 years following a whiplash trauma, and Neck Disability Index (NDI) above 10 (range 0–50, where 0 = best and 50 = worst) (20). The medical and rheumatology diagnostic evaluation had to be completed before inclusion. Numeric rating scale (NRS) (range 0–10, where 0 = best and 10 = worst) (21) and Short-Form 36 (SF-36, physical and mental component scores (range 0–100, where 0 = worst and 100 = best) were further used to describe the subjects with respect to pain and function (22). All subjects had to be able to read and understand Danish.
Inclusion criteria for matched controls. Females, each matched to one of the subjects with WAD by age ± 5 years, and an NDI of a maximum of 5. All subjects had to be able to read and understand Danish.
Exclusion criteria for both groups. Exclusion criteria were: 4 positive tests for brachial neuropathy, as tested with the Spurling-, Traction-, Valsalva- and Upper Limb Tension test (23); intrusive illnesses, such as cardiovascular disease, life-threatening and neurological diseases; pregnancy; injury/pain in the hip, knee or ankle, that could possibly influence postural control; being in progressive physical or medical treatment; being in an unstable social or work situation; or waiting for the results of unresolved insurance claim.
In total, 32 subjects were evaluated, of whom 9 subjects with WAD were excluded due to a low NDI score, and 1 due to pregnancy, and 2 control subjects were excluded due to a mismatch of age. A total of 10 female WAD (mean age 37.7 years, standard deviation (SD) 13.9 years) and 10 age matched female control subjects (mean age 35.9 years, SD 12.5 years) were included.
The Scientific Ethics Committee of Southern Denmark was notified of the study, and the study conformed to the Declaration of Helsinki 2008 (24), by fulfilling all general ethical recommendations. All subjects received information about the purpose and content of the project and gave their oral and written consent to participate, with the option to drop out of the project at any time. There was no risk of any harm to the subjects, except for general soreness after strength measurements.
Electromyography
Surface electromyography (EMG) was registered on the most affected side for WAD or dominant side if both sides were equally affected, and on the dominant side for controls, for the following 4 muscles: anterior scalene (AS), sternocleidomastoid (SCM), neck extensors (NE) and the upper part of the trapezius muscle (UT).
Bipolar electrodes (Ag/AgCl, Ambu Blue Sensor, N-00-S/25, Ballerup, Denmark), placed with an inter-electrode distance of 2 cm, were used and placed according to previously described positions, detailed below. For AS, the electrodes were placed perpendicular to the course of the SCM at the level of the lower third marking of SCM, at least 1 cm lateral to the SCM, palpated during resisted neck flexion (25). For SCM, the electrodes were placed one-third cranially to the distance between the sternal notch and the mastoid process (25), and for NE the electrodes were placed at the level of the cervical vertebra C4, on the most bulky part of the muscle during resisted neck extension (26, 27). Electrodes on UT were placed 20% medial to the halfway point between the medial border of the acromion and the cervical vertebra C7 (28). Reference electrodes were placed on the spinous processes of C7 and the bony part of the acromion.
The EMG signal was sampled at 2000 Hz (Expansion ADC12, 500k Hz, CED 3001) on a computer via laboratory interface (CED 1401mkII, Spike 2 software v. 6.02, 2006, Cambridge Electronic Design, Cambridge, UK), amplified (gain 400), and band-pass filtered with a Butterworth filter with cut-off frequencies at 10–400 Hz.
Maximum EMG
Three isometric maximum voluntary contractions (MVC) used for EMG normalization of maximum voluntary EMG (MVE) were performed during sitting for the following positions and in the following order: bi-lateral shoulder elevation, neck extension (isolated backward push with the neck) and neck flexion (isolated forward push with the head, with a slightly retracted chin). The MVC were conducted using an adjustable dynamometer (Load cell, KIS-2, 2kN, Vishay Nobel, Vishay Transducers Systems, Chelton, USA), mounted on a custom-made chair. The subjects were secured to the chair by straps around their waist and chest (Fig. 1).
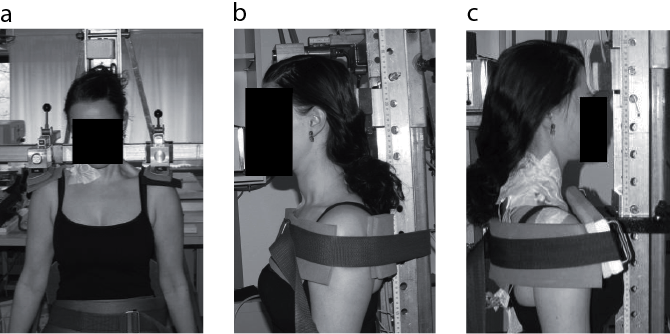
Fig. 1. Experimental set-up during maximum isometric voluntary contractions used for EMG normalization of maximum voluntary EMG (MVE) during sitting. The photographs show settings for (a) shoulder elevation, (b) neck extension and (c) neck flexion.
For MVE, the mean root-mean-square (RMS) values were calculated using a 1 s moving window, incremented in 100 ms steps, while for balance tasks the mean RMS values were calculated using a 1 s moving window, incremented in 1 s steps. For perturbation, the level at steady state in the final second preceding perturbation and the second second after perturbation were calculated as the mean RMS value, using a 100 ms moving window, incremented in 100 ms steps. During sampling of EMG (MVE and the balance tasks), a manual trigger was used for marking event start and end, whereas marking of the drop time during the perturbation test was performed with an electric trigger.
For each patient, RMS values in all 4 tests were normalized for each muscle to the relevant MVE test, calculated as the relative (%MVE) EMG level, and in perturbation the difference between the level before and after (%MVE), the peak-to-peak amplitude (µV) and time (ms) from drop to maximum peak for each muscle were further calculated. In addition, the ratio between each of the muscles activity levels was calculated.
Maximum torques were calculated for shoulder elevation (Nm, MVC × horizontal distance from the spinous processes of C7 to the dynamometer, 1 cm medial to the lateral border of the acromion), for neck extension (Nm, MVC × vertical distance from the spinous processes of C7 to the dynamometer (flat part of the back of the head)), and for neck flexion (Nm, MVC × vertical distance from the spinous processes of C7 to the dynamometer (on the flat part of the forehead)). The mean of 3 MVCs within each of the 3 different torques, and the proportion of neck extension/neck flexion torque were calculated.
Postural sway
The measurements of postural sway in Romberg (OE and CE) and one-legged stance, in addition to the perturbation test, were obtained by using a 6-degrees of freedom force platform (AMTI – force and motion OR6 – 7 – 1000, Advanced Technologies, MA, USA) with an amplifier (AMTI – MSA – 6, Advanced Technologies, MA, USA). The ground reaction forces were recorded at a sampling frequency of 125 Hz using custom-made software (Labview v. 2009, National Instruments, TX, USA). For the 3 balance tasks the centre of pressure (COP), consisting of the anterior-posterior (AP) and medio-lateral (ML) trajectories, was calculated and decomposed into rambling (the slow component of sway) and trembling (the fast component). The rambling and trembling decomposition is based on determining the instant equilibrium points (IEP). These IEPs are estimated by recording the COP positions at the instances when the horizontal ground reaction forces equal zero (29). The rambling component is then obtained by interpolating the consecutive IEP positions with a cubic spline function. The trembling component is the deviation of COP from the approximated rambling component. Subsequently, the 95% confidence ellipse areas (CEA) were calculated for the COP, and for the rambling and trembling components of the COP. The 95% CEA is the area of the 95% bivariate ellipse, entailing approximately 95% of the points of the COP path (Fig. 2) (29).
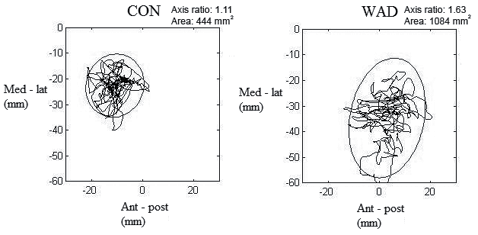
Fig. 2. An example of calculated postural sway area of healthy controls (CON) and subjects with whiplash-associated disorder (WAD), with the 95% confidence ellipse area (surrounding the trajectories). Ant-post: anterior-posterior direction; Med-Lat: medio-lateral direction.
During the 3 balance tasks, 6 parameters were calculated: (i) total sway area (mm2, 95% confidence ellipse area), (ii) sway area of rambling (slow components, mm2, 95% confidence ellipse area), (iii) sway area of trembling (fast components, mm2, 95% confidence ellipse area), (iv) proportion of rambling to trembling, (v) range of anterior-posterior displacement (mm, AP), (vi) and medial-lateral displacement (mm, ML). An example is shown of sway areas for a control and a subject with WAD (Fig. 2).
During the perturbation test, the following two parameters were calculated: (i) range of AP displacement from perturbation to maximum posterior displacement within the first second (mm), and (ii) time from perturbation to equilibrium using the AP displacement (s).
Statistical analysis
The distribution of data was tested for normality with the Kolmogorov-Smirnov Z-test, and between-group differences in demographic and self-reported variables were tested by a Student’s t-test (two-tailed). In a preliminary analysis of mean values of all 3 conditions a general linear regression model (GLM) was performed for all 6 sway parameters, with status and condition (OE, CE, OS) and interaction (status × condition) as fixed factors, and age and body mass index (BMI) as covariates. Since there were no significant interaction effects, but a significant effect of condition and status in all 6 sway parameters, these were tested separately for each of the 3 conditions.
Since only one trial was performed with eyes open, a GLM was used for this test, while a linear mixed regression model was used for eyes closed, one-legged stance and the perturbation tests, where 3 trials were performed. In all statistical models, EMG and sway variables (one at a time) were dependent factors, health status (WAD/controls) was a fixed factor, while age and BMI were used as covariates. In the linear mixed model, subject number and trial number were further used as random subject and repeated factors, respectively. Furthermore, correlations with Pearson’s r between self-reported measures (pain, disability, SF-36) and muscle activity were calculated.
Level of significance was defined as p < 0.05. All statistical analyses were performed using the Statistical Package for Social Sciences (PASW, version 18.0.0, IBM, NY, USA, released on 30 July 2009).
Results
The groups were comparable in terms of demographic parameters. In the WAD group, NRS scores were significantly higher (p < 0.001), and both dimensions of SF-36 were significantly lower than in the control group (p-values ≤ 0.004) (Table I). MVC force was significantly lower in shoulder elevation (p = 0.001), with a corresponding lower absolute EMG level (uV) in AS and UT (p = 0.017; p = 0.002) in WAD (Table II). In total, 3 subjects with WAD each failed one test, equally distributed between closed eyes, one-legged stance and the perturbation test.
|
Table I. Demographic variables, pain ratings (NRS), neck disability (NDI), and Short-Form 36 (SF-36), Physical Component Scale and Mental Health Component Scale, mean (SD), for subjects with whiplash-associated disorders (WAD) and healthy controls (CON) |
|||
|
WAD Mean (SD) |
CON Mean (SD) |
p-value |
|
|
Age, years |
37.70 (13.64) |
35.90 (12.45) |
0.761 |
|
Body weight, kg |
72.92 (22.22) |
63.88 (10.06) |
0.263 |
|
Body mass index, kg/m2 |
25.36 (8.86) |
22.88 (3.17) |
0.422 |
|
Arm length, cm |
65.05 (5.63) |
62.45 (3.15) |
0.223 |
|
Neck Disability Index, NDI, 0–50 |
20.60 (7.21) |
0.80 (0.63) |
< 0.001* |
|
Numeric rating scale, NRS, 0–10 |
4.73 (1.99) |
0.10 (0.23) |
< 0.001* |
|
SF-36 Physical Component Scale, 0–100 |
37.59 (9.10) |
57.58 (1.31) |
< 0.001* |
|
SF-36 Mental Health Component Scale, 0–100 |
41.23 (13.58) |
57.38 (3.40) |
0.004* |
|
*p < 0.05. SD: standard deviation. |
|||
|
Table II. Maximum torque (Nm) and absolute electromyography (EMG) levels (uV) for all 4 muscles (uV), mean (standard deviation; SD), for subjects with whiplash-associated disorders (WAD) and healthy controls (CON). AS (anterior scalene), SCM (sternocleidomastoid), NE (neck extensors) and UT (upper trapezius) |
|||
|
WAD Mean (SD) |
CON Mean (SD) |
p-value |
|
|
Maximum neck flexion force, Nm |
8.19 (3.84) |
11.09 (5.25) |
0.222 |
|
Maximum neck extension force, Nm |
14.03 (8.75) |
20.49 (7.00) |
0.112 |
|
Maximum shoulder elevation force, Nm |
42.48 (22.70) |
84.72 (20.32) |
0.001* |
|
Proportion of neck extension/neck flexion force |
1.72 (0.80) |
2.10 (0.73) |
0.318 |
|
Maximum EMG AS, µV |
172.52 (77.72) |
309.86 (137.82) |
0.017* |
|
Maximum EMG SCM, µV |
204.53 (141.61) |
278.35 (66.78) |
0.181 |
|
Maximum EMG NE, µV |
92.95 (67.12) |
144.99 (55.59) |
0.087 |
|
Maximum EMG UT, µV |
387.93 (175.80) |
739.87 (247.34) |
0.002* |
|
*p < 0.05. SD: standard deviation. |
|||
During 2 of the 3 balance tasks, closed eyes and one-legged stance, the relative activity for all muscles was significantly higher in WAD compared with controls (mean % MVE for 2 tests in AS: 15.6 vs 2.3; SCM: 14.2 vs 3.8; NE: 20.8 vs 7.1; UT: 8.0 vs 3.5), with p-values from 0.013 to ≤ 0.001 (Fig. 3).
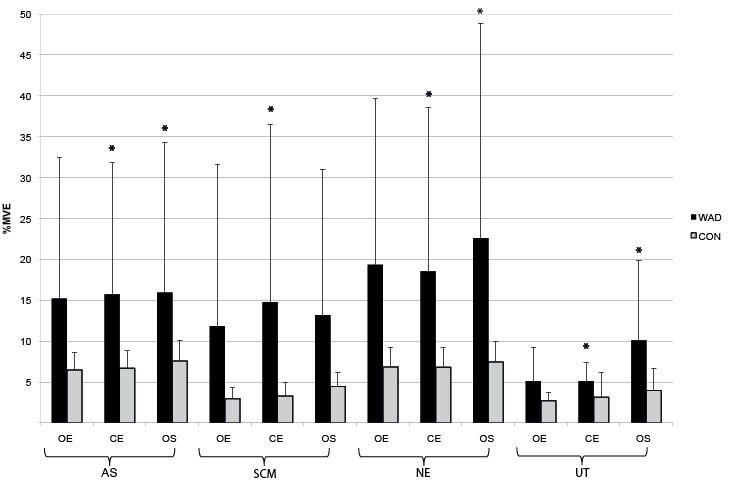
Fig. 3. Neck muscle activity during balance tasks, mean (standard deviation; SD), in %MVE (maximum voluntary electromyography), for all 4 muscles for subjects with whiplash-associated disorders (WAD) and healthy controls (CON). AS (anterior scalene), SCM (sternocleidomastoid), NE (neck extensors), and UT (upper trapezius). OE (open eyes), CE (closed eyes), OS (1-legged stance).
During perturbation also, a significantly higher muscle activity was seen in WAD, both before and after and in the difference between before and after perturbation (mean %MVE for all 4 muscles before perturbation 31.1 (WAD) vs 12.3 (controls); after perturbation 25.0 (WAD) vs 10.5 (controls); difference 10.8 (WAD) vs. 8.4 (controls)), respectively, with p-values from 0.034 to ≤ 0.001 (Fig. 4). The ratios of the muscle activity were significantly lower in subjects with WAD between Scalenus/neck-extensors and Scalenus/trapezius during one-legged stance (0.84 (WAD) vs 1.07 (controls) p = 0.020; 1.75 (WAD) vs 2.44 (controls) p = 0.042), respectively, and significantly higher in subjects with WAD between neck extensors/trapezius before perturbation (1.11 (WAD) vs 0.86 (controls) p = 0.042) (not shown in Figs).
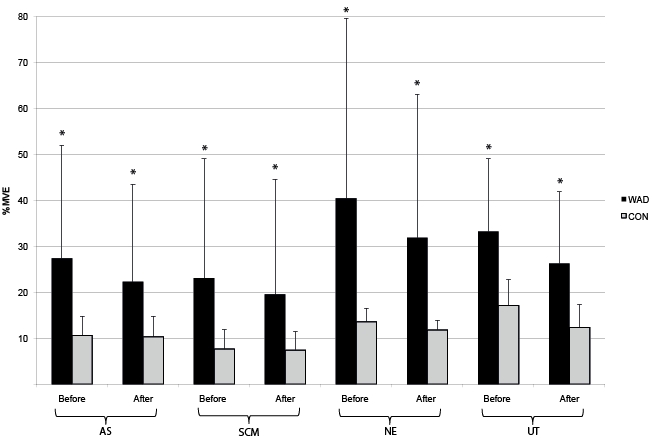
Fig. 4. Neck muscle activity during perturbation test, mean (standard deviation; SD), in %MVE (maximum voluntary electromyography) for all 4 muscles for subjects with whiplash-associated disorders (WAD) and healthy controls (CON). AS (anterior scalene), SCM (sternocleidomastoid), NE (neck extensors), and UT (upper trapezius).
Significantly negative correlations were seen between physical component scores of SF-36 and trapezius muscle activity before and after perturbation (r = –0.578, p = 0.010; r = –0.498, p = 0.030), corresponding to a lower SF-36 score with a higher muscle activity level.
There were no between-group differences in peak-to-peak muscle activity after perturbation (mean of all muscles: 458.9 vs 453.2 µV) (not shown in Figures), and in estimated time from perturbation to peak muscle activity (mean of all muscles: 221.3 vs 236.1 ms) (not shown in Figs).
Subjects with WAD had significantly higher total sway area and rambling area compared with controls during closed eyes and 1-legged stance (mean areas for CE and OS: total sway 1302 vs 782 mm2, rambling 866 vs 486 mm2), significantly larger trembling area during open eyes and closed eyes (mean areas for CE and OS: trembling 137 vs 83 mm2), and significantly larger range of displacement in closed eyes and 1-legged stance (mean displacement for CE and OS: AP 45.4 vs 36.1 mm, ML 40.8 vs 32.5 mm), with p-values between 0.048 and ≤ 0.001 (Table III). There was neither a significant difference between subjects with WAD and controls in AP range of displacement (mm) after perturbation (46.00 vs. 46.32 mm), nor in estimated time (s) from perturbation to equilibrium (0.31 vs 0.29 s).
|
Table III. Postural sway for whiplash-associated disorders (WAD) and controls (CON), during the following balance tasks: OE (open eyes), CE (closed eyes), OS (1-legged stance), and PE (perturbation) |
|||
|
WAD Mean (SD) |
CON Mean (SD) |
p-value |
|
|
OE, total area, mm2 |
625.20 (612.69) |
401.50 (327.49) |
0.322 |
|
OE, area of rambling, mm2 |
473.90 (528.11) |
299.70 (279.94) |
0.369 |
|
OE, area of trembling, mm2 |
72.70 (49.93) |
45.20 (25.78) |
0.048* |
|
OE, proportion rambling/trembling |
7.35 (5.67) |
9.03 (9.90) |
0.504 |
|
OE, range anterior-posterior, mm |
27.69 (13.01) |
19.63 (9.33) |
0.129 |
|
OE, range medio-lateral, mm |
28.50 (12.18) |
25.21 (6.49) |
0.360 |
|
CE, total area, mm2 |
1,186.37 (608.97) |
653.50 (285.96) |
< 0.001* |
|
CE, area of rambling, mm2 |
774.48 (458.35) |
418.77 (179.47) |
< 0.001* |
|
CE, area of trembling, mm2 |
206.15 (115.38) |
120.83 (81.19) |
< 0.001* |
|
CE, proportion rambling/trembling |
4.39 (2.18) |
6.10 (7.92) |
0.260 |
|
CE, range anterior-posterior, mm |
39.89 (9.47) |
29.23 (8.27) |
< 0.001* |
|
CE, range medio-lateral, mm |
42.67 (10.82) |
31.54 (9.10) |
< 0.001* |
|
OS, total area, mm2 |
1,276.56 (387.30) |
909.87 (361.23) |
0.001* |
|
OS, area of rambling, mm2 |
834.78 (318.32) |
553.10 (270.31) |
0.001* |
|
OS, area of trembling, mm2 |
240.85 (79.03) |
196.90 (81.17) |
0.084 |
|
OS, proportion/rambling trembling |
3.93 (1.47) |
3.18 (1.67) |
0.089 |
|
OS, range anterior-posterior, mm |
48.93 (9.90) |
43.02 (12.37) |
0.039* |
|
OS, range medio-lateral, mm |
37.29 (4.04) |
33.50 (5.92) |
0.004* |
|
PE, range anterior-posterior, mm |
46.00 (9.98) |
46.32 (6.63) |
0.715 |
|
PE, time until equilibrium, s |
0.31 (0.10) |
0.29 (0.08) |
0.296 |
|
*p < 0.05 due to health status. SD: standard deviation. |
|||
Discussion
Compared with controls, subjects with WAD had significantly higher relative neck muscle activity during the two most challenging balance tasks, closed eyes and 1-legged stance, and before and after perturbation. A different activity pattern was seen, with significantly lower muscle activity in subjects with WAD in the Scalenus in muscles ratios and a corresponding higher activity in the neck extensor and trapezius muscles. Furthermore, postural sway was significantly increased; i.e. larger sway area, in addition to larger range of displacement during the same two balance tasks.
Our results for muscle activation are in line with other results for WAD and subjects with neck pain lasting a minimum of 3 months (5, 11, 13), and subjects with chronic neck pain of 1–9 years’ duration (10). The current study found increased neck-shoulder activity in tasks that do not explicitly involve active movements of the neck or arms (in simple static balance tasks). Thus, the current data supplement the previous studies, reporting increased neck-shoulder muscle activity only during more physically dynamic neck-arm tasks, such as repetitive arm tasks, neck-arm tasks, and neck flexion tasks (10, 11, 13). A contribution of this study is that the high level of superficial neck muscle activity in subjects with WAD during simple balance tasks indicates a generally high activity level during normal daily activities with a low force demand. In addition, the activation pattern differed, since the least superficial flexors were less active, and the most superficial muscles most active in WAD compared with controls, during balance, perturbation and maximal contraction. It could be speculated that this high muscle activity and changed activation pattern have been present since the initial acute trauma; i.e. for at least 2 years, with associated functional implications.
Surprisingly, the perturbation task, in which the subjects experienced a sudden unloading of a weight corresponding to 3% of their body weight, also showed clear differences between WAD and CON, with higher muscle activity in WAD both before and after perturbation, and also in the difference between before and after the perturbation task, in all 4 muscles. Similarly, a study on back patients found increased trunk stiffness and trunk muscle activity in patients with recurrent low back pain (LBP) compared with healthy controls, which was interpreted as a protective mechanism of the spinal structures. It was hypothesized that this mechanism would have long-term consequences for spinal health and LBP recurrence due to compromised trunk dynamics (30). Since we also found significant correlations of a poorer physical component score of SF-36 and increased trapezius muscle activity before and after perturbation, this mechanism may also be proposed for the current patient group with chronic neck pain lasting at least 2 years.
In the perturbation task, a larger maximum peak-to-peak muscle activity response in WAD was expected, since previous studies of sudden unloading have shown such an increased response in lumbar muscles after unloading (19). Also, an increased muscle response time to peak activation in muscle activity was expected in WAD, since previous studies have shown delayed onset of muscle activity in WAD during perturbation tasks, in both deep and superficial neck flexor muscles (14, 31), as well as in the deep and superficial lumbar muscles of LBP patients (32–34). Surprisingly, such response difference could not be verified in the current data of the patients with neck pain. However, previous studies differed from ours, in using loading perturbation in contrast to our unloading, which may explain the difference in findings. Postural sway, in line with previous studies, was increased in the closed eyes condition, i.e. increased total area and range of displacement (AP and ML) in WAD compared with control subjects, with no group difference in the least challenging static balance task; i.e. with eyes open (6, 34–37).
Collectively, the current study of WAD shows the combination of increased muscle activity and sway, but no changes in muscle response activity. The increased neck muscle activity may be due to increased pain, mechanical impairment or an increased sensitivity of the muscle spindles. This increased muscle activity in the neck, where the density of muscle spindles is high, may suppress normal proprioceptive inputs, explaining the increased threshold for detection and adjustment of postural sway.
The interesting new knowledge coming from the current study is the data on the rambling area (slow components in postural sway), which were significantly increased in subjects with WAD, during both closed eyes and open eyes one-legged stance. This has not been identified previously in an open eyes task, though subjects with WAD in one study and cleaners with neck pain in another study had increased rambling area in Romberg with closed eyes (8, 17). The current deficits indicate balance difficulties in WAD both with and without vision during challenging balance tasks, as they perform their daily activities (walking in darkness, stair climbing, etc.). An increased area of slow sway components can be interpreted as noise around the central feedback processing, when perceiving sensory inputs for locating centre of mass (COM) (9, 38, 39). This may be a pain-related mechanism, since the increased rambling area was also seen in subjects without WAD, but with chronic neck pain (17).
The area of the fast components, the trembling area, was also significantly increased in WAD during tests with both open and closed eyes. These fast components are mostly ascribed to the normal COM control, based on the normal active biomechanical system acting at the ankle, which refers to the mechanical properties of muscles and joints, i.e. the myotatic reflex-component (29, 40, 41). If the trembling component normally corrects COM through proprioception inputs, the current increased trembling area may reflect a whiplash-related central impairment of proprioception inputs from the muscles. However, at present, the mechanism for the increased trembling area remains unknown.
There was no between-group difference in anterior-posterior peak-to-peak distance in sudden perturbation, in contrast to an earlier study using a similar perturbation test and procedure as the current study (6). The weight attached to the perturbation rod was the same (3% of the patient’s body weight). However, in the current study only 3 consecutive trials were performed, and this may have played a role in reducing development of fatigue, compared with using 6 tests (6). Also, time until equilibrium after perturbation was unaffected in WAD. Of note is that, despite no postural sway differences in perturbation, the current subjects with WAD maintained a constantly higher relative muscle activity both before and after the load drop compared with control subjects.
Generally, one of the challenges when comparing WAD with control subjects is that high pain levels among subjects with WAD may influence performance in the MVC tests used for normalization of muscle activity. Therefore, the relative EMG activity in WAD and control subjects should be compared with caution. Moreover, as in the current study, the variability is very often larger in patients than in healthy controls, reflecting the normal variation in the patients’ day-to-day condition.
A matching procedure by age compared with weight could be subject to bias, due to the known close relationship between weight and stability in the ankle (42). However, there was no group difference in weight and BMI. Due to the small sample size, it could be hypothesized that the current subjects with WAD would represent subjects whose life circumstances allowed for participation in such an experimental study, and thus they may not represent a general group of subjects with WAD.
Furthermore, only women were recruited.
A proper a priori power calculation involves both the assessment of a clinically relevant minimal difference as well as data on mean and SD of the variable. In the current exploratory study the clinically relevant difference was not known and we did not have access to valid data on sway analyses among patients with whiplash. An a priori power analysis, based on data among a population of cleaners with and without self-reported neck pain, was performed on rambling area during the closed eyes condition (17). In that study a 27% difference between cases and controls was found. Based on the data in that study, a power of 80% and significance level of 0.05, at least 30 subjects were needed.
Since, in the present study, we expected the difference between the more affected whiplash patients and matched controls to be larger, a study design of 20 subjects was estimated as appropriate. This assumption was confirmed as a significant difference of 85% was found in the present study. Due to the small sample size, a type 1 error cannot be excluded, but the interpretation of a significantly different postural control pattern in patients with whiplash, apart from the biological plausibility also lends support from the consistent patterns in all the 3 balance tests.
Significant group differences in this small and well-functioning sample were evident, but even larger differences may be expected in a contrasting group of subjects more severely affected by WAD.
Another limitation may be that we did not examine the subjects for vestibular deficits. They were, however, interviewed prior to entering the study and were excluded if they reported any kind of neurological disease.
Strengths of this study are the standardized test procedures, the objective measurements performed with standardized equipment, considered to be reproducible and valid (43), and responsive to training (18). Furthermore, the included subjects were tested with the same 3 experimenters, thereby minimizing inter-examiner bias.
In conclusion, subjects with WAD had higher relative neck muscle activity and larger postural sway during normal balance tasks compared with control subjects. The results indicate disturbed sensory feedback patterns in WAD, which may have negative consequences for people with this condition when performing daily activities, especially when lighting and/or the physical area of postural support is limited.
AcknowledgementS
The study was supported by the Research Fund of the Region of Southern Denmark, The Danish Rheumatism Association, and the patient organization PTU – Danish Society of Polio and Accident Victims.
References
Appendix I. Sway procedure | |
Procedure for sway testing. This procedure is a modification of a protocol, previously used in clinical trials testing the postural sway in cleaning staff with neck pain (17). | |
Introduction to the subject | Today we are going to perform 4 balance tasks, where you are standing on the platform. The tests must be performed barefoot; please therefore take off your shoes and socks. If at any time you feel tired, do not hesitate to take a break between tests. |
Preparations | Measure the subject’s weight, height and arm length – from the posterior edge of the acromion process to the perturbation rod. The weight of the perturbation weight is calculated to 3% of the total body weight. Reset the platform. |
Failure | A failure is defined as: if the subject falls, moves from the starting position or talks. The result of a failure is never saved: instead the test must be repeated. If more than 3 failures occur, mark as a failure and move on to the next test. |
Test 1: Romberg test | |
 | Description The subject is standing in the centre of the platform, with the feet parallel to the y-axis of the platform, looking at a dot 2.5 m away on the wall. The feet must be placed together heel-to-heel and toe-to-toe and with the arms lightly crossed over the chest and the back towards the platform plug. Perform 1 test with open eyes. Perform 3 tests with closed eyes. |
Instruction Please step onto the platform. Stand in the centre of the cross. Place your feet together, heel-to-heel and toe-to-toe. Cross your arms lightly over the chest. Stand as still as possible, focus on the dot in front of you and please keep quiet during the test. Ready? I will count down for you – 1, 2, 3 – Start. Closed eyes test is performed in the same way, except subject is not focusing on the dot, since the eyes are blinded. | |
Test 2: One-legged stance test | |
 | Description The subject is standing on 1 leg in the centre of the platform, with the foot parallel to the y-axis of the platform, looking at a dot 2.5 m away on the wall, and with the arms crossed lightly over the chest and the back towards the platform plug. The non-weight-bearing foot is placed towards the medial malleolus of the foot. Perform 3 tests with open eyes on 1 leg. |
Instruction Please step onto the platform. Stand in the centre of the cross. Stand on the opposite foot to your kicking leg and place the non-weight-bearing foot on the inside of the standing leg on the medial malleolus. Cross your arms lightly over the chest. Stand as still as possible, focus on the dot in front of you and please keep quiet during the testing. Ready? I will count down for you – 1, 2, 3 – Start. | |
Test 3: Perturbation test | |
 | Description The subject is standing in the centre of the platform, with the feet parallel to the y-axis of the platform, looking at a dot 2.5 m away on the wall. The feet must be placed together, heel-to-heel and toe-to-toe and with the back towards the platform plug. The arms are elevated in the sagittal plane to 90˚ shoulder flexion, holding the perturbation rod with an upper hand grip at shoulder width, corresponding to 3% of the individual’s weight. Within 20 s the weight drops automatically and the subject has to hold the starting position of the arms. A box is placed under the weight to ease the perturbation. Perform 3 tests with open eyes with a 15 s pause in between. |
Instruction Please step onto the platform. Stand in the centre of the cross. Place your feet together; heel-to-heel and toe-to-toe. Hold your arms in front of you at shoulder level and at shoulder width and hold the rod with an upper hand grip. Keep this position during the test. Within 20 s the weight will automatically drop; when this happens, try to keep your arms in the starting position until I say stop. Stand as still as possible, focus on the dot in front of you and please keep quiet during the testing. Ready? I will count down for you – 1, 2, 3 – Start. | |
