Sasha M. Scott, MPhil1, Marietta L. van der Linden, PhD1, Julie E. Hooper, PhD2, Paula Cowan, MSc3 and Thomas H. Mercer, PhD1
From the 1Queen Margaret University, 2Slateford Medical Practice and 3Kenilworth Medical Centre, United Kingdom
OBJECTIVE: To assess whether the application of Functional Electrical Stimulation improves gait kinematics and walking ability in people with multiple sclerosis who experience foot drop.
DESIGN: Acute open labelled comparative observation trial.
PARTICIPANTS: Twelve people (3 females, 9 males, EDSS 2–4) with relapsing remitting multiple sclerosis (47.8 years (standard deviation 6.6)) who were new users of functional electrical stimulation.
METHODS: Gait kinematics were recorded using 3D gait analysis. Walking ability was assessed through the 10-m walk test and the 6-min walk test. All assessments were performed with and without the assistance of functional electrical stimulation. The effect of functional electrical stimulation was analysed using paired t-tests.
RESULTS: Ankle dorsiflexion at initial contact (p = 0.026), knee flexion at initial contact (p = 0.044) and peak knee flexion during swing (p = 0.011) were significantly greater whilst walking with Functional Electrical Stimulation. The increased peak dorsiflexion in swing of nearly 4 degrees during functional electrical stimulation assisted walking approached significance (p = 0.069). The 10-m walk time was significantly improved by functional electrical stimulation (p = 0.004) but the 6 min walk test was not.
CONCLUSION: The acute application of functional electrical stimulation resulted in an orthotic effect through a change in ankle and knee kinematics and increased walking speed over a short distance in people with multiple sclerosis who experience foot drop.
Key words: functional electrical stimulation; multiple sclerosis; gait kinematics; walking ability; 3D motion analysis.
Guarantor’s address: Marietta van der Linden, Rehabilitation Sciences, Queen Margaret University, EH21 6UU Edinburgh, UK. E-mail: mvanderlinden@qmu.ac.uk
Accepted Nov 5, 2012; Epub ahead of print Feb 14, 2013
Introduction
People with multiple sclerosis (MS) frequently experience weakness (1), reduced co-ordination and spasticity of the muscles which consequently results in reduced functional capacity (2). Foot drop, which results in gait disturbance (3), is a common problem in people with MS (4). Individuals with foot drop tend to employ gait compensation strategies such as hip-hitching (pelvic elevation) and circumduction (hip abduction) (5, 6). The use of gait compensation strategies may explain that the energy cost of walking in people with MS is double that of non-MS controls as reported by Olgiati et al. (7).
Falling is a major risk for people with impaired gait (8) and the consequences of falling are serious. This is a particular concern for people with MS as steroid use is common place (9) and osteoporosis is a well recognised side effect of taking steroids (10). Therefore, in addition to an increased risk of falling people with MS also have an increased risk of sustaining a serious injury from their fall (11). Fall induced injuries are documented as one of the most common causes of restricted activity and disability among older people (12). Curtailment of activity as a consequence of fear of falling has also been documented in people with MS (13). An injurious fall can render a person with MS sedentary for a prolonged period; during this time deconditioning of skeletal muscle will occur (14). On recovery from their injury patients are likely to experience reduced functional capacity and reduced independence. It is not surprising therefore that people with MS curtail their activity as a consequence of fear of falling (13). This may, in part, account for the low levels of habitual physical activity documented in people with MS (1). Avoiding a sedentary lifestyle during adulthood not only reduces the risk of cardiovascular disease but also substantially extends total life expectancy and the cardiovascular disease-free life expectancy for men and women (15). Without an intervention to reduce fall risk, people with MS are trapped in a disuse-disability cycle. One way of potentially reducing this risk of falling is to assist patients in addressing or overcoming walking impairments such as foot drop. In turn, this may reduce the fear of falling and potentially lead to increased activity levels and independence of people with MS. The potential to arrest the disuse-disability spiral may also lead to a reduced likelihood of co-morbidity development.
Although management of foot drop has conventionally been achieved through the prescription of an ankle foot orthosis, functional electrical stimulation (FES) has recently emerged as an alternative treatment option.
FES involves the application of an extrinsically derived electrical impulse to a peripheral nerve muscle complex to elicit a contraction and produce a functional movement (16). FES can be used in patients with upper motor neurone pathology who have an intact peripheral nervous system; such as stroke (17), spinal cord injury, cerebral palsy (CP) (18) and MS. A common application of FES is the stimulation of the tibialis anterior muscle to produce dorsiflexion during the swing phase of the gait cycle. Several studies have investigated the effects of the application of FES in people after stroke (19, 20) however, research into the use of FES to aid walking ability in people with MS is limited (21, 22, 24).
In a study conducted by the manufacturers of a commercially available drop foot stimulator the regular application of FES was observed to increase walking speed by 16 % with a concurrent 24 % reduction in the physiological cost of walking for people with MS (23). Paul et al. (24) also reported that people with MS who are established users of FES walked significantly faster with the assistance of FES. Stein et al. (25) assessed participants before and after a 3 month period of daily FES use and showed that the walking ability both with and without FES improved over time. In the only study to date to have investigated the initial effects of FES in people with MS no detrimental effects of walking with FES were reported, showing that the intervention does not impede function (22). However, despite employing a battery of functional ambulatory tests to assess the 11 people with MS who had never previously used FES, the findings of this study only revealed a significant FES-assisted improvement in one test component (stairs) (22).
Although a few studies have shown that FES improves walking ability in people with MS, importantly, no study has yet quantified the orthotic effects of FES by analysing the gait kinematics in new users of the device. As a result we currently have no characterisation of the kinematic adaptations associated with improved walking performance of people with MS as a result of using FES.
We hypothesised that the application of FES would favourably alter gait kinematics towards a more normal gait pattern in people with MS who are new users of the device. Therefore, the aim of this study was to compare the kinematic characteristics and walking ability of people with MS who were new users of FES whilst walking with and without FES.
Method
The study design was an acute open-labelled comparative observation trial. Participants were recruited through a community NHS (National Health Service) physiotherapy service. People with a positive diagnosis of MS between the ages of 18 and 70 who were referred to a physiotherapist to be assessed for FES to manage foot drop were considered for participation in this study. Patients who were pregnant or breast feeding were excluded from the study. Participants who experienced a relapse were withdrawn from the study as changes in functional capacity could not be attributed to the intervention of FES.
This study was reviewed by the NHS Lothian ethics committee, research and development office and the Queen Margaret University research ethics committee; in accordance with the declaration of Helsinki. All participants provided written informed consent before testing commenced.
People with MS who were deemed suitable by their clinician for FES treatment of their dropped foot were invited to take part in this study. Participants made a maximum of 4 visits to the University motion analysis laboratory before starting to habitually use FES. Visits were separated by at least 3 clear days but no more than 14 days.
At each visit participants underwent gait analysis assessment; two 10-m timed walking tests (26), and a 6-min walk test (27). The motion analysis and 10-m walk were carried out with and without FES during each session which will be referred to as FES and No FES respectively. Gait analysis was carried out by conducting the No FES trial followed by the FES trial. The order in which the 10-m tests were conducted was counterbalanced. One 6-min walk, either with or without FES, took place at each session; again the order of these was counterbalanced. Participants were able to use additional walking aids (walking sticks) during testing if required. However, if they commenced testing with a walking aid all further testing was conducted using the same aid.
Functional Electrical Stimulation
The single channel Odstock Drop Foot Stimulator (ODFS III, Biomedical Engineering and Medical Physics, Salisbury, UK) was used to administer FES. This stimulator has an asymmetrical biphasic voltage driven wave form as the default setting; however, a symmetrical waveform can be selected. All participants but one received FES with an asymmetrical biphasic wave. The stimulator of one participant was set up to produce a symmetrical wave form as this type of stimulation lifted the foot more effectively. The intensity of the current amplitude ranged from 20 to 70 mA and was determined by the amplitude required to achieve adequate foot clearance during the swing phase of the gait. The stimulation frequency was 40 Hz. This frequency is usually used for FES as it provides a sustained smooth contraction without tiring the muscle too quickly. Output time, extension time and ramp were adjusted if required for each subject to optimise the amount and timing of ankle dorsiflexion.
Prior to the participant’s first trial the stimulator unit was fitted as part of their routine care by their physiotherapist, who was qualified to fit the stimulator unit. A FES device was fitted at each testing session using the same stimulation parameters set up by the treating clinician. The device was only switched on during FES assisted trials. One square 50 x 50 mm gel surface electrode (PALS, Platinum Blue, Nidd Valley Medical Ltd, Knaresborough Ltd) was placed over the common peroneal nerve as it passes over the head of the fibula and another over the motor point of the tibialis anterior.
Gait analysis
Participants changed into tight fitting lycra shorts on arrival and height, weight, knee width, ankle width and leg length were measured. These measurements were required for the calculation of the ankle, knee and hip joint centres by the motion analysis software (Vicon Plug in Gait). A lower back support was used to reduce skin movement around the pelvis, when required.
Three dimensional gait analysis was undertaken using a 100 Hz 8 infra-red camera Vicon Nexus 3D motion analysis system (Vicon Motion Systems, Oxford, UK). Participants had 14-mm2 passive reflective sphere makers placed on their lower limbs and the pelvis according to the Vicon Plug-In-Gait manual which is based on the Helen Hays marker system (28). A static trial was conducted using a knee alignment device (KAD) to make a direct measure of the knee flexion extension axis. The KADs were removed and standard 14-mm reflective markers were attached over the lateral epicondyle of each femur for the walking trials. Walking was assessed barefoot. Participants were asked to walk a distance of 7 m across the lab whilst the Vicon motion analysis system was recording; this constituted one trial. A minimum of 8 trials were recorded with and without FES to ensure that 6 trials for each condition were viable for analysis. Unaided walking was assessed first to eliminate any carry over effect of FES. Participants were able to sit down between trials. On completion of gait analysis participants were sat down for 10 min before commencing further testing.
Walking performance tests
Participants were asked to walk at their preferred walking speed (PWS) along a straight length of wall for 10 m in accordance with Rossier & Wade (29). Participants were also asked to walk continuously around the 16.5 m elliptical course for 6 min in accordance with Enright (30).
Kinematic data processing
Lower limb angles in the sagittal, transverse and frontal plane were derived from the 3D marker trajectories and patient measurements using the Plug in Gait software. Kinematic data was time normalised so that every trial included the data between two consecutive foot strikes which was defined as one gait cycle. The following angles were derived from each trial for statistical analysis: ankle dorsiflexion at initial contact, peak dorsiflexion in swing, knee flexion at initial contact and peak knee flexion in swing and sagittal hip range of motion (flexion minus extension). The average value of each of these angles was calculated over the 6 trials for each participant and each condition.
The Gait Deviation Index (GDI) is a multivariate measure of overall gait quality (31) developed to describe the gait of children with CP. A score of 100 is achieved by individuals without any neurological problem, while a mean score of 85 has been found for children with CP without functional limitations in their walking (Gross Motor Function Classification System I) while those with several mobility problems and using power mobility aids for longer distances (Gross Motor Function Classification System IV) were reported to have a mean score of 55 (32). The GDI was computed from pelvis, hip, knee and ankle angles derived from 3D gait analysis; the lower the score the higher the deviation from the average unimpaired person.
Statistical analysis
No accommodation or learning effects were observed over the 4 testing sessions and all analyses were based on the mean data. Comparisons between the FES and No FES trials were conducted on data collected for the most affected leg. After confirming that data was normally distributed according to the Shapiro-Wilks test differences between the No FES and FES conditions were investigated using the paired t-test. Significance was accepted at p < 0.05.
Results
The descriptive characteristics of the 12 participants recruited into this study are given in Table I. Initial comparison of unaided walking revealed a significant lower peak dorsiflexion during swing (p = 0.015) of the affected compared to the unaffected leg thus justifying the use of an assistive device to correct drop foot.
|
Table I. Participant characteristics |
|
|
Females/males, n |
3/9 |
|
Age, years, mean (SD) |
47.8 (6.6) |
|
Height, m, mean (SD) |
1.73 (0.06) |
|
Body mass, kg, mean (SD) |
85.9 (14.8) |
|
BMI, kg/m2, mean (SD) |
28.3 (3.9) |
|
Walking stick users, n |
4 |
|
SD: standard deviation. |
|
There was a significant increase in dorsiflexion (p = 0.026) and knee flexion (p = 0.044) at initial contact during FES assisted walking compared to unaided walking (Table II). Peak dorsiflexion during swing was nearly 4° degrees higher whilst walking with FES, but this difference did not reach statistical significance (p = 0.069). The application of FES resulted in an increased peak knee flexion during swing which was significantly different (p = 0.011) from the No FES condition. Figs 1 and 2 show the sagittal ankle and knee kinematics averaged over the 12 participants.
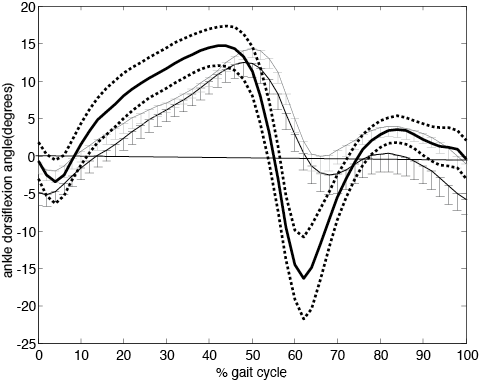
Fig. 1. Ankle dorsi-plantar flexion angle over the gait cycle (initial contact to initial contact) averaged over the 12 participants. Error bars indicate the standard error. Dorsiflexion angle is positive. Functional electric stimulation (FES) grey line, No FES black line. Healthy controls thick black line with ± one standard deviation in dotted line.
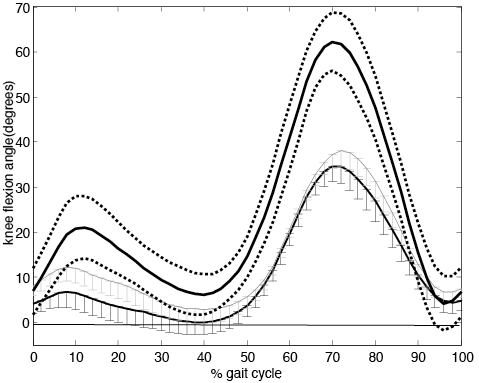
Fig. 2. Average knee flexion-extension angle over the gait cycle (initial contact to initial contact).averaged over the 12 participants. Error bars indicate the standard error. Flexion angle is positive. Functional electric stimulation (FES) grey line, No FES black line. Healthy controls thick black line with ± one standard deviation in dotted line.
There was no significant difference in hip range of motion (p = 0.089) nor stride length (p = 0.140) between FES and No FES conditions (Table II). The GDI, which is a global measure of gait quality, was not significantly different between conditions however the difference approached significance (p = 0.061) with FES conferring a positive effect on GDI (Table II).
Table II. Results of the kinematic outcome measures and p-values of paired t-tests between No FES and FES conditions | |||
Mean (SD) | p-value | ||
Ankle flexion at initial contact, ° | |||
No FES | –5.6 (6.5) | 0.026* | |
FES | –0.3 (4.5) | ||
Peak dorsiflexion during swing, ° | |||
No FES | –0.9 (6.9) | 0.069 | |
FES | 2.9 (3.5) | ||
Knee flexion at initial contact, ° | |||
No FES | 5.4 (6.7) | 0.044* | |
FES | 7.8 (5.8) | ||
Peak knee flexion during swing, ° | |||
No FES | 38.8 (10.7) | 0.011* | |
FES | 47.7 (10.8) | ||
Hip Range of Motion, ° | |||
No FES | 31.1 (5.5) | 0.089 | |
FES | 32.0 (5.8) | ||
Gait Deviation Index | |||
No FES | 79.1 (9.9) | 0.061 | |
FES | 80.7 (9.5) | ||
Stride length, m | |||
No FES | 0.92 (0.1) | 0.140 | |
FES | 0.94(0.1) | ||
*p < 0.05. FES: functional eletric stimulation; SD: standard deviation. | |||
The results of the walking performance tests are in shown Table III. Participants completed the 10-m walk test significantly quicker whilst walking with the assistance of FES (p = 0.004). However, there was no difference in 6 min walk test resultsbetween conditions (p = 0.484).
|
Table III. Results of the walking performance measures and p-values of paired t-tests between No FES and FES conditions |
||||
|
Mean (SD) |
n |
p |
||
|
10-m walk, s |
No FES |
12.7 (3.7) |
11 |
0.004* |
|
FES |
12.1 (3.5) |
11 |
||
|
6-min walk, m |
No FES |
296 (47) |
8 |
0.484 |
|
FES |
289 (40) |
8 |
||
|
FES: functional electric stimulation; SD: standard deviation. |
||||
Discussion
Initial analysis of unaided walking illustrated that participants in the current study were experiencing drop foot in their most affected leg and this thus justified the use of an assistive device to aid walking.
The acute application of FES to the tibialis anterior of people with MS with foot drop resulted in an increased knee flexion in swing and increased dorsiflexion and knee flexion at initial contact.
The significant increase in knee flexion during swing will have contributed to an improved foot clearance which allows the leg to swing through freely during the swing phase of the gait cycle. This demonstrates that improved ground clearance is achieved immediately upon application of FES. Although the improved peak dorsiflexion during swing with FES was not statistically significant, it was apparent that the application of FES favourably alters the kinematics of the affected leg and this orthotic benefit of FES has the potential to reduce the risk of tripping and falling.
The only other study to date to investigate the effect of FES on gait kinematics in people with MS reported an increase in ankle dorsiflexion at initial contact but concluded that all other parameters were variable in their response (33). This study was conducted on a small sample of 4 participants, however, the results reported were in line with that of the current study.
Peak dorsiflexion and peak knee flexion were significantly greater at initial contact which is arguably the most important phase of the gait cycle. This is the point in the gait cycle that the foot can catch on the ground and result in a trip or fall. The increased dorsiflexion and knee flexion at initial contact with the application of FES reduces the chance of this happening. Therefore the phase of the gait cycle which poses the greatest risk of patients falling shows the greatest improvement by the application of FES. This could increase patients walking confidence and therefore increase their volume of walking thereby breaking the disuse disability cycle (11, 34).
Participants walked significantly faster over the 10-m during FES assisted walking trial which reveals that the improvements in gait kinematics translate into an improvement in walking ability over short distances. The 5% improvement in walking speed over the short distances, seen in the current study, was less compared the study by Taylor et at. (23) who reported improvements of 16% in walking speed over 10 m in a group of 21 people with MS who were established users of FES. However, the 5% improvement in the 10-m test in our study agrees with the 3.9% improvement with FES in the 10-m test as reported by Stein at al. (25) at their first assessment.
In the current study no improvement in walking speed was apparent during the 6-minute walk test. This finding is inconsistent with previous research which investigated the use of FES in people with MS (24, 25). Paul et al. (24) tested established users of FES and reported a significant improvement in walking speed as measured by the 5-min walk test. However, the participants in this study were habitual users of FES therefore this may suggest a habituation period is necessary before any improvements in functional walking ability can be expected. An improved orthotic effect after a 3-month use of FES was reported by Stein et al. (25) who found that the orthotic effect on the 4-min walk was improved from 2.3% at the first assessment to 5.7% after habitual use of FES for 3 months in people with progressive neuro-disorders of which 31 out of 32 had MS. Also in the study by Stein et al. (25), effort over the 4-min walk as measured by the Physical Cost Index (PCI) showed no orthotic benefit of FES at the first assessment but a 5.9% improvement at 3 months of habitual use.
The participants in the current study were not habitual users of FES and this may account for the inconsistency in the results of the timed distance walk. Participants were not habituated to the device and may not have established a new walking pattern which may have hindered any improvement. Also due to the fact that FES was new to the participants of this study they may have experienced fatigue of the tibialis anterior (TA) during the 6-min walk test. Prior to the application of FES the TA may have experienced reduced neural innervation and as a result may have atrophied. Hence these participants were having a supramaximal contraction applied to a deconditioned muscle.
Everaert et al. (35) studied a subgroup of the participants in Stein’s study (25) establishing the neuroplastic mechanisms behind the therapeutic effects, i.e. the effect of prolonged FES on the walking ability without FES. They found large increases in the maximal voluntary contraction and motor evoked potentials after 3 months use of FES and suggested that these increases may responsible for the therapeutic effect on walking speed. It is possible that together with the local muscle adaptations discussed above, strengthening of the motor cortical areas may be responsible for an improvement of orthotic effect over time.
The study is limited by the small sample size, and the large standard deviations of the outcome measures indicating a substantial variation among the participants in both walking pattern and performance. However, the study was sufficiently powered to detect statistically significant differences in both gait kinematics and walking performance between the No FES and FES conditions at group level. Future studies are required to evaluate whether the benefit of FES depends on patient characteristics such as type and progression of MS, neuromuscular properties and gait pattern.
Clinical implications
The results of this study support the use of FES to combat foot drop in people with MS in the clinical setting as walking ability was improved over short distances. The study also demonstrates that the application of FES does not hinder walking ability. Therefore prescription of the device in the clinical setting will not impede patients’ ambulatory function.
In conclusion, this study illustrates that the acute application of FES delivers an orthotic benefit by increasing ankle dorsiflexion and knee flexion at initial contact and knee flexion in swing, thus reducing the risk of tripping and falling. Walking ability over short distances in people with MS with drop foot who are new users of the device was also improved with FES.
No beneficial effect of the acute application FES was found for the 6-min walking test, but as discussed above, this may be the lack of habituation to the device. Habitual use of FES has been shown to increase the orthotic effects and also to therapeutic effects on walking ability in people with MS (25, 35). However, no long term studies have explained this improved walking ability through the analysis of the gait kinematics.
Further appropriately powered long term studies into the effects of prolonged use of FES on the gait kinematics of people with MS are required in order the explain the altered gait mechanisms, which result in the long-term orthotic effects in people with MS.
Acknowledgements
Sasha Scott was the recipient of a Multiple Sclerosis Society PhD studentship award funded by the Multiple Sclerosis Society [grant number 873/07].
References
